Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Microsoft word - nano res-tup-research.doc



Nano Research
DOI 10.1007/s12274-015-0935-3
New approach for the treatment of CLL using
Sara Capolla1,§ (*), Nelly Mezzaroba1,§, Sonia Zorzet1, Claudio Tripodo2, Ramiro Mendoza-Maldonado3,
Marilena Granzotto4, Francesca Vita1, Ruben Spretz5, Gustavo Larsen5,6, Sandra Noriega5, Eduardo Mansilla7, Michele Dal Bo8, Valter Gattei8, Gabriele Pozzato4, Luis Núñez5,6, and Paolo Macor1,9 (*)
Nano Res., Just Accepted Manuscript • DOI: 10.1007/s12274-015-0935-3
http://www.thenanoresearch.com on November. 2, 2015
Tsinghua University Press 2015
Just Accepted
This is a "Just Accepted" manuscript, which has been examined by the peer-review process and has been accepted for publication. A "Just Accepted" manuscript is published online shortly after its acceptance, which is prior to technical editing and formatting and author proofing. Tsinghua University Press (TUP) provides "Just Accepted" as an optional and free service which allows authors to make their results available to the research community as soon as possible after acceptance. After a manuscript has been technically edited and formatted, it will be removed from the "Just Accepted" Web site and published as an ASAP article. Please note that technical editing may introduce minor changes to the manuscript text and/or graphics which may affect the content, and all legal disclaimers that apply to the journal pertain. In no event shall TUP be held responsible for errors or consequences arising from the use of any information contained in these "Just Accepted" manuscripts. To cite this manuscript please use its Digital Object Identifier (DOI®), which is identical for all formats of publication.
TABLE OF CONTENTS (TOC)

New approach for the Treatment of CLL using
anti-CD20 Nanoparticles.
Sara Capolla1 § *, Nelly Mezzaroba1 § , Sonia Zorzet1,
Mendoza-Maldonado3,
Marilena Granzotto4, Francesca Vita1, Ruben Spretz5,
Gustavo Larsen5,6, Sandra Noriega5, Eduardo Mansilla7,
Michele Dal Bo8, Valter Gattei8, Gabriele Pozzato4, Luis
Núñez5,6 and Paolo Macor1,9*
We reported a nanoplatform based on the use of biodegradable
1University of Trieste, Italy; 2University of Palermo, chemotherapeutic-loaded immune-nanoparticles for the treatment of
Italy; 3Molecular Oncology Unit, National Laboratory
Chemotherapeutic-loaded
Consorzio Interuniversitatio per le Biotecnologie (CIB),
nanoparticles were specifically targeted inside leukemic cells by an
Italy; 4University of Trieste, Italy; 5LNK Chemsolutions
anti-CD20 antibody thus improving the survival of leukemia-bearing
LLC, USA; 6Bio-Target Inc., USA; 7Centro Ùnico
mice in respect to the same amount of free drugs.
Coordinador de Ablacion e Implante Provincia de
Buenos Aires (C.U.C.A.I.B.A.), Argentina; 8Clinical and
Experimental Onco-Hematology Unit, Centro di
Riferimento Oncologico, Istituto di Ricerca e Cura a
Carattere Scientifico (I.R.C.C.S.), Italy; and 9Callerio
Foundation Onlus, Institutes of Biological Researches,
Gustavo Larsen, www.lnkchemsolutions.com
Luis Núñez, www.biotarget-ln.com



Nano Research
DOI (automatically inserted by the publisher)
Research Article
New Approach for the Treatment of CLL using
Chlorambucil/Hydroxychloroquine-loaded
anti-CD20
Sara Capolla1§(*), Nelly Mezzaroba1§, Sonia Zorzet1, Claudio Tripodo2, Ramiro Mendoza-Maldonado3, Marilena Granzotto4, Francesca Vita1, Ruben Spretz5, Gustavo Larsen5,6, Sandra Noriega5, Eduardo Mansilla7, Michele Dal Bo8, Valter Gattei8, Gabriele Pozzato4, Luis Núñez5,6 and Paolo Macor1,9(*) 1Department of Life Sciences, University of Trieste, Trieste, Italy 2Department of Human Pathology, University of Palermo, Italy 3Molecular Oncology Unit, National Laboratory Consorzio Interuniversitatio per le Biotecnologie (CIB), Trieste, Italy 4Dipartimento Universitario Clinico di Scienze mediche, Chirurgiche e della Salute, University of Trieste, Trieste, Italy 5LNK Chemsolutions LLC, Lincoln, NE 68521, USA 6Bio-Target Inc., Chicago, IL, USA; 7Centro Ùnico Coordinador de Ablacion e Implante Provincia de Buenos Aires (C.U.C.A.I.B.A.), Ministry of Health, La Plata, Buenos Aires, Argentina 8Clinical and Experimental Onco-Hematology Unit, Centro di Riferimento Oncologico, Istituto di Ricerca e Cura a Carattere Scientifico (I.R.C.C.S.), Aviano, Italy 9Cal erio Foundation Onlus, Institutes of Biological Researches, Trieste, Italy. § These authors contributed equal y to this work.
Received: day month year
ABSTRACT
Revised: day month year
Current approaches for the treatment of chronic lymphocytic leukemia (CLL)
Accepted: day month year
have greatly improved the prognosis for survival, but some patients remain
(automatically inserted by
refractive to these therapeutic regimens. Hence, there is an urgent need for
novel therapeutic strategies for difficult-to-treat leukemia cases, in addition to reducing the long-term side-effects impact of therapeutics for all leukemia
patients. Due to the cytotoxicity of drugs, currently the major challenge is to deliver the therapeutic agents to neoplastic cells while preserving the viability of non-malignant cells. In this contribution, we propose a therapeutic approach
Tsinghua University Press
in which high doses of hydroxychloroquine and chlorambucil were loaded into
and Springer-Verlag Berlin
biodegradable polymeric nanoparticles coated with an anti-CD20 antibody.
We firstly demonstrated nanoparticles' ability to target and internalize in tumor B-cells. Moreover, these nanoparticles were able to kill not only p53 mutated/deleted leukemia cell lines expressing a low amount of CD20, but also
circulating primary cells purified from chronic lymphocytic leukemia patients.
KEYWORDS
Their safety was demonstrated in healthy mice, and their therapeutic effects in a new model of aggressive leukemia. These results demonstrated that anti-CD20
nanoparticles containing hydroxychloroquine and chlorambucil can be effective
in controlling aggressive leukemia and provided a rationale for adopting this
approach for the treatment of other B-cell disorders.
tissues. The development of nanoparticles, made
1 Introduction
with biodegradable biopolymers, and loaded with chemotherapeutic agents, is an attractive method
Chronic Lymphocytic Leukemia (CLL) is a
to target neoplastic cells [13–15]. In fact,
heterogeneous disease with highly variable clinical
nanoparticles can be designed by attaching
courses and survivals ranging from months to
specific antibodies on their surface thus they are
decades. In particular, a subset of patients is
able to recognize tumor-associated antigens and
affected by a high-risk CLL form that rapidly
induce specific homing on the neoplastic cell
progresses and develops a disease that requires
surface [16, 17]. Therefore, the efficacy of
symptomatic treatment [1]. Over-represented in
high-dose chemotherapy is associated to the
this group are patients bearing mutations/deletion
specificity and the low side effects of
of the TP53 gene [2]. Moreover, a high-risk CLL
antibody-based therapy and protective nature of
patient fraction was confirmed to carry
polymeric encapsulated chemotherapeutics.
mutations/deletion of other genes, such as
On these principal characteristics, we developed
NOTCH1, BIRC3 or SF3B1 [3–7].
biodegradable nanoparticles (BNPs) coated with
For years, the standard therapy was based on the
an anti-CD20 antibody to target neoplastic B-cells,
use of alkylating agents, with not much (if any)
and loaded with hydroxychloroquine (HCQ) and
effects on the CLL natural history. The
chlorambucil (CLB) to specifically kill tumor
introduction of fludarabine signified an important
B-cells [18, 19]. For the first time, we
breakthrough in CLL therapy. The use of
demonstrated the safety and therapeutic effects
monoclonal antibodies (anti-CD20, anti-CD52 and
of targeted nanoparticles in a new leukemia
beyond) opened a new perspective overcoming
xenograft SCID mice model.
the paradigm to treat only patients with minimal complications and, alone or in combination with
2 Experimental
chemotherapy, these therapeutics increased significantly the overall survival of the patients [8,
2.1 Cells, antibodies and sera
9]. More recently, inhibitors of B-cell receptor signaling showed durable efficacy in a subset of
The CLL-like cell line MEC1 [20] (kindly
CLL patients [9–11]. Despite combined therapy
provided by prof. Josee Golay), carrying both a
advancements, CLL remains an incurable disease
TP53 mutation (i.e. c.422insC) and the 17p13
in most cases since molecular complete remission
deletion, was cultured in RPMI-1640 medium
is unachievable and, as a consequence, the disease
(Sigma-Aldrich, Milan, Italy) supplemented with
relapses invariably after some months or years. In
10% fetal bovine serum (FBS; GE Healthcare
particular, the small subgroup of patients, known
Milan, Italy). Heparinized peripheral blood
as ultra high-risk CLL, shows poor response to
samples were obtained after written informed
chemo-immunotherapy and have a life expectancy
consent from untreated CLL patients at the
of less than 2 to 3 years with conventional
University Hospital in Trieste (B-cells more than
regimens [10, 12]. These considerations indicate
90% of total circulating cells). The study was
that new therapeutic approaches are needed to
approved by the IRB of the CRO (IRCCS) of
obtain the complete recovery or at least to improve
Aviano (IRB-06-2010). The mononuclear cell
survival of CLL patients. Since most patients are
fractions were isolated by centrifugation on
older in age and often have several co-morbidities,
any new treatment approaches, in addition to
gradients [21]. MEC1 cells were suspended in
higher efficacy, must be non-toxic to organs and
serum-free RPMI-1640 medium and stained with
Female SCID mice (4–6 weeks of age) were
Healthcare) as previously reported [22]. The
provided by Charles River (Milan, Italy) and
anti-CD20 chimeric antibody Rituximab (Roche,
maintained under pathogen-free conditions.
Milan, Italy) was derived from the clinic
Animals were pretreated with cyclophosphamide
(University of Trieste, Italy). The mouse mAb to
(200mg/kg), inoculated subcutaneously with 107
CD20 and anti-PARP1 antibody were purchased
MEC1 cells or intravenously with 5x105 MEC1
from BioLegend (San Diego, CA) and Bethyl
cells after 24 hours and examined twice weekly
up to 125 days. C57/BL mice were obtained from
immunophenotypical
characterization,
the animal house of the University of Trieste. All
anti-human CD5 PE (Immunotools, Friesoythe,
the experimental procedures involving animals
Germany), anti-human CD20 (clone L26,
were done in compliance with the guidelines of
Novacastra), anti-human CD45 APC (Invitrogen,
the European and the Italian laws and were
Milan, Italy) and anti-human CD19 TC (GE
approved by the Italian Ministry of Health as well
Healthcare) mAbs were used. Anti-LC3,
as by the Administration of the University
anti-a-tubulin mAbs and all the secondary
Animal House (Prot. 42/2012).
antibodies were purchased from Sigma-Aldrich (Milan, Italy) or Aczon (Monte San Pietro,
2.4 Cytometric analysis
Bologna, Italy). Human sera from AB Rh+ blood donors (NHS - normal human serum) were
BNPs' binding was assayed incubating 10µL of
kindly provided by the Blood Transfusion Center
BNPs with 5x105 cells MEC1 cells for 1h at 37°C.
(Trieste, Italy) as a source of complement (NHS -
MEC1 localization in mouse blood was performed
normal human serum).
using anti-CD45 APC and anti-CD19 TC antibodies at 28 days after cells' injection. For
2.2 BNPs preparation
these measurements 30000 events were acquired using FACSCalibur (Becton Dickinson, San Jose,
BNPs preparation was performed using
CA) flow cytometer and data were analyzed by
chemicals reagent grade or better. Polyethylene
CELLQuest software (Becton Dickinson) [24].
glycol (PEG) was purchased from Nektar, San Carlos, CA; hydroxychloroquine sulfate (HCQ)
2.5 Transmission electron microscopy analysis
and chlorambucil (CLB) were purchased from ACROS, Gel Belgium and Sigma Aldrich (St Louis,
Samples were fixed for 1h in a solution of 2%
MO), respectively. BNPs, based on carboxylic acid
glutaraldehyde (Serva, Heidelberg, Germany) in
0.1M cacodylate buffer (pH=7.3) containing 0.03M
(PLA-b-PEG-COOH and PCL-COOH), were
CaCl2, rinsed three times (10min each wash) and
prepared with average diameter of 250nm
postfixed in 1% osmium tetroxide for 1h at 4°C.
measured by dynamic light scattering (data not
Samples were then dehydrated in ascending
shown) in an under class 100 clean room
ethanols to 100 % ethanol and embedded in Dow
conditions by implementing Bio-Target's
Epoxy Resin (DER 332; Unione Chimica Europea,
technology at LNK Chemsolutions, LLC
Milan, Italy) and DER732 (Serva), as previously
laboratories [19, 23]. All BNPs (final concentration
described by Zabucchi et al [25]. Ultrathin
of 900µg/ml) were resuspended in PBS buffer
sections were cut by an ultratome Leica Ultracut
(pH=7.4) with 10% BSA. BNPs were diluted in
UCT8 (Leica, Wirn, Austria), double stained with
serum-free RPMI-1640 medium and stained with
uranyl acetate and lead citrate and observed in a
transmission electron microscope (EM 208,
Monofunctional Dye (GE Healthcare).
Micrographs were taken with a Morada Camera (Olympus, Munster, Germany).
2.3 Animals
mice either dead from the tumor or sacrificed at day +120 were obtained at necropsy. For
2.6 Cell viability, apoptosis and autophagy
morphologic evaluation, the specimens were fixed in 10% buffered-formalin solution and
To investigate the ability of BNPs to affect cell
embedded in paraffin. Four micrometer-thick
viability, MEC1 cells (2x105) were incubated with
sections were stained with H&E. Four to 6µm
different concentrations of BNPs for 48h at 37°C
sections were fixed in cold 100% methanol for 15
(in a humidified 37°C, 5% CO2 incubator). The
minutes. Immunohistochemical analysis was
amount of residual viable cells was determined
done using the avidin-biotin-peroxidase complex
by MTT assay [26] and the percentage of dead
method according to standard procedures [29],
cells was calculated as: 100 x [(test release –
and the slides were examined under a Leica
spontaneous release)/(total release – spontaneous
DM2000 optical microscope.
release)]. Apoptosis of patient's B cells was measured using FITC-labeled recombinant
2.10 Statistical Analysis
human Annexin V assay (Apoptosis detection kit, Immunostep,
The data were expressed as mean ± SD and
analyzed for statistical significance by the
measurement 30000 events were acquired with a
two-tailed Student's t test to compare two paired
standard FACSCalibur (Becton Dickinson) flow
groups of data. The Kaplan-Meier product-limit
cytometer and analysis of data was performed
method was used to estimate survival curves and
with CellQuest (Becton Dickinson). PARP-1 and
the log-rank test was adopted to compare
different groups of mice.
immunoblotting to study apoptosis induction
3 Results and discussion
and autophagy impairment, respectively [27].
2.7 Complement-mediated lysis
3.1 Anti-CD20 BNPs Target Tumor B-cells
Current treatment strategies for leukemia involve
complement-dependent cytotoxicity (CDC) with
chemotherapy, immunotherapy, bone marrow
some modifications was used to evaluate the
transplant, and several new target therapies. These
effect of Rituximab® on complement-mediated
treatments often induce long-term side-effects,
killing of tumor B-cells [28]. The number of
resulting in impairment of vital physiological
residual viable cells was estimated by MTT assay.
functions among the survivors. This is particularly true for elderly/unhealthy CLL patients. While
2.8 Blood analysis
current treatment approaches have greatly improved the prognosis for survival, some patients
Red and white blood cells and platelets from
remain refractive to current therapeutic regimens.
treated and untreated mice were analyzed using
Hence there is an urgent need for novel
ABX Micros E660 OT/CT (Horiba ABX Diagnostic,
difficult-to-treat
Montpellier, France). Other parameters in the
leukemia cases, in addition to reducing the
animal plasma were analyzed using Integrated
long-term residual side-effects impact of
System Dx 880 (Beckman Coulter).
therapeutics for all leukemia patients. Due to the cytotoxicity of drugs, currently the major challenge
is to deliver the therapeutic agent to neoplastic
Immunohistochemical analysis
cells while preserving the viability of non-malignant cells. Research on the use of
Liver, spleen, kidneys, brain, spinal cord and
nanoparticles as drug carriers has advanced to the
bone marrow samples from leukemia-bearing
point to focus on assessing the safety and efficacy
of such drug delivery systems. In this contribution,
cells in a dose- and time-dependent manner with a
four different types of polymeric nanoparticles,
maximal uptake after 1h incubation and using
BNP0, BNP1, BNP2 and BNP3, were prepared as in
10µL of particles. Under these conditions, 74% of
Figure S-1 in the ESM and characterized as
cells appeared tagged by BNP1 (Figure 1a). On the
previously described [19]. The BNP0 were made
contrary, BNP0 did not demonstrate specific
only by polymeric carriers (PLA-b-PEG-COOH
binding after 1h incubation, suggesting the
and PCL-COOH); BNP1 were prepared
importance of the anti-CD20 antibody in BNPs'
conjugating the anti-CD20 chimeric antibody on
targeting B cells.
the surface of BNP0; BNP2 were produced encapsulating HCQ sulfate and CLB inside the core of BNP1 while BNP3 were prepared loading chemotherapeutic drugs inside BNP0. To characterize both untargeted and targeted nanoparticles, TEM and dynamic light scattering were used. In details, TEM showed that untargeted nanoparticles have a core diameter of 110±40nm while antiCD20-conjugated nanoparticles have a core diameter of 90±30nm. For what concerns dynamic light scattering analysis, untargeted nanoparticles have an hydrodynamic diameter of 190±60nm while targeted nanoparticles have a diameter of 230±70nm; moreover, ζ-potential evidenced values of -7.8±0.9 and -6.0±0.6 mV for untargeted and anti-CD20 conjugated BNPs respectively, as previously described [19]. During the experiments, nanoparticles were stored at -20°C and +4°C and than tested. We do not evidenced any significant modification in their morphology and in their capacity to target and kill tumor B cells, both in vitro and in vivo, suggesting their stability for almost 1 year since their production. To characterize the BNP's effect, the CLL-like MEC1 cell line was used. It was initially purified
Figure 1 Tumor B-cells/BNPs interaction. (a) Binding of
from a CLL patient [20] and carried a mutation in
anti-CD20 BNPs to MEC1 cells. MEC1 cells were incubated
TP53 gene and the 17p13 deletion, as demonstrated
with FITC-labeled BNPs for 1 hour at 37°C and analyzed using
by direct sequencing and FISH analysis (data not
FACS. FL1-H, green fluorescence, 530/30 nm bandpass filter.
shown). Moreover, its immunephenotype was
(b) Internalization of anti-CD20 BNPs to MEC1 cells. MEC1
studied by cytometric analysis confirming what
cells were incubated with BNPB for 1h and analyzed by
previously reported in literature [30]. In fact, more
transmission electron microscopy: ultrastructural appearance
than 95% of MEC1 cells highly expressed human
of MEC1/BNP1 interaction and internalization was
markers like CD20 and CD45 while CD5
documented. Arrows in bII and bIII indicate typical
expression was not detected (Figure S-2 in the
cytoplasmatic localization of anti-CD20 BNP. Bars represent
ESM). BNPs' functional characterization started by
200nm (bI, bIII), 2µm (bII) and 100nm (bIV).
evaluating their ability to bind to leukemia cells.
To this aim, BNP0 and BNP1 were labeled with
The BNPs' interaction with MEC1 cells was further
FITC and added to MEC1 cells at different
confirmed by confocal microscopy images
incubation times. BNP1 were able to target MEC1
incubating cells and BNPs labeled with FAST-DiO
and Cy5.5, respectively (Figure S-3 in the ESM).
elevated concentration of CLB intracellularly
Moreover, TEM studies were performed to follow
with another kind of cytotoxic drug not
BNPs' migration into tumor B cells. Two different
dependent on surviving genes could not only
types of BNPs were prepared as shown in Figure
enhance their respective killing activities but
S1, labeled as BNPA and BNPB. BNPA were
perhaps make a resistant leukemia cell sensitive
again. HCQ has demonstrated an interesting
dimeglumine (Magnevist H, Bayer HealthCare
cytotoxic effect depending on its capacity to block
Pharmaceuticals Inc) while BNPB were prepared
autophagosomes/lysosome
conjugating the anti-CD20 antibody to the surface
anti-neoplastic properties in vitro depend on its
of BNPA. Exploiting the presence of Gd in the
concentration, which however is unobtainable in
particles, BNPs' migration was followed by TEM
vivo by the usual oral administration route
analysis, incubating MEC1 cells with these two
[33–36]. The synergistic effect of HCQ and CLB
different types of BNPs (Figure S-1 in the ESM).
was previously described by our group [18] and
In details, BNPA were never seen inside the cells
it could be important especially for those CLL
(data not shown) while images showed the
patients in an already resistant disease state, or
binding of BNPB and their interaction with the cell
with poor prognostic biological characteristics.
surface. Moreover, BNPs were never documented
These drugs together were able to cause high
in the nucleus and the absence of vesicles
cytotoxic effect mainly inducing autophagy and
surrounding them suggested BNPs' internalization
apoptosis [18]. In order to evaluate the cytotoxic
through a process different from endocytosis
effect of BNPs, MEC1 cells were incubated with
(Figure 1b). This data confirmed the results
different amounts of BNP0, BNP1, BNP2 and
previously obtained both in vitro and in vivo and
BNP3 for 48h and residual viable cells were
demonstrated the importance of a targeting agent
chemotherapeutic drugs, such as BNP2 and
nanoparticle's surface [18]. BNPs' internalization
BNP3, were able to induce cell cytotoxicity in a
outside endosomes was already demonstrated for
dose-dependent manner while empty particles,
other tumor B-cell lines incubated with BNPs [18]
such as BNP1, were almost ineffective.
which passed through the membrane without
Furthermore, 2µL of BNP2 or BNP3 were
causing significantly its disruption.
sufficient to kill more than 85% of MEC1 cells suggesting
chemotherapeutic
3.2 BNP2 Induce Tumor B-Cell Cytotoxicity
maintained their cytotoxic properties even after encapsulation inside particles. On the contrary,
In this study, CLB and HCQ were loaded inside
treatment with BNP1 killed less than 20% of cells
polymeric nanoparticles because of their
in this in vitro test, showing a good safety of this
synergistic effect against cancer B cells, as we
approach (Figure 2a). Cell cytotoxicity is due to
have previously described [18]. In details, CLB is
the pro-apoptotic effect induced by the
an alkylating agent administered orally whose
chemotherapeutic drugs. Forty eight hours
rate of drug absorption can vary significantly
incubation of cells and particles loaded with
from patient to patient thus causing side effects
chemotherapeutic drugs caused high percentage
[31, 32]. Also, most B-cell malignancies will
of cell destruction, avoiding any possible
become resistant to CLB at some point, no matter
molecular studies. Thus, only 16h incubations
whether it is used at increasing doses or within
were made to further study apoptosis' induction
more aggressive regimens. In resistant situations,
and autophagy impairment. In this setting, more
it could be important to have a therapeutic
than 20% of tumor cells (2x105) incubated with
system for a better delivery of high amounts of
2ml of BNP2 showed the apoptotic profile in an
drugs specifically inside malignant B-cells in
AnnexinV/7AAD test (Figure 2b). To confirm
order to circumvent genetically driven tumor
apoptosis, the poly-(ADP-ribose) polymerase
mechanisms of resistance. The combination of an
(PARP-1) was visualized. The enzyme is cleaved
from a 113KDa molecule to fragments of 89 and
analysis, also in the presence of significant basal
24KDa during apoptosis. The PARP-1 cleavage
level in untreated cells (Figure 2c).
was detected by western blot assay using cell lysates of MEC1 cells incubated with different
3.3 Comparison Between BNP2 and Rituximab
amounts of BNP2 for 16h. These apoptotic
Cytotoxic Effects
studies demonstrated that BNP2 were able to induce PARP-1 cleavage, in particular using 2µL
Rituximab is mainly able to activate the
of particles with 5x105 cells (Figure 2c).
complement system and also to induce antibody-dependent cell cytotoxicity (ADCC) but a very low killing effect is due to its ability to activate apoptotic pathways. For this reason, we have compared the killing of MEC1 cells induced by a saturating concentration of Rituximab through complement-dependent killing, or by BNP2 acting through apoptosis/authophagy. MEC1 cells were analyzed using anti-CD20 mAb showing high amount of the antigen on cell surface (Mean Fluorescence Intensity-MFI: 784). In this particular setting, Rituximab killed up to 22% of MEC1 cells while BNP2 killed 87% of this leukemia cell line (Table 1). The BNP2 cytotoxic effect was evident also analyzing purified cells from CLL patients. Circulating CLL B-cells expressed a lower amount of CD20 on their surface with respect to MEC1 cells (MFI: 50.6 vs 784), as we documented in cells purified from 31 different untreated CLL patients, already
Figure 2 In vitro characterization of the cytotoxic effect
of BNP2. MEC1 cells (a) were incubated with 0.5, 1, and
prognosticators (Table S-1 in the ESM). Moreover,
2µl of BNPs or HCQ+CLB for 48 hours at 37°C and
as shown in Table 1, the cytotoxic effect of
residual viable cells were measured. Data are expressed as
Rituximab on purified CLL cells ranged between
mean ± SD. (b) MEC1 cells were incubated with 1µl of
0 and 38%, with a median value of 4.2%. On the
BNPs for only 16 hours at 37°C and apoptotic cells were
contrary, BNP2 killed up to 84% CLL cells, with a
analyzed using AnnexinV/7AAD test. (c) Western blot
median value of 55.1% (BNP2 vs. Rituximab:
analysis of activated PARP-1 and LC3 accumulation from
p<0.00001), and only 1 out of 31 patient did not
cell lysates obtained from MEC1 cells incubated with: 1.
respond to the treatment. Interestingly, it is also
Saline; 2. 2µl of BNP1; 3. 0.5µl of BNP2; 4. 2µl of BNP2.
possible to perform these studies in whole blood
samples, as a predictive functional test. Apoptosis
The same pattern was demonstrated studying
was evaluated by Annex V/7AAD test after 16h of
autophagy, which is impaired by HCQ. This
incubation with BNP2. In all the samples more
mechanism was analyzed taking in consideration
than 98% of CD5/CD19 positive BNP2-treated
the LC3 protein, which is processed to a cytosolic
cells resulted in an apoptotic state in comparison
version (LC3-I, 18KDa) and then converted to
with only 11% of BNP1-treated cells. These
activate forms commonly used as a marker of
results seem to be independent from CD20
autophagosome accumulation. The effect of HCQ
expression or from specific biological features; in
on MEC1 cells was evident detecting increased
fact, TP53 mutated/deleted or NOTCH1 mutated
amount of LC3-III in lysates of cells incubated for
patients' cells, that usually have poor response to
only 16h with 0.5 or 2µL of BNP2 by western blot
standard therapies (Table S-2 in the ESM), were
anyway killed by BNP2.
evident in our experiments on healthy mice.
The reduction of side effects was addressed by
Table 1 Comparison between BNP2 and Rituximab effects
including CLB+HCQ drugs in BNPs produced
RITUXIMAB
from biocompatible and biodegradable polymers.
(% of killing)
(% of killing)
The development of these nanoparticles with an average diameter of 250nm as drug delivery
agents has several advantages, including specific
targeting via receptor-mediated mechanisms and
microenvironment [19]. BNP2 in particular can
transport and release into tumor B-cells enough
amount of drugs to kill cancer cell, overcoming
multidrug resistance overexpressed in several
B-cell disorders [39].
The toxic effects induced by the intra-peritoneal
injection of BNPs were evaluated in groups of
five C57/BL mice receiving 8 injections of saline, 8
injections of BNP2 (containing 400µg of CLB and
HCQ) or 4 injections free HCQ+CLB (400µg each).
We have previously documented that 8 injections
of free drugs killed all the animals [18].
Mice were followed for 28 days. Animal survival,
total body weight but also circulating cells and several tissue markers in the blood were analyzed.
All the animals survived during the experiment
but free drugs-treated mice evidenced a strong
reduction in their body weight, with a median of
about 20%. Blood samples were collected 3 days
after the end of the treatments in order to
evaluate complete blood count, hemoglobin, urea,
transaminase (ALT), alkaline phosphatase (ALP),
lactate-dehydrogenase
phosphokinase (CPK), creatinine and aldolase
concentration (Table 2). We did not evidence any
significant differences during all the experiment
between controls and animals receiving 8
injections of BNP2; only platelets seem to be
increased after the treatments, remaining in a
Median (Pt)
physiological range. On the contrary, animals
CLL patients (Pt); MFI: mean fluorescence intensity: mean;
receiving only 4 times free HCQ+CLB showed a
percentage of killing: mean (n=3).
reduction in white blood cells, mainly due to the
low number of circulating lymphocytes, and a significant reduction in erythrocytes.
3.4 BNPs Show a Safe Toxicological Profile
Table 2 Toxicological studies
Side effects induced by HCQ and CLB are well described in the literature [37, 38] and were also
Body weight
(% variation)
A xenograft model of human CLL was previously described by Bertilaccio et al. [40], who challenged
intravenously or subcutaneously Rag-/-γc-/- mice
(103/mm3)
with 107 MEC1 cells. Unfortunately, we were
LYMPHOCYTES
unable to repeat these results in SCID mice; in fact,
(103/mm3)
the intravenous injection of 107 cells rapidly killed
MONOCYTES
all the animals from respiratory problems. On the
other hand, cells' subcutaneous challenging
(103/mm3)
induced only the formation of a localized tumor
mass at the site of injection without colonizing
(103/mm3)
other tissues and inducing the death of the animals
in about 70 days (Figure 3).
(106/mm3)
(103/mm3)
Hemoglobin
Urea (mg/dL)
Creatinine
Figure 3 Development of human/SCID leukemia model.
MEC1 were injected subcutaneously (SC, 107 cells) or
intravenously (IV, 5x105 cells) after cyclophosphamide
pre-treatment. Animal survival was studied and reported as
Kaplan Mayer curves.
However, an intravenous injection of only 5x105
MEC1 cells 24 hours after a pre-treatment with
cyclophosphamide (200mg/kg intraperitoneally)
which reduce immune effects, in particular via NK
cells [41, 42], developed a diffuse leukemia model characterized by the colonization of different
Aldolase
organs and also the blood. In details, MEC1 cells'
biodistribution pattern was evaluated by
Total body weight, red blood cells (RBC), white blood cells
immunohistochemical analysis staining tissues
(WBC), platelets (PLT), and other plasma parameters from
with H&E and detecting human B cells with an
treated and untreated mice were compared. *= p<0.05 vs
anti-human CD45 antibody 28 days after MEC1
control; §= p<0.05 vs BNP2.
injection (Figure 4a). MEC1 cells were detected in
liver, spleen, kidney, bone marrow, spinal cord
At the same time, we observed reduced
and brain. Moreover, the accumulation of MEC1
concentration of hemoglobin, creatinine, ALP and
cells in mice bloodstream was confirmed by
LDH, with increased values of aldolase (Table 2).
cytometric analysis using anti-human CD19 and
anti-human CD45 antibodies. Human B cells
3.5 Development of a Disseminated Leukemia
presence was detected from the 20th day after
Model Using MEC1 Cells
tumor cells injection (Figure 4b).
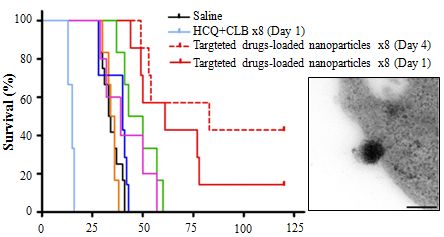
animals per group, and followed for 120 days (Figure 5).
Figure 5 Therapeutic effect of BNPs and HCQ+CLB. SCID
mice (n = 6-10 per group) received (5x106) MEC1 cells
intravenously and BNP1, BNP2, BNP3 or HCQ+CLB as
described in the results; animal survival was represented as
Figure 4 Characterization of diffuse leukemia model in
Kaplan Mayer curve
SCID mice. MEC1 (5x105 cells) were injected intravenously in
SCID mice and human tumor cells' distribution was analyzed
Group 1 did not receive any treatment; all mice
after 28 days by H&E and by exploiting human CD45 (a).
died within 30-40 days after tumor cell injection
Original magnification 200X. (b) Human tumor B-cells were
with a median survival of 33.5 days. The
also detected in the circulation by FACS analysis using labeled
therapeutic protocol followed our previous data
anti-CD45 and anti-CD19 mAbs.
and derived from toxicological profile obtained
with free HCQ+CLB. Thus, group 2 and group 3
All animals died between 30 and 37 days after
received both 80µL of BNP2 (corresponding to
tumor cell injection (Figure 3), as the evidence of a
400µg of each encapsulated chemotherapeutic
very reproducible and very aggressive leukemia
agent targeted via anti-CD20 antibody) for 8 times
human/SCID model, which was useful for the
in 17 days from the 1st and the 4th day after MEC1
characterization of the therapeutic effect of
cell injection, respectively. The overall survival of
targeted nanoparticles but also for the
group 2 was 83 days and 3 mice out of 7 were
development of new recombinant antibodies, as
cured at the end of the study (BNP2x8 (day 1) vs
already performed for other B-cell malignancies
Untreated: p<0.0001; BNP2x8 (day 1) vs BNP1x8:
p<0.0002; BNP2x8 (day 1) vs BNP3x8: p<0.0001; BNP2x8 (day 1) vs HCQ+CLB: p<0.01; BNP2x8
3.6 BNP2 Therapeutic Effect in a Disseminated
(day 1) vs BNP2x4: p<0.03; BNP2x8 (day 1) vs
Leukemia Human/Mouse Model
BNP2x8 (day 4): Not Significant). Group 3 received the same treatment but starting from day 4,
The BNP2 demonstrated their ability to target
resulting in a overall survival of 61 days and 1 out
cancer B-cell in vivo and also their potential efficacy
of 7 mice was cured (BNP2x8 (day 4) vs Untreated:
in the treatment of tumor-bearing mice, as already
p<0.0001; BNP2x8 (day 4) vs BNP1x8: p<0.0002;
evidenced in other B-cell xenograft [18, 19]. To
BNP2x8 (day 4) vs BNP3x8: p<0.0001; BNP2x8 (day
study BNP2 efficacy in the treatment of the
4) vs HCQ+CLB: p<0.03; BNP2x8 (day 4) vs
human/SCID leukemia model, MEC1 cells were
BNP2x4: p<0.04). These results demonstrate BNP2
injected in SCID mice, divided into 8 groups of 6–8
ability to treat this aggressive human/mouse leukemia model with a better outcome when the
treatment started at the early stage of the
Research (AIRC Project n° 12965/2012), Italian
Ministry of Health (GR‐2011‐ 02346826 and
Group 4 received only 4 injections of 80µL of BNP2
GR‐2011‐ 02347441), Fondazione Casali – Trieste,
in 8 days starting from the 4th day after cell
Italy and Stiftung Foundation – Liechtenstein.
injection. This treatment improved the overall
Nanoparticles fabrication at LNK Chemsolutions,
survival of about 13.5 days (BNP2x4 vs Untreated:
USA, was possible in part by Grant
p<0.002; BNP2x4 vs HCQ+CLB: p<0.03). Group 5 and group 6 received 8 injections of 80µL
2R44CA135906-02 (SBIR Phase II) from the
of BNP1 and BNP3, respectively. Both these
National Institutes of Health (USA) to Ruben
treatments did not significantly increased mice
Spretz, Gustavo Larsen, Sandra Noriega and Luis
survival demonstrating both BNPs' safety and the
inability of BNP3 to bind cancer cells due to the
Conflict-of-interest disclosure: Ruben Spretz,
absence of the anti-CD20 antibody on the surface
Gustavo Larsen, Sandra Noriega and Luis Núñez
of these particles, as already demonstrated by our
working in Biotarget Inc.
group [19]. In vitro, untargeted nanoparticles
Chemsolutions LLC have commercial interests in
(BNP3) evidenced cell cytotoxicity but their effect
the particle systems described in this work. No
was not confirmed in vivo. This was probably due
conflicts of interest for the other authors.
to the blood flow (for circulating tumor cells) and reduced
Correspondence: Sara Capolla and Paolo Macor,
nanoparticles in tumor microenvironment.
Department of Life Sciences, University of Trieste,
Finally, group 7 received 8 injections of
via L. Giorgeri, 5 – 34127, Trieste, Italy. Phone:
HCQ+CLB (400µg each) in 17 days starting from
+39 040 5588682; FAX: +39 040 5584023; e-mail:
day 1. This treatment improved survival of 2 days
[email protected], [email protected]
and showed that BNP2 (groups 2) were more
effective than free drugs in the treatment of this
Electronic
Material:
aggressive human/mouse leukemia model.
Supplementary material about CLL patients'
In the group 8, three animals received 8 injections
characterization (confocal microscopy, cytometry,
of free drugs but all the mice died for the toxicity
sequencing, killing test) is available in the online
of the treatment in less than 20 days, as already
4 Conclusions
In conclusion, the results of the present study demonstrated that anti-CD20 nanoparticles
References
containing HCQ+CLB can be effective as a single agent in controlling a new disseminated model of
aggressive leukemia. It also provides a rationale
Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.;
for adopting this therapeutic approach for the
Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.;
treatment of other B-cell disorders with BNP2 or
Kipps, T.J., Guidelines for the diagnosis and treatment
different types of tumors, using other monoclonal
of chronic lymphocytic leukemia: A report from the
antibodies to specifically deliver cytotoxic
International Workshop on Chronic Lymphocytic
agent-loaded nanoparticles in cancer cells.
Acknowledgements
Institute-Working Group 1996 guidelines. Blood 2008,
111, 5446–5456.
This study has been made possible by research
Zenz, T.; Eichhorst, B.; Busch, R.; Denzel, T.; Häbe,
grants from Italian Association for Cancer
S.; Winkler, D.; Bühler, A.; Edelmann, J.; Bergmann,
M.; Hopfinger, G.; Hensel, M.; Hallek, M.; Döhner, H.;
identifies new prognostic subgroups in chronic
Stilgenbauer, S., TP53 mutation and survival in
lymphocytic leukemia. Blood 2013, 121, 1403–1412.
chronic lymphocytic leukemia. Journal of Clinical
[6] Rossi, D.; Rasi, S.; Fabbri, G.; Spina, V.; Fangazio, M.;
Oncology : Official Journal of the American Society of
Forconi, F.; Marasca, R.; Laurenti, L.; Bruscaggin, A.;
Clinical Oncology 2010, 28, 4473–4479.
Cerri, M.; Monti, S.; Cresta, S.; Famà, R.; De Paoli, L.;
Puente, X.S.; Pinyol, M.; Quesada, V.; Conde, L.;
Bulian, P.; Gattei, V.; Guarini, A.; Deaglio, S.; Capello,
Ordóñez, G.R.; Villamor, N.; Escaramis, G.; Jares, P.;
D.; Rabadan, R.; Pasqualucci, L.; Dalla-Favera, R.;
Beà, S.; González-Díaz, M.; Bassaganyas, L.;
Foà, R.; Gaidano, G., Mutations of NOTCH1 are an
Baumann, T.; Juan, M.; López-Guerra, M.; Colomer,
D.; Tubío, J.M.C.; López, C.; Navarro, A.; Tornador,
lymphocytic leukemia. Blood 2012, 119, 521–529.
C.; Aymerich, M.; Rozman, M.; Hernández, J.M.;
Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.;
Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko,
Gutiérrez-Fernández, A.; Costa, D.; Carrió, A.;
A.; DeLuca, D.S.; Zhang, L.; Zhang, W.; Vartanov,
Guijarro, S.; Enjuanes, A.; Hernández, L.; Yagüe, J.;
A.R.; Fernandes, S.M.; Goldstein, N.R.; Folco, E.G.;
Nicolás, P.; Romeo-Casabona, C.M.; Himmelbauer, H.;
Cibulskis, K.; Tesar, B.; Sievers, Q.L.; Shefler, E.;
Castillo, E.; Dohm, J.C.; de Sanjosé, S.; Piris, M.A.; de
Gabriel, S.; Hacohen, N.; Reed, R.; Meyerson, M.;
Alava, E.; San Miguel, J.; Royo, R.; Gelpí, J.L.;
Golub, T.R.; Lander, E.S.; Neuberg, D.; Brown, J.R.;
Torrents, D.; Orozco, M.; Pisano, D.G.; Valencia, A.;
Getz, G.; Wu, C.J., SF3B1 and other novel cancer
Guigó, R.; Bayés, M.; Heath, S.; Gut, M.; Klatt, P.;
genes in chronic lymphocytic leukemia. The New
Marshall, J.; Raine, K.; Stebbings, L.A.; Futreal, P.A.;
England Journal of Medicine 2011, 365, 2497–506.
López-Guillermo, A.; Estivill, X.; Montserrat, E.;
Dreger, P.; Schetelig, J.; Andersen, N.; Corradini, P.;
Gelder, M. van; Gribben, J.; Kimby, E.; Michallet, M.;
sequencing identifies recurrent mutations in chronic
Moreno, C.; Stilgenbauer, S.; Montserrat, E.,
lymphocytic leukaemia. Nature 2011, 475, 101–105.
Managing high-risk CLL during transition to a new
treatment era: stem cell transplantation or novel agents?
Fabbri, G.; Rasi, S.; Rossi, D.; Trifonov, V.;
Blood 2014, 124, 3841–3849.
Khiabanian, H.; Ma, J.; Grunn, A.; Fangazio, M.;
Capello, D.; Monti, S.; Cresta, S.; Gargiulo, E.;
Siddiqi, T.; Rosen, S., Novel Biologic Agents for
Forconi, F.; Guarini, A.; Arcaini, L.; Paulli, M.;
Non-Hodgkin Lymphoma and Chronic Lymphocytic
Laurenti, L.; Larocca, L.M.; Marasca, R.; Gattei, V.;
Leukemia - Part 1. Oncology (Williston Park) 2015, 29,
Oscier, D.; Bertoni, F.; Mullighan, C.G.; Foá, R.;
Pasqualucci, L.; Rabadan, R.; Dalla-Favera, R.;
[10] Woyach, J.; Johnson, A., Targeted therapies in CLL:
Gaidano, G., Analysis of the chronic lymphocytic
leukemia coding genome: role of NOTCH1 mutational
management. Blood 2015.
activation. The Journal of Experimental Medicine
[11] Jain, N.; O'Brien, S., Initial treatment of CLL:
2011, 208, 1389–1401.
integrating biology and functional status. Blood 2015,
Rossi, D.; Rasi, S.; Spina, V.; Bruscaggin, A.; Monti,
126, 463–70.
S.; Ciardullo, C.; Deambrogi, C.; Khiabanian, H.;
[12] Hallek, M., Chronic lymphocytic leukemia: 2013
Serra, R.; Bertoni, F.; Forconi, F.; Laurenti, L.;
update on diagnosis, risk stratification and treatment.
Marasca, R.; Dal-Bo, M.; Rossi, F.M.; Bulian, P.;
American Journal of Hematology 2013, 88, 803–816.
Nomdedeu, J.; Del Poeta, G.; Gattei, V.; Pasqualucci,
L.; Rabadan, R.; Foà, R.; Dalla-Favera, R.; Gaidano,
[13] Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.;
G., Integrated mutational and cytogenetic analysis
State-of-the-art in design rules for drug delivery
B-chronic lymphocytic leukaemia in prolymphocytoid
platforms: Lessons learned from FDA-approved
nanomedicines. Journal of Controlled Release 2014,
187, 133–144.
[21] Dereani, S.; Macor, P.; D'Agaro, T.; Mezzaroba, N.;
[14] Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.;
Dal-Bo, M.; Capolla, S.; Zucchetto, A.; Tissino, E.;
Davis, M.E., Impact of tumor-specific targeting on the
Del Poeta, G.; Zorzet, S.; Gattei, V.; Bomben, R.,
biodistribution and efficacy of siRNA nanoparticles
Potential therapeutic role of antagomiR17 for the
measured by multimodality in vivo imaging.
treatment of chronic lymphocytic leukemia. J Hematol
Proceedings of the National Academy of Sciences of
Oncol 2014, 7, 79.
the United States of America 2007, 104, 15549–15554.
[22] Biffi, S.; Garrovo, C.; Macor, P.; Tripodo, C.; Zorzet,
[15] Sanna, V.; Pala, N.; Sechi, M., Targeted therapy using
S.; Secco, E., In Vivo Biodistribution and Lifetime
nanotechnology: Focus on cancer. International
Analysis of Cy5.5-Conjugated Rituximab in Mice
Journal of Nanomedicine 2014, 9, 467–483.
Bearing Near-Infrared Optical Imaging. Molecular
Imaging 2008, 7, 272–282.
[16] Chattopadhyay, N.; Fonge, H.; Cai, Z.; Scollard, D.;
Lechtman, E.; Done, S.J.; Pignol, J.P.; Reilly, R.M.,
[23] Marín, G.H.; Mansilla, E.; Mezzaroba, N.; Zorzet, S.;
Role of antibody-mediated tumor targeting and route
Núñez, L.; Larsen, G.; Tau, J.M.; Maceira, A.; Spretz,
of administration in nanoparticle tumor accumulation
R.; Mertz, C.; Ingrao, S.; Tripodo, C.; Tedesco, F.;
Molecular
Pharmaceutics
Macor, P., Exploratory study on the effects of
biodegradable nanoparticles with drugs on malignant B
cells and on a human/mouse model of Burkitt
lymphoma. Current Clinical Pharmacology 2010, 5,
Nanoparticle Delivery of Cancer Drugs. Annual
Review of Medicine 2012, 63, 185–198.
[24] Macor, P.; Secco, E.; Mezzaroba, N.; Zorzet, S.;
[18] Mezzaroba, N.; Zorzet, S.; Secco, E.; Biffi, S.; Tripodo,
Durigutto, P.; Gaiotto, T.; De Maso, L.; Biffi, S.;
C.; Calvaruso, M.; Mendoza-Maldonado, R.; Capolla,
Garrovo, C.; Capolla, S.; Tripodo, C.; Gattei, V.;
S.; Granzotto, M.; Spretz, R.; Larsen, G.; Noriega, S.;
Marzari, R.; Tedesco, F.; Sblattero, D., Bispecific
Lucafò, M.; Mansilla, E.; Garrovo, C.; Marìn, G.H.;
antibodies targeting tumor-associated antigens and
Baj, G.; Gattei, V.; Pozzato, G.; Nunez, L.; Macor, P.,
neutralizing complement regulators increase the
New Potential Therapeutic Approach for the Treatment
efficacy of antibody-based immunotherapy in mice.
Leukemia 2015, 29, 406–414.
Anti-CD20 Nanoparticles. PLoS ONE 2013, 8, e74216.
[25] Zabucchi, G.; Soranzo, M.R.; Menegazzi, R.; Vecchio,
M.; Knowles, A.; Piccinini, C.; Spessotto, P.; Patriarca,
[19] Capolla, S.; Garrovo, C.; Zorzet, S.; Lorenzon, A.;
P., Eosinophil peroxidase deficiency: morphological
Rampazzo, E.; Spretz, R.; Pozzato, G.; Nunez, L.;
immunocytochemical
Macor, P.; Biffi, S., Targeted tumor imaging of
eosinophil-specific
anti-CD20-polymeric nanoparticles developed for the
diagnosis of B-cell malignancies. International
Journal of Nanomedicine 2015, 10, 4099–109.
[26] Tripodo, C.; Florena, A.M.; Macor, P.; Di Bernardo,
A.; Porcasi, R.; Guarnotta, C.; Ingrao, S.; Zerilli, M.;
[20] Stacchini, A.; Aragno, M.; Vallario, A.; Alfarano, A.;
Secco, E.; Todaro, M.; Tedesco, F.; Franco, V.,
Circosta, P.; Gottardi, D.; Faldella, A.; Rege-Cambrin,
P-selectin glycoprotein ligand-1 as a potential target
G.; Thunberg, U.; Nilsson, K.; Caligaris-Cappio, F.,
for humoral immunotherapy of multiple myeloma.
MEC1 and MEC2: Two new cell lines derived from
Current Cancer Drug Targets 2009, 9, 617–625.
[27] Mendoza-Maldonado, R.; Paolinelli, R.; Galbiati, L.;
Giadrossi, S.; Giacca, M., Interaction of the
hydroxychloroquine, delivered in a biodegradable
retinoblastoma protein with orc1 and its recruitment to
nanoparticle system, overcomes drug resistance of
human origins of DNA replication. PLoS ONE 2010, 5,
B-chronic lymphocytic leukemia cells in vitro. Cancer
Biotherapy & Radiopharmaceuticals 2010, 25,
[28] Macor, P.; Tripodo, C.; Zorzet, S.; Piovan, E.; Bossi,
F.; Marzari, R.; Amadori, A.; Tedesco, F., In vivo
Hydroxychloroquine,
targeting of human neutralizing antibodies against
chloroquine, and all-trans retinoic acid regulate growth,
CD55 and CD59 to lymphoma cells increases the
survival, and histone acetylation in breast cancer cells.
antitumor activity of rituximab. Cancer Research 2007,
Anti-Cancer Drugs 2009, 20, 736–745.
67, 10556–10563.
[37] Tehrani, R.; Ostrowski, R.A.; Hariman, R.; Jay, W.M.,
[29] Florena, A.M.; Tripodo, C.; Iannitto, E.; Porcasi, R.;
Ocular toxicity of hydroxychloroquine. Seminars in
Ingrao, S.; Franco, V., Value of bone marrow biopsy in
Ophthalmology 2008, 23, 201–9.
thrombocythemia.
[38] Stein, M.; Bell, M.J.; Ang, L.C., Hydroxychloroquine
Haematologica 2004, 89, 911–919.
neuromyotoxicity. J Rheumatol 2000, 27, 2927–2931.
[30] Bertilaccio, M.T.S.; Scielzo, C.; Simonetti, G.; Hacken,
[39] Rao, D.A.; Forrest, M.L.; Alani, A.W.; Kwon, G.S.;
E.T.; Apollonio, B.; Ghia, P.; Caligaris-Cappio, F.,
Xenograft models of chronic lymphocytic leukemia:
nanoparticles for sustained regional lymphatic drug
problems, pitfalls and future directions. Leukemia 2013,
delivery. J Pharm Sci 2010, 99, 2018–2031.
[40] Bertilaccio, M.T.S.; Scielzo, C.; Simonetti, G.;
[31] Kalil, N.; Cheson, B.D., Management of chronic
Ponzoni, M.; Apollonio, B.; Fazi, C.; Scarfò, L.;
lymphocytic leukaemia. Drugs and Aging 2000, 16,
Rocchi, M.; Muzio, M.; Caligaris-Cappio, F.; Ghia, P.,
A novel Rag2-/-gammac-/--xenograft model of human
[32] Zhou, Y.; Hileman, E.O.; Plunkett, W.; Keating, M.J.;
CLL. Blood 2010, 115, 1605–1609.
Huang, P., Free radical stress in chronic lymphocytic
[41] Nonaka, Y.; Ishibashi, H.; Nakai, M.; Shibata, H.; Kiso,
leukemia cells and its role in cellular sensitivity to
Y.; Abe, S., Effects of the antlered form of Ganoderma
ROS-generating anticancer agents. Blood 2003, 101,
lucidum on tumor growth and metastasis in
Bioscience,
[33] Amaravadi, R.K.; Lippincott-Schwartz, J.; Yin, X.-M.;
Biotechnology,
Biochemistry
Weiss, W.A.; Takebe, N.; Timmer, W.; DiPaola, R.S.;
Lotze, M.T.; White, E., Principles and current
[42] Zorzet, S.; Perissin, L.; Rapozzi, V.; Giraldi, T.,
strategies for targeting autophagy for cancer treatment.
Restraint Stress Reduces the Antitumor Efficacy of
Clin Cancer Res. 2011, 17, 654–666.
Ciclophosphamide in Tumor-Bearing Mice. Brain,
[34] Lagneaux, L.; Delforge, A.; Dejeneffe, M.; Massy, M.;
Behavior, and Immunity 1998, 12, 23–33.
Bernier, M.; Bron, D., Hydroxychloroquine-induced
[43] Macor, P.; Secco, E.; Zorzet, S.; Tripodo, C.;
apoptosis of chronic lymphocytic leukemia involves
Celeghini, C.; Tedesco, F., An update on the xenograft
and mouse models suitable for investigating new
Bcl-2/bax/ratio.
therapeutic compounds for the treatment of B-cell
malignancies. Current Pharmaceutical Design 2008,
[35] Mansilla, E.; Marin, G.H.; Nuñez, L.; Drago, H.;
14, 2023–2039.
Sturla, F.; Mertz, C.; Rivera, L.; Ichim, T.; Riordan, N.;
Electronic Supplementary Material
New Approach for the Treatment of CLL using
Chlorambucil/Hydroxychloroquine-loaded
anti-CD20
Sara Capolla1§(*), Nelly Mezzaroba1§, Sonia Zorzet1, Claudio Tripodo2, Ramiro Mendoza-Maldonado3, Marilena Granzotto4, Francesca Vita1, Ruben Spretz5, Gustavo Larsen5,6, Sandra Noriega5, Eduardo Mansilla7, Michele Dal Bo8, Valter Gattei8, Gabriele Pozzato4, Luis Núñez5,6 and Paolo Macor1,9(*) 1Department of Life Sciences, University of Trieste, Trieste, Italy 2Department of Human Pathology, University of Palermo, Italy 3Molecular Oncology Unit, National Laboratory Consorzio Interuniversitatio per le Biotecnologie (CIB), Trieste, Italy 4Dipartimento Universitario Clinico di Scienze mediche, Chirurgiche e della Salute, University of Trieste, Trieste, Italy 5LNK Chemsolutions LLC, Lincoln, NE 68521, USA 6Bio-Target Inc., Chicago, IL, USA; 7Centro Ùnico Coordinador de Ablacion e Implante Provincia de Buenos Aires (C.U.C.A.I.B.A.), Ministry of Health, La Plata, Buenos Aires, Argentina 8Clinical and Experimental Onco-Hematology Unit, Centro di Riferimento Oncologico, Istituto di Ricerca e Cura a Carattere Scientifico (I.R.C.C.S.), Aviano, Italy 9Cal erio Foundation Onlus, Institutes of Biological Researches, Trieste, Italy. § These authors contributed equally to this work.
Supporting information to DOI 10.1007/s12274-****-****-* (automatically inserted by the publisher)
Electronic supplementary materials (ESM) contain three figures and two tables. Figure S-1 showed types of nanoparticles produced and used for this project; Figure S-2 represented the immunophenotype in terms of CD5, CD20 and CD45 expression on the CLL-cell line MEC1; and Figure S-3 confirmed BNPs' binding on MEC1 cells. Moreover, Table S-1 showed the genetic features of analyzed CLL patients and Table S-2 put in evidence the differences between Rituximab and BNP2 treatments on cells derived from difficult-to-treat CLL-patients' samples.
Address correspondence to Capolla S., [email protected]; Macor P., [email protected]
Silver Nanowires with Semiconducting Ligands for
Low Temperature Transparent Conductors
Brion Bob,1 Ariella Machness,1 Tze-Bin Song,1 Huanping Zhou,1 Choong-Heui Chung,2 and Yang
1 Department of Materials Science and Engineering and California NanoSystems Institute,
University of California Los Angeles, Los Angeles, CA 90025 (USA)
2 Department of Materials Science and Engineering, Hanbat National University, Daejeon
Abstract
Metal nanowire networks represent a promising candidate for the rapid fabrication of transparent electrodes with high transmission and low sheet resistance values at very low deposition temperatures. A commonly encountered obstacle in the formation of conductive nanowire electrodes is establishing high quality electronic contact between nanowires in order to facilitate long range current transport through the network. A new system of nanowire ligand removal and replacement with a semiconducting sol-gel tin oxide matrix has enabled the fabrication of high performance transparent electrodes at dramatically reduced temperatures with minimal need for post-deposition treatments of any kind.
Keywords: Silver Nanowires, Sol-Gel, Transparent Electrodes, Nanocomposites
1. Introduction.
Silver nanowires (AgNWs) are long, thin, and possess conductivity values on the same order of magnitude as bulk silver
(Ag) [1]. Networks of overlapping nanowires allow light to easily pass through the many gaps and spaces between nanowires, while transporting current through the metallic conduction pathways offered by the wires themselves. The high aspect ratios achievable for solution-grown AgNWs has allowed for the fabrication of transparent conductors with very promising sheet resistance and transmission values, often approaching or even surpassing the performance of vacuum-processed materials such as indium tin oxide (ITO) [2-6].
Significant electrical resistance within the metallic nanowire network is encountered only when current is required to pass
between nanowires, often forcing it to pass through layers of stabilizing ligands and insulating materials that are typically used to assist with the synthesis and suspension of the nanowires [7, 8]. The resistance introduced by the insulating junctions between nanowires can be reduced through various physical and chemical means, including burning off ligands and partially melting the wires via thermal annealing [9, 10], depositing additional materials on top of the nanowire network [11-14], applying mechanical forces to enhance network morphology [15-17], or using various other post-treatments to improve the contact between adjacent wires [18-21]. Any attempt to remove insulating materials the network must be weighed against the risk of damaging the wires or blocking transmitted light, and so many such treatments must be reined in from their full effectiveness to avoid endangering the performance of the completed electrode.
We report here a process for forming inks with dramatically enhanced electrical contact between AgNWs through the use
of a semiconducting ligand system consisting of tin oxide (SnO2) nanoparticles. The polyvinylpyrrolidone (PVP) ligands introduced during AgNW synthesis in order to encourage one-dimensional growth are stripped from the wire surface using ammonium ions, and are replaced with substantially more conductive SnO2, which then fills the space between wires and enhances the contact geometry in the vicinity of wire/wire junctions. The resulting transparent electrodes are highly conductive immediately upon drying, and can be effectively processed in air at virtually any temperature below 300 °C. The capacity for producing high performance transparent electrodes at room temperature may be useful in the fabrication of devices that are damaged upon significant heating or upon the application of harsh chemical or mechanical post-treatments.
2. Results and Discussion
2.1. Ink Formulation and Characterization
Dispersed AgNWs synthesized using copper chloride seeds represent a particularly challenging material system for
promoting wire/wire junction formation, and often require thermal annealing at temperatures near or above 200 °C to induce long range electrical conductivity within the deposited network [22, 23]. The difficulties that these wires present regarding junction formation is potentially due to their relatively large diameters compared to nanowires synthesized using other seeding materials, which has the capacity to enhance the thermal stability of individual wires according to the Gibbs-Thomson effect. We have chosen these wires as a demonstration of pre-deposition semiconducting ligand substitution in order to best illustrate the contrast between treated and untreated wires.
Completed nanocomposite inks are formed by mixing AgNWs with SnO2 nanoparticles in the presence of a compound
capable of stripping the ligands from the AgNW surface. In this work, we have found that ammonia or ammonium salts act
as effective stripping agents that are able to remove the PVP layer from the AgNW surface and allow for a new stabilizing
matrix to take its place. Figure 1 shows a schematic of the process, starting from the precursors used in nanowire and
nanoparticle synthesis and ending with the deposition of a completed film. The SnO2 nanoparticle solution naturally contains
enough ammonium ions from its own synthesis to effectively peel the insulating ligands from the AgNWs and allow the
nanoparticles to replace them as a stabilizing agent. If not enough SnO2 nanoparticles are used in the mixture, then the wires
will rapidly agglomerate and settle to the bottom as large clusters. Large amounts of SnO2 in the mixture gradually begin to
increase the sheet resistance of the nanowire network upon deposition, but greatly enhance the uniformity, durability, and
wetting properties of the resulting films. We have found that AgNW:SnO2 weight ratios ranging between 2:1 and 1:1
produce well dispersed inks that are still highly conductive when deposited as films.
The nanowires were synthesized using a polyol method that has been adapted from the recipe described by Lee et al. [22,
23] Silver nitrate dissolved in ethylene glycol via ultrasonication was used as a precursor in the presence of copper chloride
and PVP to provide seeds and produce anisotropic morphologies in the reaction products. Synthetic details can be found in
the experimental section. Distinct from previous recipes, we have found that repeating the synthesis two times without
cooling down the reaction mixture generally produces significantly longer nanowires than a single reaction step. The lengths
of nanowires produced using this method fall over a wide range from 15 to 65 microns, with diameters between 125 and 250
nm. This range of diameters is common for wires grown using copper chloride seeds, although the double reaction produces
a number of wires with roughly twice their usual diameter. The morphology of the as-deposited AgNWs as determined via
SEM is shown in Figure 2(a), higher magnification images are also provided in Figures 2(c) and 2(d).
The SnO2 nanoparticles were synthesized using a sol-gel method typical for multivalent metal oxide gelation reactions. A
large excess of deionized water was added to SnCl4·5H2O dissolved in ethylene glycol along with tetramethylammonium chloride and ammonium acetate to act as surfactants. The reaction was then allowed to progress for at least one hour at near reflux conditions, after which the resulting nanoparticle dispersion can be collected, washed, and dispersed in a polar solvent of choice. The material properties of SnO2 nanoparticles formed using a similar synthesis method have been reported previously [24], although the present recipe uses excess water to ensure that the hydrolysis reaction proceeds nearly to completion.
After mixing with SnO2 nanoparticles, films deposited from AgNW/SnO2 composite inks show a largely continuous
nanoparticle layer on the substrate surface with some nanowires partially buried and some sitting more or less on top of the
film. Representative scanning electron microscopy (SEM) images of nanocomposite films are shown in Figure 2(b).
Regardless of their position relative to the SnO2 film, all nanowires show a distinct shell on their outer surface that gives
them a soft and slightly rough appearance, as is visible in the higher magnification images shown in Figure 2(e) and 2(f).
The SnO2 nanoparticles do a particularly good job coating the regions near and around junctions between wires, and
frequently appear in the SEM images as bulges wrapped around the wire/wire contact points.
The precise morphology of the SnO2 shell that effectively surrounded each AgNW was analyzed in more detail using
transmission electron microscopy (TEM) imaging. Figures 3(a) to 3(c) show individual nanowires in the presence of
different ligand systems: as-synthesized PVP in Figure 3(a), inactive SnO2 in Figure 3(b), and SnO2 activated with trace
amounts of ammonium ions in Figure 3(c). The as-synthesized nanowires show sharp edges, and few surface features. In the
presence of inactive SnO2, which is formed by repeatedly washing the SnO2 nanoparticles in ethanol until all traces of
ammonium ions are removed, the nanowires coexist with somewhat randomly distributed nanoparticles that deposit all over
the surface of the TEM grid. When AgNWs are mixed with activated SnO2, a thick and continuous SnO2 shell is formed
along the nanowire surface. In when sufficiently dilute SnO2 solutions are used to form the nanocomposite ink, nearly all of
the nanoparticles are consumed during shell formation and effectively no nanoparticles are left to randomly populate the rest
of the image.
As the AgNWs acquire their metal oxide coatings in solution, the properties of the mixture change dramatically. Freshly
synthesized AgNWs coated with residual PVP ligands slowly settle to the bottom of their vial or flask over a time period of several hours to one day, forming a dense layer at the bottom. The AgNWs with SnO2 shells do not settle to the bottom, but remain partially suspended even after many weeks at concentrations that are dependent on the amount of SnO2 present in the solution.
A comparison of the settling behavior of various AgNW and SnO2 mixtures after 24 hours is shown in Figures 3(d) and
3(e). The ratios 8:4, 8:16, and 8:8 indicate the concentrations of AgNWs and SnO2 (in mg/mL) present in each solution. The
8:8 uncoupled solution, in which the PVP is not removed from the AgNW surface with ammonia, produces a situation in
which the nanowires and nanoparticles do not interact with one another, and instead the nanowires settle as in the isolated
nanowire solution while the nanoparticles remain well-dispersed as in the solution of pure SnO2. The mixtures of nanowires
and nanoparticles in which trace amounts of ammonia are present do not settle to the bottom, but instead concentrate
themselves until repulsion between the semiconducting SnO2 clusters is able to prevent further settling.
Our current explanation for the settling behavior of the wire/particle mixtures is that the PVP coating on the surface of the
as-synthesized wires is sufficient to prevent interaction with the nanoparticle solution. The addition of ammonia into the solution quickly strips off the PVP surface coating and allowing the nanoparticles to coordinate directly with the nanowire surface. This explanation is in agreement with the effects of ammonia has on a solution of pure AgNWs, which rapidly begin to agglomerate into clusters and sink to the bottom as soon as any significant quantity of ammonia is added to the ink.
We attribute the stripping ability of ammonia in these mixtures to the strong dative interactions that
occur via the lone pair on the nitrogen atom interacting with the partially filled d-orbitals of the Ag atoms
on the nanowire surface. These interactions are evidently strong enough to displace the existing
coordination of the five-membered rings and carbonyl groups contained in the original PVP ligands and
allow the ammonia to attach directly to the nanowire surface. Since ammonia is one of the original
surfactants used to stabilize the surface of the SnO2 nanoparticles, we consider it reasonable that ammonia
coordination on the nanowire surface would provide an appropriate environment for the nanoparticles to
adhere to the AgNWs.
Scanning Energy Dispersive X-ray (EDX) Spectroscopy was also conducted on nanoparticle-coated AgNWs in order to
image the presence of Sn and Ag in the nanowire and shell layer. The line scan results are shown in Figure 3(f), having been
normalized to better compare the widths of the two signals. The visible broadening of the Sn lineshape compared to that of
Ag is indicative of a Sn layer along the outside of the wire. The increasing strength of the Sn signal toward the center of the
AgNW is likely due to the enhanced interaction between the TEM's electron beam and the dense AgNW, which then
improves the signal originating from the SnO2 shell as well. It is also possible that there is some intermixing between the Ag
and Sn x-ray signals, but we consider this to be less likely as the distance between their characteristic peaks should be larger
than the detection system's energy resolution.
2.2. Network Deposition and Device Applications
For the deposition of transparent conducting films, a weight ratio of 2:1 of AgNWs to SnO2 nanoparticles was chosen in
order to obtain a balance between the dispersibility of the nanowires, the uniformity of coated films, and the sheet resistance of the resulting conductive networks. Nanocomposite films were deposited on glass by blade coating from an ethanolic solution using a scotch tape spacer, with deposited networks then being allowed to dry naturally in air over several minutes.
The as-dried nanocomposite films are highly conductive, and require only minimal thermal treatment to dry and harden
the film. Without the use of activated SnO2 ligands, deposited nanowire networks are highly insulating, and become
conductive only after annealing at above 200 °C. The sheet resistance values of representative films are shown in Figure
4(a). The capability to form transparent conductive networks in a single deposition step that remain useful over a wide range
of processing temperatures provides a high degree of versatility for designing thin film device fabrication procedures.
Figure 5(a) shows the sheet resistance and transmission of a number of nanocomposite films deposited from inks
containing different nanowire concentrations. The deposited films show excellent conductivity at transmission values up to
85%, and then rapidly increase in sheet resistance as the network begins to reach its connectivity limit. The optimum
performance of these networks at low to moderate transmission values is a consequence of the relatively large nanowire
diameters, which scatter a noticeable amount of light even when the conditions required for current percolation are just
barely met. Nonetheless, the sheet resistance and transmission of the completed nanocomposite networks place them within
an acceptable range for applications in a variety of optoelectronic devices. Figure 5(b) shows the wavelength dependent
transmission spectra of several nanowire networks, which transmit light well out into the infrared region. The presence of
high transmission values out to wavelengths well above 1300 nm, where ITO or other conductive oxide layers would
typically begin to show parasitic absorption, is due to the use of semiconducting SnO2 ligands, which is complimentary to
the broad spectrum transmission of the silver nanowire network itself.
Avoiding the use of highly doped nanoparticles has the potential to provide optical advantages, but can create difficulties
when attempting to make electrical contact to neighboring device layers. In order to investigate their functionality in thin
film devices, we have incorporated AgNW/SnO2 nanocomposite films as electrodes in amorphous silicon (a-Si) solar cells.
Two contact structures were used during fabrication: one with the nanocomposite film directly in contact with the p-i-n
absorber structure and one with a 10 nm Al:ZnO (AZO) layer present to assist in forming Ohmic contact with the device.
The I-V characteristics of the resulting devices are shown in Figure 6(a).
The thin AZO contact layers typically show sheet resistance values greater than 2.5 kΩ/⧠, and so cannot be responsible
for long range lateral current transport within the electrode structure. However, their presence is clearly beneficial in improving contact between the nanocomposite electrode and the absorber material, as the SnO2 matrix material is evidently not conductive enough to form a high quality contact with the p-type side of the a-Si stack. We hope that future modifications to the AgNW/SnO2 composite, or perhaps the use of islands of high conductivity material such as a discontinuous layer of doped nanoparticles will allow for the deposition of completed electrode stacks that provide both rapid fabrication and good performance.
Figure 6(b) contains the top view image of a completed device. The enhanced viscosity of the nanowire/sol-gel composite
inks allows for films to be blade coated onto substrates with a variety of surface properties without reductions in network uniformity. In contrast with traditional back electrodes deposited in vacuum environments, the nanocomposite can be blade coated into place in a single pass under atmospheric conditions and dried within moments. We anticipate that the use of sol-gel mixtures to enhance wetting and dispersibility may prove useful in the formulation of other varieties of semiconducting and metallic inks for deposition onto a variety of substrate structures.
3. Conclusions
In summary, we have successfully exchanged the insulating ligands that normally surround as-synthesized AgNWs with
shells of substantially more conductive SnO2 nanoparticles. The exchange of one set of ligands for the other is mediated by
the presence of ammonia during the mixing process, which appears to be necessary for the effective removal of the PVP ligands that initially cover the nanowire surface. The resulting nanowire/nanoparticle mixtures allow for the deposition of nanocomposite films that require no annealing or other post-treatments to function as high quality transparent conductors with transmission and sheet resistance values of 85% and 10 Ω/⧠, respectively. Networks formed in this manner can be deposited quickly and easily in open air, and have been demonstrated as an effective n-type electrode in a-Si solar cells when a thin interfacial layer is deposited first to ensure good electronic contact with the rest of the device. The ligand management strategy described here could potentially be useful in any number of material systems that presently suffer from highly insulating materials that reside on the surface of otherwise high performance nano and microstructures.
4. Experimental Details
Tin oxide nanoparticle synthesis. Tin chloride pentahydrate was dissolved in ethylene glycol by
stirring for several hours at a concentration of 10 grams per 80 mL to serve as a stock solution. In a typical
synthesis reaction, 10 mL of the SnCl4·5H2O stock solution is added to a 100 mL flask and stirred at room
temperature. Still at room temperature, 250 mg ammonium acetate and 500 mg ammonium acetate were
added in powder form to regulate the solution pH and to serve as coordinating agents for the growing
oxide nanoparticles. 30 ml of water was then added, and the flask was heated to 90 °C for 1 to 2 hours in
an oil bath, during which the solution took on a cloudy white color. The gelled nanoparticles were then
washed twice in ethanol in order to keep trace amounts of ammonia present in the solution. Additional
washing cycles would deactivate the SnO2, and then require the addition of ammonia to coordinate with
as-synthesized AgNWs.
Silver nanowire synthesis. Copper(ii) chloride dihydrate was first dissolved in ethylene glycol at
1 mg/ml to serve as a stock solution for nanowire seed formation. 20 ml of ethylene glycol was then added
into a 100 ml flask, along with 200 µL of copper chloride solution. the mixture was then heated to 150 °C
while stirring at 325 rpm, and .35g of PVP (MW 55,000) was added. In a small separate flask, .25 grams of
silver nitrate was dissolved in 10 ml ethylene glycol by sonicating for approximately 2 minutes, similar to
the method described here.22 The silver nitrate solution was then injected into the larger flask over
approximately 15 minutes, and the reaction was allowed to progress for 2 hours. After the reaction had
reached completion, the various steps were repeated without cooling down. 200 µL of copper chloride
solution and .35g PVP were added in a similar manner to the first reaction cycle, and another .25g silver
nitrate were dissolved via ultrasonics and injected over 15 minutes. The second reaction cycle was allowed
to progress for another 2 hours, before the flask was cooled and the reaction products were collected and
washed three times in ethanol.
Nanocomposite ink formation. After the synthesis of the two types of nanostructures is complete,
the double washed SnO2 nanoparticles and triple-washed nanowires can be combined at a variety of weight
ratios to form the completed nanocomposite ink. The dispersibility of the mixture is improved when more
SnO2 is used, although the sheet resistance of the final networks will begin to increase if they contain
excessive SnO2. AgNW agglomeration during mixing is most easily avoided if the SnO2 and AgNW
solutions are first diluted to the range of 10 to 20 mg/ml in ethanol, with the SnO2 solution being added
first to an empty vial and the AgNW solution added afterwards. The dilute mixture was then be allowed to
settle overnight, and the excess solvent removed to concentrate the wires to a concentration that is
appropriate for blade coating.
Film and electrode deposition. The completed nanocomposite ink was deposited onto any desired
substrates using a razor blade and scotch tape spacer. The majority of the substrates used in this study were
Corning soda lime glass, but the combined inks also deposited well on silicon, SiO2, and any other
substrates tested. Electrode deposition onto a-Si substrates was accomplished by masking off the desired
cell area with tape, and then depositing over the entire region. The p-i-n a-Si stacks and 10 nm AZO
contact layers were deposited using PECVD and sputtering, respectively.
The authors would like to acknowledge the use of the Electron Imaging Center for Nanomachines
(EICN) located in the California NanoSystems Institute at UCLA.
REFERENCES
[1]
Sun, Y.; Gates, B.; Mayers, B.; Xia, Y., Crystalline silver nanowires by soft solution
processing. Nano Lett. 2002, 2, 165-168.
Kim, T.; Kim, Y. W.; Lee, H. S.; Kim, H.; Yang, W. S.; Suh, K. S., Uniformly
interconnected silver-nanowire networks for transparent film heaters. Adv. Funct.
Mater. 2013, 23, 1250-1255.
Hu, L.; Wu, H.; Cui, Y., Metal nanogrids, nanowires, and nanofibers for transparent
electrodes. MRS Bull. 2011, 36, 760-765.
van de Groep, J.; Spinelli, P.; Polman, A., Transparent conducting silver nanowire
networks. Nano Lett. 2012, 12, 3138-3144.
Yang, L.; Zhang, T.; Zhou, H.; Price, S. C.; Wiley, B. J.; You, W., Solution-processed
flexible polymer solar cells with silver nanowire electrodes. ACS Appl. Mater.
Interfaces 2011, 3, 4075-4084.
Scardaci, V.; Coull, R.; Lyons, P. E.; Rickard, D.; Coleman, J. N., Spray deposition of
highly transparent, low-resistance networks of silver nanowires over large areas. Small
2011, 7, 2621-2628.
Wiley, B.; Sun, Y.; Xia, Y., Synthesis of silver nanostructures with controlled shapes
and properties. Acc. Chem. Res. 2007, 40, 1067-1076.
Korte, K. E.; Skrabalak, S. E.; Xia, Y., Rapid synthesis of silver nanowires through a
cucl- or cucl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437-441.
Anuj, R. M.; Akshay, K.; Chongwu, Z., Large scale, highly conductive and patterned
transparent films of silver nanowires on arbitrary substrates and their application in
touch screens. Nanotechnology 2011, 22, 245201.
[10] Lee, J.-Y.; Connor, S. T.; Cui, Y.; Peumans, P., Solution-processed metal nanowire
mesh transparent electrodes. Nano Lett. 2008, 8, 689-692.
[11] Zhu, R.; Chung, C.-H.; Cha, K. C.; Yang, W.; Zheng, Y. B.; Zhou, H.; Song, T.-B.;
Chen, C.-C.; Weiss, P. S.; Li, G.; Yang, Y., Fused silver nanowires with metal oxide
nanoparticles and organic polymers for highly transparent conductors. ACS Nano 2011,
5, 9877-9882.
[12] Chung, C.-H.; Song, T.-B.; Bob, B.; Zhu, R.; Duan, H.-S.; Yang, Y., Silver nanowire
composite window layers for fully solution-deposited thin-film photovoltaic devices.
Adv. Mater. 2012, 24, 5499-5504.
[13] Kim, A.; Won, Y.; Woo, K.; Kim, C.-H.; Moon, J., Highly transparent low resistance
zno/ag nanowire/zno composite electrode for thin film solar cells. ACS Nano 2013, 7,
[14] Ajuria, J.; Ugarte, I.; Cambarau, W.; Etxebarria, I.; Tena-Zaera, R. n.; Pacios, R.,
Insights on the working principles of flexible and efficient ito-free organic solar cells
based on solution processed ag nanowire electrodes. Sol. Energy Mater. Sol. Cells
2012, 102, 148-152.
[15] Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.; Aso, Y.; Suganuma, K.,
Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res.
2011, 4, 1215-1222.
[16] Lim, J.-W.; Cho, D.-Y.; Jihoon, K.; Na, S.-I.; Kim, H.-K., Simple brush-painting of
flexible and transparent ag nanowire network electrodes as an alternative ito anode for
cost-efficient flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 107,
[17] De, S.; Higgins, T. M.; Lyons, P. E.; Doherty, E. M.; Nirmalraj, P. N.; Blau, W. J.;
Boland, J. J.; Coleman, J. N., Silver nanowire networks as flexible, transparent,
conducting films: Extremely high dc to optical conductivity ratios. ACS Nano 2009, 3,
[18] Hu, L.; Kim, H. S.; Lee, J.-Y.; Peumans, P.; Cui, Y., Scalable coating and properties of
transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955-2963.
[19] Garnett, E. C.; Cai, W.; Cha, J. J.; Mahmood, F.; Connor, S. T.; Greyson Christoforo,
M.; Cui, Y.; McGehee, M. D.; Brongersma, M. L., Self-limited plasmonic welding of
silver nanowire junctions. Nat. Mater. 2012, 11, 241-249.
[20] Yu, Z.; Zhang, Q.; Li, L.; Chen, Q.; Niu, X.; Liu, J.; Pei, Q., Highly flexible silver
nanowire electrodes for shape-memory polymer light-emitting diodes. Adv. Mater.
2011, 23, 664-668.
[21] Song, T.-B.; Chen, Y.; Chung, C.-H.; Yang, Y.; Bob, B.; Duan, H.-S.; Li, G.; Tu,
K.-N.; Huang, Y., Nanoscale joule heating and electromigration enhanced ripening of
silver nanowire contacts. ACS Nano 2014, 8, 2804-2811.
[22] Lee, P.; Lee, J.; Lee, H.; Yeo, J.; Hong, S.; Nam, K. H.; Lee, D.; Lee, S. S.; Ko, S. H.,
Highly stretchable and highly conductive metal electrode by very long metal nanowire
percolation network. Adv. Mater. 2012, 24, 3326-3332.
[23] Lee, J. H.; Lee, P.; Lee, D.; Lee, S. S.; Ko, S. H., Large-scale synthesis and
characterization of very long silver nanowires via successive multistep growth. Cryst.
Growth Des. 2012, 12, 5598-5605.
[24] Bob, B.; Song, T.-B.; Chen, C.-C.; Xu, Z.; Yang, Y., Nanoscale dispersions of gelled
Sno2: Material properties and device applications. Chem. Mater. 2013, 25, 4725-4730.
Figure 1. Process flow diagram showing the synthesis of AgNWs and SnO2 nanoparticles followed
by stirring in the presence of ammonium salts to create the final nanocomposite ink. Transparent
conducting films were produced by blade coating the completed inks onto the desired substrate.
Figure 2. (a,c,d) SEM images of as-synthesized AgNWs at various magnifications. (b,e,f) SEM
images of nanocomposite films, showing the tendency of the SnO2 nanoparticles to coat the entire
outer surface of the AgNWs, increasing their apparent diameter and giving them a soft appearance.
Figure 3. Schematic diagrams and TEM images of (a) a single untreated AgNW, (b) an AgNW in the
presence of uncoupled SnO2 (all ammonium ions removed), and (c) an AgNW with a coordinating
SnO2 shell. Scale bars in images (a), (b), and (c) are 300 nm, 400 nm, and 600 nm, respectively. (d,e)
Optical images of AgNW and SnO2 nanoparticle dispersions mixed in varying amounts (d) before and
(e) after settling for 24 hours. The numbers associated with each solution represent the AgNW:SnO2
concentrations in mg/ml. The uncoupled solution contains AgNWs and non-coordinating SnO2
nanoparticles, and shows settling behavior similar to the pure AgNW and pure SnO2 solutions. (f)
Normalized Ag and Sn EDX signal mapped across the diameter of a single nanowire, with the inset
showing the scanning path across an isolated wire.
Figure 4. Sheet resistance versus temperature for films deposited using (red) AgNWs that have been
washed three times in ethanol and (blue) mixtures of AgNW and SnO2 with weight ratio of 2:1. The
annealing time at each temperature value was approximately 10 minutes. The large sheet resistance
values of the bare AgNWs when annealed below 200 °C is typical for nanowires fabricated using
copper chloride seeds, which clearly illustrate the impact of SnO2 coordination at low treatment
Figure 5. (a) Sheet resistance and transmission data for samples deposited from solutions of varying
nanostructure concentration. Each of these samples were fabricated starting from the same
nanocomposite ink, which was then diluted to a range of concentrations while maintaining the same
AgNW to SnO2 weight ratio. (b) Transmission spectra of several transparent conducting networks
chosen from the plot in plot (a).
Figure 6. (a) I-V characteristics of devices made with AgNW/SnO2 rear electrodes with (blue) and
without (red) a 10 nm AZO contact layer. The dramatic double diode effect is likely a result of a
significant barrier to charge injection at the electrode/a-Si interface. (b) Top view SEM image of the
AgNW/SnO2 composite films on top of the textured a-Si absorber. (c) Schematic cross section of the
a-Si device architecture used in solar cell fabrication. The thickness of the thin AZO contact layer is
exaggerated for clarity.
Source: http://www.thenanoresearch.com/upload/justPDF/0935.pdf
AREA DRUGS & THERAPEUTICS COMMITTEE: 8 DECEMBER 2014 ADTC(M) 14/05 Minutes: 62 - 75 NHS GREATER GLASGOW AND CLYDE Minutes of a Meeting of the Area Drugs and Therapeutics Committee held in the Boardroom, JB Russell House on Monday, 8 December 2014 at 2.00 p.m. Dr J Gravil (in the Chair) Miss F Qureshi . Observer
Guideline for oral healthcare of adults with Huntington's disease Graham Manley1, Helen Lane1, Annette Carlsson2, Bitte Ahlborg2, Åsa Mårtensson2, Monica B Nilsson2, Sheila A Simpson3,4 & Daniela Rae*3,4; On behalf of the contributing members of the European Huntington's Disease Networks Standards of Care Dental Care Group A preventive dentistry regime should be implemented at the earliest possible opportunity and maintained throughout development of the condition. The use of high fluoride toothpaste is essential.











