Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels
nature publishing group
intervention and Prevention
Resistance Training Improves Cardiovascular
Risk Factors in Obese Women Despite
a Significative Decrease in Serum
Adiponectin Levels
Javier Ibáñez1, Mikel Izquierdo1, Cristina Martínez-Labari1, Francisco Ortega2, Ana Grijalba3,
Luis Forga4, Fernando Idoate5, Marisol García-Unciti6, José M. Fernández-Real2
and Esteban M. Gorostiaga1
Increased circulating adiponectin and insulin sensitivity are usually observed after body fat loss induced by a weight‑loss diet. Progressive resistance training (PRT) without a concomitant weight‑loss diet significantly decreases visceral fat, thus improving insulin sensitivity. Therefore, the purpose of this study was to ascertain the effects of combined 16‑week PRT and weight‑loss diet on circulating adiponectin and insulin sensitivity index. Thirty‑four obese (BMI: 30–40 kg/m2) women, aged 40–60 year, were randomized to three groups: a control group (C;
n = 9); a diet group (WL;
n = 12) with a caloric restriction of 500 kcal/d; and a diet plus resistance training group (WL+RT;
n = 13) with the same caloric restriction as group WL and a 16‑week supervised whole body PRT of two sessions/week. Both WL and WL+RT groups showed similar decreases in body mass (−6.3% and −7.7%) and visceral fat (−19.9% and −20.5%). WL resulted in an expected increase in circulating levels of adiponectin (
P = 0.07) and insulin sensitivity. However, circulating total adiponectin decreased (
P < 0.05) in WL+RT group, whereas an improvement in different cardiovascular risk factors (insulin sensitivity, low‑density lipoprotein cholesterol (LDL‑C), etc.) was observed. In conclusion, in obese women a 16‑week combined PRT and weight‑loss diet is accompanied by significant improvements in different cardiovascular risk factors in spite of a significant decrease of circulating adiponectin.
Obesity (2009)
Lifestyle changes such as weight loss and regular physical
At present, adiponectin may be the most important and promis-
activity are recognized as effective nonpharmacological inter-
ing adipocytokine for obtaining a better understanding of the link ventions with beneficial effects on metabolic and cardio vascular
between obesity and metabolic and cardiovascular disease (1,2). risk factors (6,10). In this context, different studies have dem-
Recent studies have revealed that this adipocytokine exhibits onstrated that, without a concomitant weight-loss diet, pro-
antidiabetic (3), anti-inflammatory, and antiatherogenic proper-
gressive resistance training (PRT) significantly decreases
ties (4). Adiponectin has been shown to be secreted principal y visceral fat in older men (11) and women (12), improving
by visceral adipose tissue (VAT), the size of this visceral fat depot insulin sensitivity (11). However, at present both the effect
being an important correlate of plasma adiponectin levels (5). of PRT on circulating adiponectin and the physiological role
Moreover, it is known that an excess of abdominal adipose tis-
of adiponectin on the improvement of metabolic and cardio-
sue is a better predictor of the development of insulin resistance, vascular risk factors with either body or fat mass loss are as yet
type 2 diabetes and cardiovascular disease than the total amount incompletely understood (1,13).
of adipose tissue (6,7). Circulating levels of adiponectin may be
In the present study, we hypothesized that a twice-weekly
the link between visceral obesity and certain related metabolic PRT would be particularly useful in the context of a weight-
abnormalities which contribute to the development of insulin loss diet in adult obese women, increasing the visceral fat
resistance, type 2 diabetes and atherosclerosis (3,8,9).
loss usually observed with a hypocaloric diet in women (14).
1Studies, Research and Sports Medicine Center, Government of Navarra, Pamplona, Spain; 2Department of Diabetes, Endocrinology and Nutrition. Institut d'Investigació Biomédica de Girona (IdIBGi) CIBER Fisiopatología de la Obesidad y Nutrición, Girona, Spain; 3Department of Clinical Biochemistry, Hospital of Navarra, Pamplona, Spain; 4Department of Endocrinology, Hospital of Navarra, Pamplona, Spain; 5Department of Radiology, Clinica San Miguel, Pamplona, Spain; 6Department of Nutrition and Food Sciences, Physiology and Toxicology, University of Navarra, Pamplona, Spain. Correspondence:
Received 10 February 2009; accepted 13 July 2009; advance online publication 27 August 2009.
This could yield highly favorable changes in circulating
anthropometric variables
adiponectin and, as a result, to the metabolic and cardio-
Height of the barefoot subjects was measured to the nearest 0.1 cm.
vascular risk.
Body mass was measured on the same standard medical scale to an
accuracy of ±100 g. Waist and hip circumferences were measured with
the subject standing erect with arms at the sides and feet together, wear-
Methods and Procedures
ing only underwear. The measurer placed an inelastic tape around the
subject, without compressing the skin, on a horizontal plane at the level
Thirty-four sedentary, nonsmoking, obese (BMI: 30–40 kg/m2) women,
of the last false rib and the buttocks, respectively. The measurement was
aged 40–60 years, were recruited through an advertisement in a local
recorded to the nearest 0.1 cm.
newspaper. Before inclusion in the study, all candidates were thor-
oughly screened using an extensive medical history, resting and maxi-
mal exercise electrocardiogram and blood pressure measurements.
The volumes of visceral and subcutaneous adipose tissue (SAT)
Cardiovascular, neuromuscular, arthritic, pulmonary, or other debili-
(abdominal and thigh) and muscle volume in the thigh were meas-
tating diseases as determined by one or al of the screening tools were
ured by magnetic resonance (MR). MR imaging was performed with
reasons for exclusion from the study. None of the subjects received any
a 1T magnet (Magnetom Impact Expert; Siemens, Erlangen, Germany)
medication. Al the subjects were informed in detail about the possible
using body coil The subjects were examined in a supine posi-
risks and benefits of the project, and they then signed a written consent
tion with both arms positioned paral el along the sides of the body. We
form before participating in the study. This project was approved by
obtained a spoiled T1-weighted gradient-echo sequence with repetition
the ethical committee of the regional Health Department. Participants
time = 127 ms and echo time = 6 ms. Each half body volume was scanned
were randomized to three groups: a control group (C;
n = 9); a diet
using two stacks, each containing 10 contiguous 10 mm thick slices.
group (WL;
n = 12) with a caloric restriction of 500 kcal/day; and a diet
Each stack was acquired in 20 s and interleaved slice order was used. A
plus resistance training group (WL+RT;
n = 13) with the same caloric
field of view of 500 mm was used and al the stacks were acquired with
restriction as group WL and a 16-week supervised whole-body resist-
breath- holding. The total investigation time was about 5 min.
ance training program of two sessions/week. Perimenopausal women
MR imaging of both thighs was then obtained. T1-weighted sequence
were balanced among groups. The subjects were tested on two differ-
was used with a repetition time of 645 min and a spin echo time of 20
ent occasions (weeks 0 and 16) using identical protocols. During the
min. The field of view was 500 × 500 mm and the matrix was 512 × 192.
16 weeks of the study the subjects maintained their customary recrea-
The slices were 10 mm thick, with no gap between the slices. The thighs
tional physical activities (e.g., walking). The baseline characteristics of
were scanned using two stacks, each containing 15 contiguous 10 mm
the subjects are presente
thick slices; the scan was performed axial y from the articular boundary
table 1 selected anthropometric, muscle strength, and energy intake and expenditure variables, before and after the 16 weeks of
the intervention (week 0 and week 16)
Control group (
n = 9)
WL group (
n = 12)
WL+RT group (
n = 13)
Body weight (kg)
Subcutaneous fat (cc)
11,832 ± 3,326***
11,954 ± 2,446***
Visceral fat (cc)
2,724 ± 1,052***
2,633 ± 1,000***
Subcutaneous fat (cc)
84,886 ± 23,867 73,085±20,427*** 10,3912 ± 16,407 87,520 ± 13,826***
Energy intake (kcal/d)
Carbohydrates (g/d)
Habitual energy
expenditure (Kcal/d)
1-RM bench-press (kg)
44.4 ±7.7***,†
1-RM half-squat (kg)
291 ± 73.4***,†
Values are expressed as means ± s.d.
*
P < 0.05, **
P < 0.01, ***
P < 0.001 between values in weeks 0 and 16. Differences between groups after intervention: †
P < 0.001 between C−WL+RT and WL−WL+RT.
intervention and Prevention
measured by Lipoprint System (Quantimetrix, Redondo Beach, CA).
This method is based on electrophoresis of lipid stained serum (Sudan
black) in a nondenaturing gel gradient of polyacrylamide. The intra-
and interassay coefficients of variation was <2%.
energy intake and energy expenditure analysis
At weeks 0 and 16 all subjects were interviewed by an experienced dieti-
tian and given instructions on how to complete food records accurately.
Three-day dietary food records (including 1 weekend day) were com-
pleted, the records being fil ed out on the actual day of consumption of
All food records were analyzed by DIETSOURCE (DietSource
program; version 1.0; Novartis, Barcelona, Spain).
Similarly, habitual physical activity was evaluated by accelerometry
(TriTrac-R3D System, version 2.04; Madison, WI). The TriTrac-R3D was
worn on a belt that was firmly attached to the anterior torso of the subject
at the level of the waist. TriTrac monitoring was recorded on a minute-
by-minute basis over 2 weekdays and 2 weekend days, coinciding with
the days of dietary food records.
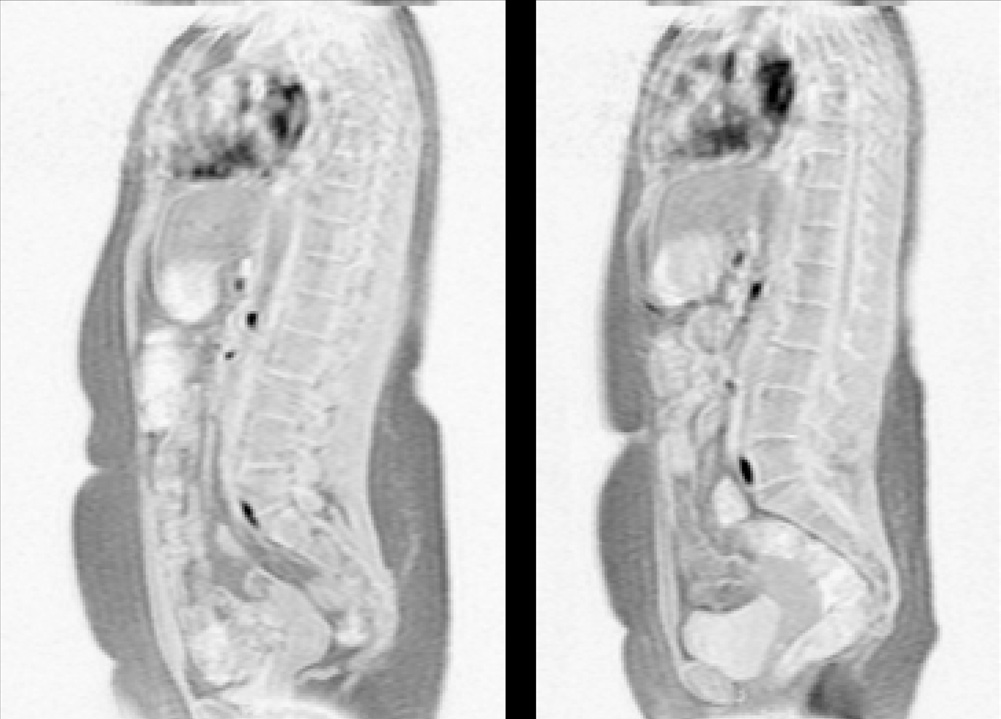
Figure 1 Magnetic Resonance of an obese woman's trunk (a) before
and (b) after a 16-week combined progressive resistance training and
weight-loss diet.
Each subject in the WL and WL+RT groups received a varied and well-
balanced hypocaloric diet (55% of calories as carbohydrates, 15% as
of the lowest external femoral condyle. The images were retrieved from
proteins, and the rest as fat) of 500 kcal/day, according to the previous
the scanner according to a DICOM (Digital Imaging and Communica-
analysis of individual daily energy expenditure by accelerometry. This
tions in Medicine) protocol. The acquired axial MR images were trans-
diet was designed to elicit a 0.5 kg weight loss per week. The control
ferred to an external personal computer running Windows XP. The level
group was asked to maintain body weight. Throughout the 16-week
of each abdominal image was labeled using sagittal scout images, referred
intervention period body weight was recorded once every 2 weeks in
to the discal level. We used special y designed image analysis software
both WL and WL+RT groups. Also, every 2 weeks each subject of the
(SliceOmatic 4.3; Tomovision, Montreal, Quebec, Canada) for quantita-
intervention groups participated in a series of 1-h seminars in which
tive analysis of the images.
the dietitian taught proper food selection and preparation, eating
behavior, control of portion sizes, and modification of binge eating and
other adverse habits.
Resting blood samples were drawn at weeks 0 and 16. Venous blood
samples were obtained at rest between 8:00 and 9:00 am from the
strength testing and training protocol
antecubital vein. In premenopausal women, all blood samples were
Lower and upper body maximal strength was assessed using 1 rep-
obtained during days 5–9 of the follicular phase to check for possible
etition concentric maximum (1-RM) action in a half-squat and in a
effects of menstrual phases. Blood was drawn after 12 h of fasting and 1
bench-press position, respectively. A detailed description of the 1-RM
day of minimal physical activity. The postintervention (week 16) blood
testing procedure can be found elsewhere (15). In brief, in the half-
draw occurred 72–96 h after the last exercise session. Whole blood
squat the subjects began the test by lifting a bar in contact with the
was centrifuged at 3,000 rpm for 15 min and the resulting serum was
shoulders with weight plates added to both ends of the bar. On com-
then removed and stored at −80 °C until subsequent analysis. Basal
mand, the subject performed a concentric extension (as fast as pos-
glycemia was analyzed using an enzymatic hexokinase method (Roche
sible) of the leg muscles starting from a knee angle of 90° to reach the
Diagnostics, Mannheim, Germany). Serum insulin levels were meas-
full extension of 180°. In the bench-press the bar was positioned 1 cm
ured in duplicate by monoclonal immunoradiometric assay (INSI-CTK
above the subject's chest and supported by the bottom stops of the
Irma; DiaSorin, Madrid, Spain). Intraassay and interassay coefficients
measurement device. Maximal strength half-squat was defined as the
of variation were <5%. To estimate insulin resistance, the homeo stasis
maximum weight that could be lifted through a full range of motion
model assessment (HOMA) index was calculated as fasting insulin
with proper form. In all tests, strong verbal encouragement was given
concentration (μU/ml) × fasting glucose concentration (mmol/l)/22.5.
to each subject to motivate them to perform each test action as maxi-
Serum levels of estradiol and progesterone were radioimmunologi-
mally and as rapidly as possible. Maximal strength variables showed
cal y measured using commercial kits (Immunotech SAS, Marseil e,
reliability coefficients ranging from 0.80 to 0.99, and the coefficients
France) according to the manufacturer's procedures. The intra- and
of variation ranged from 2 to 7%. Before testing and training, each
interassay accuracies were 6.2–9.5% and 6.6–10.2% of coefficients of
subject was familiarized with the testing procedure of voluntary force
variation for estradiol and 3.5–5.8% and 5.1–9.0% of coefficients of
production in several submaximal and maximal actions.
variation for progesterone. Serum adiponectin and leptin levels were
The strength training program used in the present study was similar to
measured by commercial y available enzyme-linked immunosorbent
that reported previously (15). Briefly, the subjects were asked to report to
assay kits (Linco Research, St Charles, MO). The intra- and interassay
the training facility twice a week to perform dynamic resistance exercise
coefficients of variation were <8 and 7%, respectively. The lowest lev-
for 45–60 min per session. A minimum of 2 days elapsed between two
els of adiponectin and leptin that can be detected by these assays are
consecutive training sessions.
0.78 and 0.50 ng/ ml, respectively. No significant cross-reactivity with
Each training session included two exercises for the leg extensor
other cytokines or hormone molecules was detected. Serum triglycer-
muscles (bilateral leg press and bilateral knee extension exercises), one
ides were measured using Infinity Triglycerides Liquid Stable reagent
exercise for the arm extensor muscle (the bench-press) and four to
(ThermoElectron, Noble Park, Australia). High-density lipoprotein
five exercises for the main muscle groups of the body. Only resistance
cholesterol (HDL-C) concentration was analyzed by a homogeneous
machines (Technogym, Gambettola, Italy) were used throughout the
method (ITC Diagnostics, Barcelona, Spain). Total cholesterol (TC) con-
training period. Resistance in this study was progressively increased or
centration was determined in serum according to the IL test cholesterol
decreased every week for the 16-week training period using a repeti-
Trinders method 181618–10 (Instrumentations Laboratory, Lexington,
tion maximum approach, so that the loads that brought about a given
MA). Low-density lipoprotein cholesterol (LDL-C) concentration was
relative intensity remained unchanged from week to week.
During the first 8 weeks of the training period the subjects trained with
group. In turn, body mass was significantly diminished after
loads of 50–70% of the individual 1-RM, and during the last 8 weeks of
16 week of intervention in WL and WL+RT groups, by −6.3
the training period the loads were 70–80% of the maximum. In addition,
from week 8 to week 16 the subjects performed a part (20%) of the leg
and −7.7%, respectively; whereas abdominal SAT decreased
extensor and bench-press sets with loads ranging from 30 to 50% of the
by −18.3 and −21.4% (P < 0.001) and thigh subcutaneous fat
maximum. In all the individual exercise sessions performed one of the
by −16.4% and −18.9% (P < 0.001), respectively. Likewise, vis-
researchers was present to direct and assist each subject towards perform-
ceral fat mass was reduced in WL and WL+RT groups by −19.9
ing the appropriate work rates and loads. In all subjects average compli-
and −20.5% (P < 0.001), respectively. As expected, in the WL
ance with the diet classes and exercise sessions was above 95%.
group a significant loss in thigh muscle mass (−5%, P < 0.05) was
observed; however, thigh muscle mass was maintained in the
Standard statistical methods were used for the calculation of the means,
WL+RT group Final y, by week 16 no significant dif-
standard deviation and Pearson product-moment correlation coeffi-
ferences between WL and WL+RT groups were observed, either
cient. One-way ANOVA was used to determine any differences among
the three groups' initial measurements. The resistance training and/or
in body mass or abdominal fat, thigh fat or thigh muscle mass.
diet related effects were assessed using a two-way ANOVA with repeated
measures (groups × time). When a significant F-value was achieved,
Basal relationships between adipocytokines levels
Bonferroni post hoc procedures were performed to locate the pairwise
and anthropometric and metabolic variables
differences between the means. Selected relative changes were analyzed
In the whole group (n = 34), no correlation was observed
via one-way ANOVA. Analyses of covariance (ANCOVA) were used
to adjust post-interventional values to compare the data between the
between baseline adiponectin levels and VAT or any other
groups. For this purpose, preinterventional values were used as covari-
measured fat compartment. Adiponectin correlated negatively
ates so that the effects of the covariance could be observed.
with waist-to-hip ratio (r = −0.359, P < 0.05). In turn, base-
Multiple regression models were used to assess the influence of muscle
line leptin levels correlated with VAT (r = 0.352, P < 0.05) and
mass and adipose tissue compartment changes on HOMA index vari-
with basal insulin and HOMA values (r = 0.397, and r = 0.427,
ation and TC changes, taking into account potential factors associated
with this variable such as age, changes in BMI, energy intake, fat in diet,
respectively, P < 0.05). Leptin also presented a marked
adiponectin, leptin, LDL-C, and HDL-C. The models were built in a cus-
association with BMI (r = 0.753, P < 0.01), waist (r = 0.606, P <
tomized way by the stepwise method. Statistical power calculations for
0.01), hip (r = 0.697, P < 0.01), abdominal SAT (r = 0.720, P <
this study ranged from 0.75 to 0.80. The P < 0.05 criterion was used for
0.01), and thigh SAT (r = 0.456, P < 0.01).
relationships between percentage variations in adipocytokine
levels and anthropometric and metabolic variables
Baseline characteristics were similar in the three groups In the WL group the difference between baseline and final
andAfter 16 weeks, no significant changes were concentration of adiponectin (Δadiponectin) was significantly
observed in the different parameters evaluated in the control correlated to the concomitant changes in ΔHDL-C (r = 0.699,
table 2 selected metabolic variables, and lipoprotein profiles before and after the 16-week of the intervention (weeks 0 and 16,
respectively)
Control group (n = 9)
WL group (n = 12)
WL+RT group (n = 13)
Metabolic variablesFasting plasma glucose
(mg/dl)Insulin (μU/ml)
HOMA index (10−14/
μU/ml)Leptin (ng/ml)
Adiponectin (μg/ml)
Estradiol (μg/ml)
Progesterone (μg/ml)
Lipoprotein profilesTriglycerides (mg/dl)
Values are expressed as means ± s.d.
HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein; TC, total cholesterol; VLDL, very low-density lipoprotein.
*P < 0.05, **P < 0.01, ***P < 0.001 between values before and after the 16 weeks of intervention.
intervention and Prevention
P < 0.05). In turn, a marked association was observed between the decrease in leptin levels that were mediated by the loss of
changes in leptin (Δleptin) and decrease in waist (Δwaist) (r = body fat. It is known that leptin regulates insulin sensitivity and
0.749, P < 0.01), hip (r = 0.734, P < 0.01), thigh subcutane-
glucose homeostasis via two different pathways: one through
ous fat (r = 0.757, P < 0.01), abdominal subcutaneous fat (r = an adiposity-dependent mechanism, by controlling energy bal-
0.649, P < 0.05), visceral fat (r = 0.663, P < 0.05), and thigh ance and body fat (increased body adiposity leads to insulin
muscle (r = 0.592, P < 0.05). In the WL+RT group, by week 16 resistance), and the other through an adiposity-independent
a negative relation was only observed between Δadiponectin pathway mediated by the CNS (18,19). Moreover, in our study
and concomitant changes in TC (−0.587, P < 0.05). In turn, a the change in total body fat (P < 0.001) was the most significant
marked association was observed between changes in leptin factor impacting on the variation in baseline insulin levels (64%
and those observed in body mass (r = 0.729, P < 0.01), hip (r = of its variance), even after accounting for the effects of age and
0.731, P < 0.01), thigh subcutaneous fat (r = 0.809, P < 0.01), changes in energy intake, thigh muscle, leptin, fat in diet, VAT
abdominal subcutaneous fat (r = 0.589, P < 0.05), thigh muscle and SAT in a multiple linear regression analysis.
mass (r = 0.592, P < 0.05), HOMA value (r = 0.666, P < 0.05),
and basal insulin levels (r = 0.612, P < 0.05).
effect of resistance training on tc and ldl-c
The results of the present study demonstrate a favorable
response of plasma TC and LDL-C to resistance training.
The main result of this study shows that a whole-body resist-
Indeed, after 16 weeks of intervention, while the lipid profile
ance training plus a weight-loss diet in middle-aged obese showed no modification in the WL group, the hypercholeste-
women were accompanied by significant improvements in dif-
rolemic women in the WL+RT group experienced a signifi-
ferent cardiovascular risk factors (insulin sensitivity, baseline cant decrease in TC and LDL-C (Table 2). These results are
insulin levels, TC or LDL-C), in spite of a significant decrease in disagreement with most interventional studies, which show
in serum adiponectin levels. Indeed, in the WL+RT group a no improvement in lipid profiles after PRT in adult men (20)
decrease in circulating total adiponectin was found in 11 of 13 or women (12,21). Indeed, two recent reviews have concluded
subjects (P < 0.05), whereas a parallel improvement in differ-
that resistance training does not seem to alter blood lipid and
ent cardiovascular risk factors (insulin sensitivity, LDL-C, etc.) lipoprotein levels (10,22). For Braith and Stewart (10) a pos-
was observed. In turn, in the WL group a similar diet in terms sible explanation for the lack of significant lipoprotein-lipid
of caloric content and nutrient composition, and a loss of body changes with PRT may be the fact that TC values for most
mass and adipose tissue of similar magnitude were accompa-
study groups have been ≤200 mg/dl at study entry. Individuals
nied by no changes in serum adiponectin levels or lipid profile, with normal lipid profiles may require greater exercise stimulus
although an increase in insulin sensitivity and a decrease in and energy expenditure, coupled with significant reductions in
baseline insulin levels were observed.
body weight, to further improve lipid profiles. Surprisingly, no
change in lipid profile was found in the WL group even though
dissociation between insulin metabolism
no significant differences were observed between the WL and
and circulating adiponectin
WL+RT groups either in improvement in insulin sensitivity or
Two mechanisms could explain the dissociation of improve-
in diet and habitual caloric expenditure, or in body/fat mass
ment in insulin metabolism from the circulating adiponectin loss. Whereas no clear dose–response relation between weight
observed in the WL+RT group. First, a decreased amount of loss and lipid modulations could be determined, it would
circulating adiponectin, in parallel to an insulin- sensitizing appear that trials that experience a weight reduction >5%
effect of resistance training associated with increases in adi-
of initial body weight seem to observe the most significant
ponectin receptor expression should not be discarded. Available changes in TC and LDL-C concentrations (23). However, this
evidence suggests that a relationship between aerobic exercise was not the case with our WL group, in which a decrease of
and adiponectin-mediated increases in insulin sensitivity is 6% was not translated into an improvement in lipid profile. In
more likely to occur at the level of receptor expression in skel-
view of these findings, it may be assumed that chronic resist-
etal muscle (16). Second, Abbasi et al. (17) reported that it is ance exercise was the main factor responsible for the lipid pro-
possible to dissociate improvements in insulin metabolism with file improvement in our WL+RT group. This suggestion is in
weight loss from circulating adiponectin, concluding that other agreement with the results of Fahlman et al. (24), who reported
factors (e.g., other adipokynes or cytokines produced in adi-
that 10 weeks of resistance training at a rate of three sessions/
pose tissue or elsewhere) may play a role in the improvement of week in overweight older women significantly improved the
insulin sensitivity. In our study, a marked relation was observed lipid profile without concurrent changes in weight or diet.
in the WL+RT group between changes in leptin (Δleptin) and
concomitant changes in circulating insulin levels and HOMA effect of resistance training on hdl-c
value, these correlations being also present in the whole group As to the significant decrease of HDL-C in the WL+RT group,
(n = 34) before the start of the study. These results agree with a this finding agrees with most of the results in the literature.
previous study by Ryan (6) that reported the effects of 16 weeks Indeed, a review by Durstine et al. (22) concluded that in
of a WL+RT program in obese postmenopausal women, con-
women, when bodyweight loss is induced by either dietary
cluding that the increase in insulin action may be related to intervention, alone or in combination with exercise, a majority
of studies indicate that HDL-C will decrease or not change.
In conclusion, our results agree with some previous studies
At present, we are unable to find any plausible explanation indicating that there are major controversies around the real
for this decrease in serum HDL-C. In our study no correla-
physiological meaning of increases in circulating adiponectin
tion was found between the variation of this lipid variable and that need to be solved. Moreover, future studies are needed to
any other physiological or metabolic factors. Different authors determine whether the mechanism of the decreased circulat-
have reported that in nondiabetic individuals, and also in type ing adiponectin found in the present study can be explained
2 diabetic patients, circulating adiponectin is positively associ-
by a change in the multiisoforms and/or the levels of receptor
ated with plasma HDL-C and negatively correlated with LDL-C expression of this hormone.
and triglycerides (25). In this context, a recent study reported
that hypoadiponectinemia was independently associated with acknowledgMents
increased postheparin plasma hepatic lipase activity, which in The study was supported by grant no. 04/1594 from the Instituto de Salud
turn could result in reductions in HDL-C (26). Of note in our Carlos III, Ministerio de Sanidad y Consumo, Spain.
study was that the difference between baseline and final con-
dIsclosure
centration of adiponectin (Δadiponectin) in the WL group sig-
The authors declared no conflict of interest.
nificantly correlated to the concomitant changes in ΔHDL-C
(r = 0.699, P < 0.05). However, no relation was found between 2009 The Obesity Society
serum adiponectin and any lipid profile variable, either in the
whole group (n = 34) before the start of the study or after 16 reFerences
1. Ahima RS. Metabolic actions of adipocyte hormones: focus on adiponectin.
weeks of intervention in the WL+RT group. Interestingly, the
Obesity 2006;14(Suppl 1):S9–S15.
most significant factor impacting on TC in this WL+RT group 2. Pischon T, Rimm EB. Adiponectin: a promising marker for cardiovascular
(95% of its variance) was LDL-C, even after accounting for the
disease. Clin Chem 2006;52:797–799.
3. Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin
effects of age, BMI, fat in diet, thigh muscle, VAT, SAT, and
multimeric complexes and the metabolic syndrome trait cluster. Diabetes
adiponectin in a multiple linear regression analysis.
4. Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB
activation and IL-6 production and increases PPARgamma2 expression in
Increased serum adiponectin levels may not always be
adipocytes. Am J Physiol Regul Integr Comp Physiol 2005;288:R1220–R1225.
beneficial in terms of good health
5. Motoshima H, Wu X, Sinha MK et al. Differential regulation of adiponectin
One could speculate about the physiological meaning of a
secretion from cultured human omental and subcutaneous adipocytes: effects
significant decrease in serum adiponectin in hypercholes-
of insulin and rosiglitazone. J Clin Endocrinol Metab 2002;87:5662–5667.
6. Ryan AS. Insulin resistance with aging. Effects of diet and exercise. Sports
terolemic obese women submitted to a PRT program plus a
weight-loss diet. Although a state of opinion has been created 7. Lapidus L, Bengtsson C, Larsson B et al. Distribution of adipose tissue and
assuming that increased adiponectin levels are beneficial in
risk of cardiovascular disease and death: a 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Br Med J
terms of good health and low circulating concentrations of this
(Clin Res Ed) 1984;289:1257–1261.
hormone have been shown associated to metabolic and cardio-
8. Hotta K, Funahashi T, Arita Y et al. Plasma concentrations of a novel,
vascular disease (3,8,9), in recent years different authors have
adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20:1595–1599.
emphasized that increased adiponectin levels may not always 9. Vilarrasa N, Vendrell J, Maravall J et al. Distribution and determinants of
be beneficial (27,28). Hadjadj et al. (27) reported increased
adiponectin, resistin and ghrelin in a randomly selected healthy population.
levels of adiponectin associated with diabetes-related microv-
Clin Endocrinol (Oxf) 2005;63:329–335.
ascular complications. More recently, Dekker et al. (28) found 10. Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention
of cardiovascular disease. Circulation 2006;113:2642–2650.
that, after adjustment for cardiovascular risk factors, high 11. Ibañez J, Izquierdo M, Argüelles I et al. Twice-weekly progressive resistance
adipo nectin was significantly associated with increased all-
training decreases abdominal fat and improves insulin sensitivity in older
cause and cardiovascular mortality.
men with type 2 diabetes. Diabetes Care 2005;28:662–667.
12. Treuth MS, Hunter GR, Kekes-Szabo T et al. Reduction in intra-abdominal
The present study has several limitations. First, the lack
adipose tissue after strength training in older women. J Appl Physiol
of multiisoforms and receptors of adiponectin. In fact, the
results found in this study may be explained by a change in 13. Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic
review. Obesity (Silver Spring) 2008;16:241–256.
these multiisoforms and/or the expression of adiponectin 14. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight
receptors. However, although the mechanism responsible for
loss on regional fat distribution and insulin sensitivity in obesity. Diabetes
the decreased circulating adiponectin cannot be discerned, to
the best of our knowledge this is the first time in which such a 15. Izquierdo M, Häkkinen K, Ibañez J et al. Effects of strength training on
muscle power and serum hormones in middle-aged and older men. J Appl
physiological adaptation (i.e., decrease in serum adiponectin
with a parallel improvement in insulin sensitivity and other 16. Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin
cardiovascular risk factors) has been observed as a result of
receptor expression in skeletal muscle: effects of exercise. Diabetes Metab Res Rev 2007;23:600–611.
an ordinary nonpharmacological treatment of obesity. Second, 17. Abbasi F, Chang SA, Chu JW et al. Improvements in insulin resistance with
the different ovarian functional status in women. However,
weight loss, in contrast to rosiglitazone, are not associated with changes
this limitation was avoided measuring basal circulating estra-
in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol 2006;290:R139–R144.
diol levels (Table 2) and balancing menopausal and perimeno-
18. Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance.
pausal women between groups.
J Clin Endocrinol Metab 2004;89:447–452.
intervention and Prevention
19. Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain
24. Fahlman MM, Boardley D, Lambert CP, Flynn MG. Effects of
adipocytokine action and metabolic regulation. Diabetes 2006;55
endurance training and resistance training on plasma lipoprotein
profiles in elderly women. J Gerontol A Biol Sci Med Sci 2002;57:
20. Kokkinos PF, Hurley BF, Vaccaro P et al. Effects of low- and high-repetition
resistive training on lipoprotein-lipid profiles. Med Sci Sports Exerc
25. Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin
concentrations in women with dyslipidemia. J Clin Endocrinol Metab
21. Blumenthal JA, Matthews K, Fredrikson M et al. Effects of exercise training
on cardiovascular function and plasma lipid, lipoprotein, and apolipoprotein
26. Schneider JG, von Eynatten M, Schiekofer S, Nawroth PP, Dugi KA. Low
concentrations in premenopausal and postmenopausal women. Arterioscler
plasma adiponectin levels are associated with increased hepatic lipase
activity in vivo. Diabetes Care 2005;28:2181–2186.
22. Durstine JL, Grandjean PW, Davis PG et al. Blood lipid and lipoprotein
27. Hadjadj S, Aubert R, Fumeron F et al. Increased plasma adiponectin
adaptations to exercise: a quantitative analysis. Sports Med
concentrations are associated with microangiopathy in type 1 diabetic
23. Varady KA, Jones PJ. Combination diet and exercise interventions for
28. Dekker JM, Funahashi T, Nijpels G et al. Prognostic value of adiponectin
the treatment of dyslipidemia: an effective preliminary strategy to lower
for cardiovascular disease and mortality. J Clin Endocrinol Metab
cholesterol levels? J Nutr 2005;135:1829–1835.
Source: http://www2.unavarra.es/gesadj/depCSalud/mikel_izquierdo/Obesity%20(2009).pdf
FARREN.DOC 12/22/2008 10:23:30 AM REMOVING THE WRINKLE IN COSMETICS AND DRUG REGULATION: A NOTICE RATING SYSTEM AND EDUCATION PROPOSAL FOR ANTI-AGING COSMECEUTICALS Victoria Farren Anti-aging skincare products often make unrealistic anti-aging claims that mislead consumers, particularly older consumers. Because of the different premarket testing standards for cosmetics and drugs, companies often classify and market their anti-aging skincare products as cosmetics to the FDA in order to avoid more rigorous standards, yet simultaneously emphasize the drug-like qualities of the products to consumers, suggesting that these products are equivalent to drugs. The FDA allows these products, known as "cosmeceuticals," to be classified as cosmetics despite their drug-like appearances and qualities, such as high-tech anti-aging skincare products that use nanotechnology, stem cell research, or DNA. The weakness of the present classification system in handling cosmeceuticals, which fall into the gray area between clearly defined cosmetics and drugs, creates unknown health risks and confuses and misleads consumers about the actual physiological effects of these products. To resolve these two problems, the Federal Food and Drug Administration need not extensively amend its regulations of the cosmetic and drug categories. Rather, the FDA should instate a notice system paired with consumer education, as well as more carefully regulate product claims.
OPEN Mechanical competence of ovariectomy-induced compromised bone after single or combined Received: 10 February 2015 Accepted: 28 April 2015 treatment with high-frequency Published: 01 June 2015 loading and bisphosphonatesCamargos G. V.1,3, Bhattacharya P.2, van Lenthe G. H.2, Del Bel Cury A. A.3, Naert I.1, Duyck J.1 & Vandamme K.1




