Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Vedafil.co.nz
power, performance, price
Erectile dysfunction
Erectile dysfunction is a common condition affecting over
50% of men over the age of 40, at some time1. How many
of your patients are hiding in their sheds or workshops,
afraid or too embarrassed to discuss their erectile
difficulties and not receiving treatment?Men need good tools. So let's get your male patients out
of their sheds and workshops and give them the power.
Sildenafil is the most common PDE5 inhibitor treatment
available2, and with Vedafil®, your patients have a cost
effective alternative to help restore their normal sex life
Sildenafil use significantly
improves erectile function
and is well tolerated3.
The efficacy and safety of sildenafil has been established
in a large number of clinical trials. In addition to
improvements in erectile function, analysis of the data
showed that sildenafil treatment also improved the
domains of orgasm, satisfaction with intercourse and
Vedafil®
• The alternative choice in erectile dysfunction (ED)
• Bioequivalent to Viagra®• Sildenafil citrate is the most common PDE5I treatment
• Patient support • Visit www.vedafil.co.nz







Sildenafil citrate 25mg, 50mg and 100mg tablets
Vedafil® is an oral therapy for erectile dysfunction which restores impaired erectile
function by increasing blood flow to the penis, resulting in a natural response to sexual
stimulation in adult males.
Vedafil® offers your patients a cost effective choice in their ED medication options. While
ED treatment options can be expensive, Vedafil® is priced to ensure cost savings to your
patient, assisting with any anxiety, stress or worry around the cost of treatment.
The Vedafil® Buzz Card loyalty programme provides your patients with a free pack of
Vedafil® with every four packs purchased – that's every fifth pack of Vedafil® for free5.
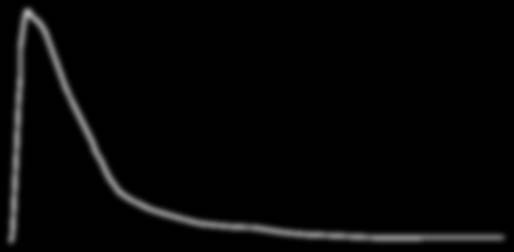
Bioequivalence6 to Viagra®
Single dose fasting bioequivalence study. Mean plasma concentration of sildenafil citrate vs time (N=41)
Viagra® 100mg tablet (Pfizer)
Vedafil® 100mg tablet (Mylan)
ation (ng/ml)tr 300
00.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00 26.00
Avigra® is an identical formulation to Viagra®. Avigra® was registered as an additional name with Viagra® as the parent product in February 2011.
Vedafil® is an approved and registered medicine having been thoroughly evaluated by
the required NZ authorities according to international standards.
As part of the registration process, bioequivalence was investigated to compare and
evaluate the single-dose oral bioavailability of Vedafil® (sildenafil citrate) 100mg tablets
(Mylan Ltd) and Viagra® (sildenafil citrate) 100mg tablets (Pfizer Australia Pty Ltd), as well
as to monitor the safety of subjects.
The study was a randomized, open label, two treatment, two period, two sequence,
single dose, crossover, bioequivalence study in healthy human adult male subjects, under
fasting conditions. A wash out period of 7 days was maintained between consecutive
dosing periods.
Sildenafil citrate 25mg, 50mg and 100mg tablets
Use in adults
The recommended starting dose is 50mg taken approximately one hour before sexual
activity. Based on efficacy and toleration, the dose may be increased to 100mg or
decreased to 25mg. The maximum recommended dose is 100mg. The maximum
recommended dosing frequency is once per day.
When sildenafil is taken with a high fat meal, the rate of absorption is reduced and
patients may need to individualise their dosing relative to their food intake based on
their own experienced clinical response.
Use in patients with impaired renal or hepatic function, or elderly patients
A 25mg starting dose should be considered in these patient groups. Based on efficacy
and toleration, the dose may be increased to 50mg and 100mg.
Use in patients using other medications
Concomitant use of potent CYP 3A4 inhibitors has been associated with increased
plasma levels of sildenafil. Since higher plasma levels may increase both the efficacy
and incidence of adverse events, a starting dose of 25mg should be considered in these
In order to minimise the potential for developing postural hypotension, patients should
be stable on alpha-blocker therapy prior to initiating sildenafil treatment. In addition,
initiation of sildenafil at lower doses should be considered.
Refer to the Vedafil® data sheet available at www.medsafe.govt.nz for further information on medications that may be affected by sildenafil or may affect how sildenafil works.
Buzz Card Loyalty Programme
Vedafil® offers your patients additional cost
savings with the Buzz Card Loyalty Programme.
With every four packs of Vedafil® purchased,
we provide the fifth pack free5.
How it works.
The patient completes a Buzz Card
application form and posts in to Mylan
New Zealand with their four pharmacy
purchase receipts.
2. Mylan New Zealand sends a free pack of
Vedafil® to the nominated pharmacy for
collection by the patient.
Buzz Card application forms can be obtained from www.vedafil.co.nz or from a
pharmacy or doctor. If you would like to request Buzz Cards for your patients, please
contact us; [email protected] or Freephone 0800 579 811.
Vedafil® GP Sampling
Mylan New Zealand offers a sampling programme to GP's, enabling your patients to trial
Vedafil® prior to purchase.
To join the Vedafil® GP sampling programme, go to the healthcare professional section of
www.vedafil.co.nz to send us your request.
Please note that certain criteria apply and must be verified prior to product samples
being sent.
Establishing a diagnosis and treatment for ED can be an embarrassing and often confusing
time for men who are experiencing erectile difficulties, as well as their partners.
Vedafil.co.nz offers a variety of information from causes of ED through to what to expect
at your doctors visit. Patient resources such as the patient information booklet and Buzz
Card can also be downloaded.
Hypersensitivity to any component of the product
Men for whom sexual intercourse is inadvisable
ED patients with a previous episode of non-arteritic anterior ischaemic optic neuropathy (NAION)
Severe hepatic impairment
Hypotension (<90/50 mmHg)
Hypertension (>170/110 mmHg)
Recent history of stroke or myocardial infarction
Hereditary degenerative retinal disorders
Concomitant use with nitric oxide donors, organic nitrates or organic nitrites in any form, including
glyceryl trinitrate, isosorbide salts, sodium nitroprusside, amyl nitrite, nicorandil or organic nitrates
Vedafil® is not indicated for use by women.
Viagra® is a registered trademark of Pfizer Products Inc.
1. Feldman HA et al. J. of Urol. 1994 151:54-61
2. IMS Analyser data May 2014, G04E Erectile Dysfunction Prd, MAT Units
3. Fink H et al. Arch Intern Med. 2002; 162:1349-1360
4. Vedafil® data sheet
5. A valid prescription is required and normal doctor's fees and pharmacy dispensing fees apply
6. Mylan NZ data on file
Vedafil (sildenafil citrate) 25 mg, 50 mg, 100 mg Tablets.
Medicine Classification: Prescription medicine. Indications: For the treatment of erectile dysfunction in adult males. Contraindications:
Hypersensitivity to any component of the tablet; Men for whom sexual intercourse is inadvisable; ED patients with a previous
episode of non-arteritic anterior ischaemic optic neuropathy (NAION); severe hepatic impairment; hypotension (<90/50 mmHg);
hypertension (>170/110 mmHg); recent history of stroke or myocardial infarction; hereditary degenerative retinal disorders.
Concomitant use with nitric oxide donors, organic nitrates or organic nitrites in any form, including glyceryl trinitrate, isosorbide
salts, sodium nitroprusside, amyl nitrite, nicorandil or organic nitrates. Not indicated for use by women. Precautions: Cardiovascular
status including recent onset angina, patients with left ventricular outflow obstruction and patients with multiple system atrophy
manifesting as severely impaired autonomic control of blood pressure. Caution in patients with: anatomical deformation of the penis;
priapism; bleeding disorders; active peptic ulceration and diabetic retinopathy. Advise caution when driving or operating machinery.
Interactions: Caution with CYP3A4 or CYP2C9 inhibitors or inducers and alpha-blockers. Use with other treatments for erectile
dysfunction is not recommended. Adverse Effects: Headache, flushing, dyspepsia, nasal congestion, diarrhoea, abnormal/
decreased vision, cardiovascular events. Refer to data sheet for full list of adverse effects. Dosage & Administration:
Average dose is 50 mg once daily taken approximately one hour before sexual activity. Vedafil is an unfunded prescription
medicine. Your patient will need to pay for this medicine. Before prescribing please refer to the full datasheet, available from
www.medsafe.govt.nz. Mylan NZ Ltd., Auckland. DA0114JL-18.
MEN NEED GOOD TOOLS
Source: http://www.vedafil.co.nz/userDocuments/Vedafil%20Health%20Care%20Professional%20Brochure.pdf
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 2010, p. 2075–2085 Copyright © 2010, American Society for Microbiology. All Rights Reserved. Monitoring the Effects of Chiral Pharmaceuticals on Aquatic Microorganisms by Metabolic Fingerprinting䌤 Emma S. Wharfe, Catherine L. Winder, Roger M. Jarvis, and Royston Goodacre* School of Chemistry and Manchester Interdisciplinary Biocentre, University of Manchester,
SAIC/CHCS Doc. TC-4.5-0359 29 Jul 1996 PHR: OUTPATIENT PHARMACY FUNCTIONS Copyright 1996 SAIC License is granted under Contract DAHC94-88-D-0005 and the provisions of DFAR 52.227-7013 (May 1987) to the U.S. Government and third parties under its employ to reproduce this document, in whole or in part, for Government purposes only.











