Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Http://www.uptodate.com/contents/guidelines-for-pediatric-advan
Guidelines for pediatric advanced life support
Official reprint from UpToDate® www.uptodate.com 2014 UpToDate®
Guidelines for pediatric advanced life support
Section Editor
Deputy Editor
Eric Fleegler, MD, MPH
Susan B Torrey, MD
James F Wiley, II, MD, MPH
Monica Kleinman, MD
All topics are updated as new evidence becomes available and our peer review process is complete.
Literature review current through: May 2014.
This topic last updated: Jan 31, 2013.
INTRODUCTION — The American Heart Association (AHA) Pediatric Advanced Life Support (PALS)
program provides a structured approach to the assessment and treatment of the critically ill pediatric
patient [1]. The AHA guidelines for pediatric resuscitation were updated in 2010 to reflect advances
and research in clinical care using new evidence from a variety of sources ranging from large clinical trials to animal models [2]. The PALS content includes:
■ Overview of assessment■ Recognition and management of respiratory distress and failure■ Recognition and management of shock■ Recognition and management of cardiac arrhythmias■ Recognition and management of cardiac arrest■ Postresuscitation management of patients with pulmonary and cardiac arrest■ Review of pharmacology
The clinician should primarily focus on prevention of cardiopulmonary failure through early recognition
and management of respiratory distress, respiratory failure, and shock that can lead to cardiac arrest
from hypoxia, acidosis, and ischemia.
This topic will discuss the advanced components of recognition and treatment of respiratory failure, shock, cardiopulmonary failure, and cardiac arrhythmias in children. Basic life support in children and
guidelines for cardiac resuscitation in adults are discussed separately. (See "Basic life support in
infants and children" and "Advanced cardiac life support (ACLS) in adults".)
OVERVIEW OF ASSESSMENT — The assessment of respiratory distress and circulatory
compromise in children, including the common findings, is covered in greater detail separately. (See
"Initial assessment and stabilization of children with respiratory or circulatory compromise".)
PALS uses an assessment model that facilitates rapid evaluation and intervention for life-threatening
conditions. In infants and children, most cardiac arrests result from progressive respiratory failure and/or shock, and one of the aims of this rapid assessment model is to prevent progression to cardiac
arrest. The evaluation includes:
■ General assessment via the pediatric assessment triangle (brief visual and auditory observation
of child's overall appearance, work of breathing, circulation) (see "Initial assessment and
stabilization of children with respiratory or circulatory compromise")
■ Primary assessment (rapid evaluation of cardiopulmonary and neurologic function)
■ Secondary assessment (focused medical history using SAMPLE mnemonic and thorough head
to toe physical exam)
■ Tertiary assessment (laboratory, radiographic, and other ancillary studies)
Guidelines for pediatric advanced life support
Primary assessment — The clinician should in rapid sequence assess:
■ Airway (patent, patent with maneuvers/adjuncts, partially or completely obstructed)
■ Breathing (respiratory rate, effort, tidal volume, lung sounds, pulse oximetry)
■ Circulation (skin color and temperature, heart rate and rhythm, blood pressure, peripheral and
central pulses, capillary refill time)
• AVPU pediatric response scale: Alert, Voice, Pain, Unresponsive
■ Glasgow Coma Scale: Eye Opening, Verbal Response, Motor Response (table 1)
• Pupillary response to light
• Presence of hypoglycemia (rapid bedside glucose or response to empiric administration of
■ Exposure (fever or hypothermia, skin findings, evidence of trauma)
Secondary assessment — This portion of the evaluation includes a thorough head to toe physical
examination, as well as a focused medical history that consists of the "SAMPLE" history:
■ S: Signs and symptoms■ A: Allergies■ M: Medications■ P: Past medical history■ L: Last meal■ E: Events leading to current illness
Tertiary assessment — Injury and infection are common causes of life-threatening illness in children.
Thus, ancillary studies are frequently directed towards identifying the extent of trauma or an infectious focus. (See "Trauma management: Approach to the unstable child", section on 'Adjuncts to the
primary survey' and "Trauma management: Approach to the unstable child", section on 'Adjuncts to
the secondary survey' and "Approach to the septic-appearing infant", section on 'Ancillary studies' and "Initial evaluation of shock in children", section on 'Evaluation'.)
RESPIRATORY DISTRESS AND FAILURE — A major goal of PALS is to recognize and treat
respiratory conditions amenable to simple measures (eg, supplemental oxygen, inhaled albuterol).
The clinician may also have to treat rapidly progressive conditions and intervene with advanced therapies to avoid cardiopulmonary arrest in patients with respiratory failure. Early detection and
treatment improve overall outcome.
There are many causes of acute respiratory compromise in children (table 2). The clinician should
strive to categorize respiratory distress or failure into one or more of the following (see "Emergent evaluation of acute respiratory compromise in children"):
■ Upper airway obstruction (eg, croup, epiglottitis)■ Lower airway obstruction (eg, bronchiolitis, status asthmaticus)■ Lung tissue (parenchymal) disease (eg, bronchopneumonia)■ Disordered control of breathing (eg, seizure, coma, muscle weakness)
Initial management supports airway, breathing, and circulation:
Guidelines for pediatric advanced life support
■ Airway – Key steps in basic airway management include (see "Basic airway management in
• Provide 100 percent inspired oxygen
• Allow child to assume position of comfort or manually open airway
• Clear airway (suction)
• Insert oropharyngeal airway or nasopharyngeal airway if consciousness impaired
■ Breathing – The clinician should assist ventilation manually in patients not responding to basic
airway maneuvers, monitor oxygenation by pulse oximetry, monitor ventilation by end-tidal CO2
if available, and administer medications as needed (albuterol, epinephrine). In preparation for
intubation, positive pressure ventilation should be initiated with a bag-valve-mask to preoxygenate and improve ventilation. (See "Basic airway management in children".)
Children who cannot maintain their airway, oxygenation, or ventilatory requirements should
undergo placement of an artificial airway, usually via endotracheal intubation and less
commonly with a laryngeal mask airway or alternative device. A rapid overview describes the steps in performing rapid sequence intubation (table 3). (See "Emergent endotracheal
intubation in children" and "Rapid sequence intubation (RSI) in children".)
■ Circulation – Key interventions consist of monitoring heart rate and rhythm and establishing
vascular access (see "Vascular (venous) access for pediatric resuscitation and other pediatric
SHOCK — The goal is to recognize and categorize the type of shock in order to prioritize treatment
options (algorithm 1). Early treatment of shock may prevent the progression to cardiopulmonary failure
(algorithm 2).
Shock results from the inadequate delivery of oxygen to the tissues relative to tissue metabolic
demand, usually characterized by inadequate perfusion. Shock may occur with normal, increased, or decreased systolic blood pressure. Shock in children is usually related to low cardiac output, but some
patients may have high cardiac output, such as with sepsis or severe anemia. (See "Initial evaluation
of shock in children".)
Shock severity is usually categorized by its effect on systolic blood pressure:
■ Compensated shock occurs when compensatory mechanisms (including tachycardia,
increased systemic vascular resistance, increased inotropy, and increased venous tone)
maintain a systolic blood pressure within a normal range. The calculators provide the percentile of blood pressure by height for boys, age 2 to 17 years (calculator 1) and girls, age 2 to 17
years (calculator 2).
■ Hypotensive shock (or decompensated shock) occurs when compensatory mechanisms fail to
maintain systolic blood pressure. In children 1 to 10 years of age, hypotension is defined as:
Systolic pressure (5th percentile) < 70 mmHg + (child's age in years x2)
For infants 1 to 12 months of age, hypotension is defined by systolic pressure <70 mmHg, and, in term infants 0 to 1 month of age, systolic pressure <60 mmHg [2]. Systolic blood pressure
<90 mmHg indicates hypotension in children over 10 years of age. Hypotensive shock may
rapidly progress to cardiopulmonary failure.
Guidelines for pediatric advanced life support
■
Shock categorization – There are four major categories of shock (see "Initial evaluation of
shock in children"):
• Hypovolemic shock – Hypovolemic shock is characterized by inadequate circulating blood
volume. Common causes of fluid loss include diarrhea, hemorrhage (internal and external),
vomiting, inadequate fluid intake, osmotic diuresis (eg, diabetic ketoacidosis), third-space
losses, and burns.
• Distributive shock – Distributive shock describes inappropriately distributed blood volume
typically associated with decreased systemic vascular resistance. Common causes include
septic shock, anaphylactic shock, and neurogenic shock (eg, head injury, spinal injury).
• Cardiogenic shock – Cardiogenic shock refers to impairment of heart contractility. Common
causes include congenital heart disease, myocarditis, cardiomyopathy, arrhythmias, sepsis, poisoning or drug toxicity, and myocardial injury (trauma).
• Obstructive shock – In this form of shock, hypotension arises from obstructed blood flow to
the heart. Common causes include cardiac tamponade, tension pneumothorax, ductal dependent congenital heart lesions, and massive pulmonary embolism.
Any given patient may suffer from more than one type of shock. For example, a child in septic shock may develop hypovolemia during the prodrome phase, distributive shock
during the early phase of sepsis, and cardiogenic shock later in the course.
■
Shock management – The approach to undifferentiated shock in children requires careful
attention to history and physical examination in order to arrive at the type of shock present (algorithm 2). Goal-directed therapy for shock is discussed separately. (See "Initial
management of shock in children", section on 'Early goal-directed therapy'.)
Early treatment can greatly improve outcome. Goals are to improve oxygen delivery and to
reduce oxygen consumption. Specific measures include increasing circulating volume, increasing cardiac contractility, improving distribution of cardiac output, and reducing oxygen
demand. Methods include:
• Administration of high concentration of oxygen
• Support of respirations to decrease the work of breathing
• If rapid sequence intubation is necessary, a sedating agent should be chosen so that
hemodynamic stability is not worsened (see "Initial management of shock in children",
section on 'Airway management')
• Rapid intravenous administration of fluids (eg, boluses of normal saline 20 mL/kg) up to
three times or more as needed for persistent hypotension. For septic shock in children in
resource-rich settings, it is optimal to deliver 60 mL/kg within the first 60 minutes of management (see "Septic shock: Rapid recognition and initial resuscitation in children",
section on 'Intravenous fluid therapy')
• Administration of vasoconstrictors in selected patients
• Use of inotropic medications in selected patients
• Reversal of identified obstructions
• Other adjunct methods include treatment of underlying infection, fever, pain and anxiety,
and treatment of metabolic derangements (hypoglycemia, hypocalcemia, hyperkalemia, metabolic acidosis)
Guidelines for pediatric advanced life support
CARDIOPULMONARY FAILURE — Respiratory failure and hypotensive shock are the most common
conditions preceding cardiac arrest.
■ Causes of respiratory failure include:
• Upper airway obstruction (choking, infection)
• Lower airway obstruction (asthma, foreign body aspiration)
• Parenchymal disease (pneumonia, acute pulmonary edema)
■ Causes of hypotensive shock include:
• Hypovolemia (dehydration, hemorrhage)
• Cardiac disease
• Distributive shock (septic, neurogenic)
• Metabolic/electrolyte disturbances
• Acute myocardial infarction/ischemia
• Toxicologic ingestions
• Pulmonary embolism
■ The following physical findings often precede cardiopulmonary failure:
•
Airway – Possible upper airway obstruction secondary to decreased level of
consciousness or anatomic obstruction from foreign body or infection
•
Breathing – Bradypnea, irregular, ineffective respiration, gasping
•
Circulation – Bradycardia, capillary refill >5 seconds, weak central pulses, no peripheral
pulses, hypotension, cool extremities, mottled/cyanotic skin
•
Disability – Diminished level of consciousness
The patient in cardiopulmonary failure will progress rapidly to cardiac arrest without aggressive
intervention. Positive pressure ventilations with 100 percent inspired oxygen, chest compressions for heart rate <60 beats per minute in patients with poor perfusion, and administration of intravenous
fluids and medications tailored to treat the underlying cause are indicated. (See "Basic airway management in children" and "Basic life support in infants and children".)
APPROACH TO CARDIAC ARRHYTHMIAS — Arrhythmias are classified as bradyarrhythmias,
tachyarrhythmias, and pulseless arrest. Evaluation requires knowledge of the child's typical heart rate
(table 4) and baseline rhythm as well as level of activity and clinical condition. The approach presented here is based on the consensus 2010 international resuscitation guidelines developed by
the American Heart Association (AHA) and the International Liaison Committee on Resuscitation
(ILCOR) [3-5].
■ Definition – Bradycardia is defined as a heart rate that is slow compared with normal heart
rates for the patient's age (table 4). Primary bradycardia is the result of congenital and acquired
heart conditions that directly slow the spontaneous depolarization rate of the heart's pacemaker or slow conduction through the heart's conduction system. Secondary bradycardia is the result
of conditions that alter the normal function of the heart, including hypoxia, acidosis,
hypotension, hypothermia, and drug effects. Bradyarrhythmias are common prearrest rhythms in children and are often due to hypoxia.
Guidelines for pediatric advanced life support
■ Signs and symptoms – Pathologic bradycardia frequently causes a change in the level of
consciousness, lightheadedness, dizziness, syncope, or fatigue. Shock associated with bradycardia can manifest with hypotension, poor end-organ perfusion, altered consciousness,
and/or sudden collapse. Bradycardia with symptoms of shock (eg, poor systemic perfusion,
hypotension, altered consciousness) requires urgent treatment to prevent cardiac arrest. ECG findings associated with bradycardia include (see "Bradycardia in children"):
• Slow heart rate relative to normal rates (table 4)
• P waves that may or may not be visible
• QRS complex that is narrow (electrical conduction arising from the atrium or high nodal
area) or wide (electrical conduction from low nodal or ventricular region)
• P wave and QRS complex may be unrelated (ie, atrioventricular dissociation) or have an
abnormally long period between them (atrioventricular block)
Sinus bradycardia — Sinus bradycardia is commonly an incidental finding in healthy children as a
normal consequence of reduced metabolic demand (sleep, rest) or increased stroke volume (well-conditioned athlete). Pathologic causes include hypoxia, hypothermia, poisoning, electrolyte
disorders, infection, sleep apnea, drug effects, hypoglycemia, hypothyroidism, and increased
intracranial pressure. (See "Bradycardia in children", section on 'Sinus bradycardia'.)
Atrioventricular block — Atrioventricular (AV) block is defined as a delay or interruption in the
transmission of an atrial impulse to the ventricles due to an anatomical or functional impairment in the
conduction system. Heart block is categorized into three types:
■
First degree – First degree AV block is characterized by a prolonged PR interval for age
caused by slow conduction through the AV node without missed ventricular beats (waveform
1). Of note, first degree AV block does not cause bradycardia. In general, the normal PR-intervals are: 70 to 170 msec in newborns, and 80 to 200 msec in young children and adults.
(See "Bradycardia in children", section on 'First degree AV block'.)
■
Second degree – In second-degree AV block, the organized atrial impulse fails to be
conducted to the ventricle in a 1:1 ratio. There are two types of second degree AV block (see
"Bradycardia in children", section on 'Second degree AV block'):
• Mobitz type I (Wenckebach phenomenon) – On ECG, there is progressive prolongation of
the PR-interval until a P wave fails to be conducted (waveform 2). The block is located at the level of the AV node and is usually not associated with other significant conduction
system disease or symptoms.
• Mobitz type II – This block occurs below the AV node and has consistent inhibition of a
specific proportion of atrial impulses, usually with a 2:1 atrial to ventricular rate (waveform
3). It has a less predictable course and frequently progresses to complete heart block.
■
Third degree – In third-degree AV block, also referred to as complete heart block, there is
complete failure of the atrial impulse to be conducted to the ventricles (waveform 4). The atrial and ventricular activity is independent of one another. The ventricular escape rhythm that is
generated is dictated by the location of the block. It is usually slower than the lower limits of
normal for age, resulting in clinically significant bradycardia. (See "Bradycardia in children", section on 'Third degree AV block'.)
Bradyarrhythmia management — The management of bradycardia focuses on reestablishing or
optimizing oxygenation and ventilation, supporting circulation with chest compressions if needed, and
using medications to increase heart rate and cardiac output (algorithm 3). (See "Basic airway
Guidelines for pediatric advanced life support
management in children" and "Basic life support in infants and children" and "Primary drugs in
pediatric resuscitation", section on 'Epinephrine' and "Primary drugs in pediatric resuscitation", section
If these measures fail, transcutaneous pacing can be attempted; however, the same factors that are
producing refractory bradycardia (eg, hypoxia, hypothermia, electrolyte disturbance, drug overdose) may prevent effective electrical capture. (See "Bradycardia in children", section on 'Acute
■
Definition – Tachyarrhythmias are fast abnormal rhythms originating in the atria or the
ventricles. Relative tachycardia is a heart rate that is too fast for the child's age, level of activity,
and clinical condition (table 4). In children, sinus tachycardia usually represents hypovolemia, fever, physiologic response to stress or fear, or drug effect (such as with beta agonists). (See
"Approach to the child with tachycardia".)
Certain arrhythmias, such as supraventricular tachycardia and ventricular tachycardia, can lead
to shock and cardiac arrest. Unstable rhythms lead to poor tissue perfusion with a fall in cardiac
output, poor coronary artery perfusion, and increased myocardial oxygen demand, which can all lead to cardiogenic shock.
■
Signs and symptoms – Clinical findings in children with tachycardia are often nonspecific and
vary by age. They may include palpitations, lightheadedness, dizziness, fatigue and syncope. In infants, prolonged tachycardia may cause poor feeding, tachypnea, and irritability with signs
of heart failure. (See "Approach to the child with palpitations" and "Emergent evaluation of syncope in children and adolescents".)
Important ECG findings include:
• Heart rate that is fast compared with normal rates (table 4)
• P waves that may or may not be visible
• QRS interval that is narrow or wide
■
Classification – Treatment priorities in managing tachycardias rely on differentiating between
tachycardia with narrow QRS complex (sinus tachycardia, supraventricular tachycardia, atrial flutter) and wide QRS complex tachycardias (ventricular tachycardia, supraventricular
tachycardia with aberrant intraventricular conduction).
Sinus tachycardia — Sinus tachycardia is characterized by a rate of sinus node discharge that is
faster than normal for the patient's age (table 4). This rhythm usually represents the body's increased
need for cardiac output or oxygen delivery. The heart rate is not fixed and varies with other factors,
including fever, stress, and level of activity. Causes include tissue hypoxia, hypovolemia, fever, metabolic stress, injury, pain, anxiety, toxins/poisons/drugs, and anemia. Less common causes
include cardiac tamponade, tension pneumothorax, and thromboembolism. (See "Approach to the
child with tachycardia".)
Typical ECG findings in patients with sinus tachycardia include:
■ Heart rate is usually <220/min in infants, <180/min in children, and exhibits beat to beat
variability in rate
■ P waves are present with normal appearance■ PR interval is constant and exhibits a normal duration for age■ R-R interval is variable
Guidelines for pediatric advanced life support
■ QRS complex is narrow
Supraventricular tachycardia — Supraventricular tachycardia (SVT) can be defined as an
abnormally rapid heart rhythm originating above the ventricles, often (but not always) with a narrow
QRS complex; it conventionally excludes atrial flutter and atrial fibrillation. The two most common
forms of SVT in children are atrioventricular reentrant tachycardia (AVRT), including the Wolff-Parkinson-White (WPW) syndrome (waveform 5), and atrioventricular nodal reentrant tachycardia
■
Signs and symptoms – SVT typically has an abrupt onset and intermittent presentation. Signs
and symptoms in infants include poor feeding, tachypnea, irritability, increased sleepiness,
diaphoresis, pallor, and/or vomiting. Older children may have palpitations, shortness of breath, chest pain/discomfort, dizziness, lightheadedness, and/or fainting. Infants and children with
prolonged SVT may display clinical findings of heart failure. (See "Supraventricular tachycardia
in children: AV reentrant tachycardia (including WPW) and AV nodal reentrant tachycardia", section on 'Clinical features'.)
Typical ECG findings in patients with SVT include:
• Heart rate that is usually >220/min in infants, >180/min in children, and has NO beat to
• P waves are absent or abnormal
• PR interval may not be present or short PR interval with ectopic atrial tachycardia
• R-R interval is usually constant
• QRS is usually narrow. Conduction delay along the ventricular system may lead to an
appearance of wide complex tachycardia, known as SVT with aberrant conduction.
Ventricular tachycardia — Ventricular tachycardia (VT) originates from the ventricular myocardium
or Purkinje cells below the bifurcation of the bundle of His (waveform 6). VT is associated with sudden cardiac death. As a result, patients who develop VT or are at risk for developing VT must be identified,
evaluated, and treated if necessary. Some forms of VT found primarily in infants and young children may be benign, but this conclusion is reached only after other more serious causes of VT are
excluded. VT may present with or without pulses. (See "Causes of wide QRS complex tachycardia in
children", section on 'Ventricular tachycardia' and 'Pulseless arrest' below.)
VT with pulses can vary in rate from near normal to >200 beat per minute. Faster rates can
compromise stroke volume and cardiac output leading to pulseless VT or ventricular fibrillation (VF). Causes of VT include underlying heart disease or cardiac surgery, prolonged QT syndrome, or
myocarditis/cardiomyopathy. Other causes include hyperkalemia and toxic ingestions (eg, tricyclic antidepressants, cocaine) (table 5).
Findings of ventricular tachycardia on ECG include (waveform 6):
■ Ventricular rate is >120 beats per minute and regular■ P waves are often not identifiable, may have AV dissociation, or may have retrograde
■ QRS is typically wide (>0.09 sec) ■ T waves are often opposite in polarity from the QRS complex
Tachyarrhythmia management — The management of sinus tachycardia focuses on treatment of
the underlying physiologic derangement and is largely supportive. The management of
tachyarrhythmias that are not sinus in origin is guided by the appearance of the QRS complex, and by the patient's status, whether compensated or uncompensated (algorithm 4):
Guidelines for pediatric advanced life support
■ Patients with either narrow or wide complex tachycardia who have significantly impaired
consciousness and hypotensive shock should be treated with synchronized cardioversion (Initial dose: 0.5 to 1 J/kg). (See "Defibrillation and cardioversion in children (including
automated external defibrillation)", section on 'Methods: Manual defibrillator use'.)
■ Patients who are mentating and not hypotensive may receive a trial of anti-arrhythmic therapy,
based on whether the arrhythmia is believed to originate from above the AV node (narrow
complex) or below the AV node (wide complex). For narrow complex tachycardia, the first recommended medication is adenosine, 0.1 mg/kg (maximum dose 6 mg) administered rapidly
IV/IO and followed by a rapid saline flush. Anti-arrhythmic therapy of wide-complex tachycardia
involves agents with significant side effects (eg: amiodarone) and consultation with a pediatric cardiology specialist is recommended. If the wide-complex rhythm is monomorphic and
regular, it is acceptable to administer a dose of adenosine to determine if the rhythm is actually
supraventricular tachycardia with aberrant conduction. (See "Management of supraventricular tachycardia in children", section on 'Antiarrhythmic drugs' and "Management and evaluation of
wide QRS complex tachycardia in children", section on 'Initial management' and "Primary drugs
in pediatric resuscitation", section on 'Adenosine' and "Primary drugs in pediatric resuscitation", section on 'Amiodarone' and "Primary drugs in pediatric resuscitation", section on
PULSELESS ARREST — Pulseless arrest refers to cessation of blood circulation caused by absent
or ineffective cardiac mechanical activity.
Most pediatric cardiac arrests are hypoxic/asphyxial arrests that result from a progression of
respiratory distress, respiratory failure, or shock rather than from primary cardiac arrhythmias ("sudden cardiac arrest"). Thus, the presenting rhythm is typically pulseless electrical activity or
Children with pulseless arrest appear apneic or display a few agonal gasps. They have no palpable
pulses, and are unresponsive. Overall survival from pediatric cardiac arrest is poor, and the incidence
of neurologic deficits in survivors is high.
Epidemiology and presenting rhythm — Out-of-hospital arrests in children six months to young
adulthood often occur at or near home [6,7]. The most common cause of death is from trauma,
leading to respiratory compromise and/or shock. Massive head injury and severe multiple systems
trauma are common in nonsurvivors. Sudden infant death syndrome (SIDS) is a leading cause of death in infants <6 months [6].
Sudden cardiac arrest due to ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT)
occurs in up to 18 percent of all pediatric prehospital cardiac arrests, but is less commonly the
presenting rhythm in younger children between the ages of one and eight years (8 percent) and infants [8]. Predisposing conditions or causes of ventricular rhythms in pediatric patients with
pulseless arrest include hypertrophic cardiomyopathy, anomalous coronary artery (from the pulmonary artery), long QT syndrome, myocarditis, drug intoxication (eg, digoxin, ephedra, cocaine),
and commotio cordis (ie, sharp blow to chest). These patients may have intact survival if defibrillation
is performed within minutes of arrest.
For in-hospital cardiac arrest, shockable rhythms are present at some point during the resuscitation in
up to 27 percent of children, with 10 to 15 percent having VF/VT as the initial arrest rhythm [9,10]. In one observational study of 1005 in-hospital cardiac arrest reported to a multicenter cardiac arrest
registry, survival was higher if VF or VT was the presenting arrhythmia (35 percent survival) versus "non-shockable" rhythms, such as asystole (11 percent) (algorithm 5) [9]. In another multicenter
observational study of 1031 children, risk-adjusted survival for all patients in cardiopulmonary arrest
was 43 percent, with significantly improved adjusted survival for those whose initial arrest rhythms
Guidelines for pediatric advanced life support
were pulseless VT or pulseless electrical activity when compared to asystole (risk ratio 1.6 and 1.2,
respectively) [10].
Arrest rhythms — Asystole, pulseless electrical activity (PEA), ventricular fibrillation (VF), and
pulseless ventricular tachycardia (VT) comprise the potential arrest rhythms.
Asystole — Children with asystole have cardiac standstill with no discernible electrical activity
(waveform 7). The most common cause is respiratory failure progressing to critical hypoxemia, bradycardia, and then cardiac standstill. Underlying conditions include pneumonia, submersion,
hypothermia, sepsis, and poisoning (eg, carbon monoxide poisoning, sedative-hypnotics) leading to
hypoxia and acidosis.
Pulseless electrical activity — Pulseless electrical activity (PEA) consists of any organized
electrical activity observed on ECG in a patient with no central palpable pulse. Reversible conditions
may underlie PEA, including:
■ Hypovolemia■ Hypoxia■ Hydrogen ion (acidosis)■ Hypo-/hyperkalemia■ Hypoglycemia■ Hypothermia■ Toxins■ Tamponade, cardiac■ Tension pneumothorax■ Thrombosis (coronary or pulmonary)■ Trauma
These can be remembered as the H's and T's of PEA [2].
Ventricular fibrillation — Ventricular fibrillation is characterized by no organized rhythm and no
coordinated contractions (waveform 7). Electrical activity is chaotic. Causes overlap with etiologies of
ventricular tachycardia, including hyperkalemia, congenital or acquired heart disease, toxic exposures,
electrical or lightning shocks, and submersion.
Pulseless ventricular tachycardia — Pulseless VT is a cardiac arrest of ventricular origin
characterized by organized, wide QRS complexes (waveform 6). Any cause of VT with pulses can
lead to pulseless VT. (See 'Ventricular tachycardia' above.)
Torsades de pointes — Torsades de pointes or polymorphic VT displays a QRS complex that
changes in polarity and amplitude, appearing to rotate around the ECG isoelectric line (translation: "twisting of the points") (waveform 8). This arrhythmia is associated with markedly prolonged QTc
interval from congenital conditions (long QT syndrome), drug toxicity (antiarrhythmic drugs, tricyclic
antidepressants, calcium channel blockers, phenothiazine), and electrolyte disturbances (eg, hypomagnesemia arising from anorexia nervosa). Ventricular tachycardia, including torsades de
pointes, can deteriorate into ventricular fibrillation.
Management — For highly effective chest compressions, the individual performing the compressions
needs to push hard, push fast, allow complete chest recoil, and minimize interruptions in chest compressions. The clinician should only interrupt compressions for ventilation, rhythm check, and
shock delivery. While chest-compression only CPR is effective as the initial treatment for adult out-of-
hospital cardiac arrest, infants and children should still receive both chest compressions and ventilations. (See "Basic life support in infants and children", section on 'Chest compressions'.)
Advanced management — Once basic cardiopulmonary resuscitation is established, treatment of
pulseless arrest requires rapid assessment of rhythm, performance of defibrillation as indicated, and
Guidelines for pediatric advanced life support
pharmacotherapy aimed at increasing coronary artery circulation and restoration of organized cardiac
conduction (algorithm 5):
■ Patients with ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) should
receive immediate CPR and defibrillation as soon as a device is available. After delivering the
shock, perform approximately two minutes of CPR (10 cycles for two person CPR or 5 cycles
for one person CPR) before checking the rhythm. If the rhythm has not converted with defibrillation, the patient should receive a repeated defibrillation at a higher dose. Persistent VF
or pVT requires the addition of medications such as parenteral epinephrine and antiarrhythmic
therapy (eg, amiodarone for VF or pVT; magnesium sulfate for torsades de pointes). See the algorithm for drug dosing (algorithm 5). Details of drug dosing and administration are provided
separately. (See "Primary drugs in pediatric resuscitation", section on 'Amiodarone' and "Primary drugs in pediatric resuscitation", section on 'Magnesium sulfate'.)
■ Patients with asystole or pulseless electrical activity should receive cardiopulmonary
resuscitation and epinephrine (IV/IO administration preferred over endotracheal). See the algorithm for drug dosing (algorithm 5). The dosing and administration of epinephrine in
children is discussed in more detail separately. (See "Primary drugs in pediatric resuscitation",
section on 'Epinephrine'.)
■ During the course of the resuscitation, the clinician should evaluate for underlying causes (H's
and T's) for the pulseless arrest as indicated on the algorithm (algorithm 5).
Vascular access — Establishment of reliable vascular access is a critical step in pediatric
resuscitation. During pulseless arrest, intraosseous cannulation and peripheral venous access should
be pursued simultaneously. (See "Vascular (venous) access for pediatric resuscitation and other pediatric emergencies" and "Intraosseous infusion".)
Resuscitation medications given through a peripheral IV should be followed with a 5 mL flush of normal saline to move the drug from the peripheral to the central circulation.
Many clinicians regard intraosseous cannulation as the preferred initial route of vascular access,
especially in young infants with pulseless arrest. Doses of medications administered via an
intraosseous line are the same as peripheral IV doses and should be followed by a 5 to 10 mL flush of normal saline. (See "Intraosseous infusion".)
Intraosseous cannulation becomes progressively more difficult as children age because the cortex of
bone becomes thicker and the tibial bone marrow cavity becomes smaller. However, the development
of devices, such as spring-loaded single-deployment devices and battery-operated handheld drills, has made intraosseous cannulation feasible for patients of all ages, including adults. (See
"Intraosseous infusion", section on 'Preparation'.)
Attempts at peripheral and central venous access in the head, neck, and chest should NOT interrupt
chest compressions. Central lines are more secure than peripheral access and provide more rapid onset and higher peak concentration of medications but are NOT required during initial resuscitation
attempts. If central lines are used, the femoral route is preferred by many. (See "Vascular (venous)
access for pediatric resuscitation and other pediatric emergencies", section on 'Femoral vein'.)
Endotracheal drug administration — Although lipid soluble drugs, such as lidocaine,
epinephrine, atropine, and naloxone ("LEAN"), may be administered via endotracheal tube (ETT), the
intravascular route is always preferred. Optimal drug dosing via endotracheal tube is unknown, with
unpredictable drug absorption leading to lower blood levels when compared with the same dose given intravascularly. Several key actions are needed when giving drugs via ETT:
Guidelines for pediatric advanced life support
■ Increase the epinephrine dose ten-fold (by using the 1:1000 concentration instead of the
1:10,000 concentration used for IV delivery) and the dose of other medications (atropine, lidocaine, naloxone) two- to threefold. (See "Primary drugs in pediatric resuscitation", section
on 'Epinephrine' and "Primary drugs in pediatric resuscitation", section on 'Atropine' and "Opioid
intoxication in children and adolescents", section on 'Dosing and administration'.)
■ Hold compressions during ETT administration.
■ Follow drug administration with 3 to 5 mL of normal saline.
■ Provide five positive pressure ventilations after instilling the drug.
Defibrillation — Defibrillation does not restart the heart; the shock "stuns" the heart by
depolarizing all of the myocardial cells, hopefully terminating VF and allowing the heart's natural pacemaker cells to resume an organized rhythm. After delivering a shock, the caregivers should
perform approximately two minutes of CPR (10 cycles for two person CPR or 5 cycles of one person
CPR) before checking the rhythm. CPR may be discontinued when a perfusing rhythm has been established. The dose of energy for pediatric defibrillation is 2 joules/kg for the initial shock, 4
joules/kg for the second shock, and 4 joules/kg or more for subsequent shocks with a maximum of 10
joules/kg or the adult dose (maximum dose differs between defibrillator models). (See "Defibrillation and cardioversion in children (including automated external defibrillation)", section on 'Methods:
Manual defibrillator use'.)
Biphasic defibrillators have a high first shock efficacy rate for ventricular fibrillation (VF) of short
duration in adults. This procedure eliminates VF but may not lead to a perfusing rhythm (often the patient will be in asystole or PEA after the shock) or myocardial ischemia prevents proper cardiac
contractility. For this reason, after delivering the shock, chest compressions should resume immediately for approximately two minutes as above. Cardiopulmonary resuscitation is required to
maintain blood flow to the heart, coronary circulation, and brain until effective cardiac contractility
Although manual defibrillators or automated external defibrillators with pediatric attenuating devices
are preferred for use in infants and children, automated external defibrillators without pediatric attenuating devices may be used if they are the only option available. (See "Basic life support in
infants and children", section on 'Automated external defibrillator'.)
Pharmacologic therapy — A detailed discussion of pediatric resuscitation drugs is provided
separately. (See "Primary drugs in pediatric resuscitation".)
Epinephrine is the most commonly used medication in children with pulseless arrest. It is classified as a catecholamine, vasopressor, and inotrope. (See "Primary drugs in pediatric resuscitation", section
on 'Epinephrine'.)
For pulseless arrest, the IV/IO dosing is 0.01 mg/kg (0.1 mL/kg of the 1:10,000 concentration) given
every three to five minutes; maximum single dose: 1 mg (10 mL). High dose intravenous epinephrine is no longer recommended. When epinephrine is administered via ET tube, use a 10-fold higher dose
or 0.1 mg/kg (0.1 mL/kg of the 1:1000 concentration) every three to five minutes. The IV/IO route is
always preferred (algorithm 5).
EARLY POSTRESUSCITATION MANAGEMENT — The early postresuscitation period involves the
time soon after return of spontaneous circulation or recovery from circulatory or respiratory failure.
During this time, the clinician must continue to treat the underlying cause of the life-threatening event
and monitor for common respiratory or circulatory problems that may cause secondary morbidity or death [5]:
Guidelines for pediatric advanced life support
■
Oxygen administration – Once return of spontaneous circulation has been achieved, the
clinician should titrate inspired oxygen to maintain arterial oxyhemoglobin saturation between 94 to 99 percent to avoid hyperoxemia. In two large, multicenter observational studies
hyperoxia has been associated with clinically significant increased risk of death [11,12].
■
Intubated patients – All intubated children require continued assessment to ensure proper
endotracheal tube positioning, continuous monitoring of oxygenation (pulse oximetry), and
ongoing monitoring of ventilation (eg, continuous end-tidal CO2 monitoring, if available, and/or intermittent blood gas assessment). Insertion of a gastric tube helps to reduce gastric
The causes of sudden decompensation in a child who has been successfully intubated with an
artificial airway is described by the mnemonic "DOPE":
•
D: Dislodged or displaced endotracheal tube (right mainstem or esophageal location)
•
O: Obstructed endotracheal tube (eg, mucous plug, kinked endotracheal tube)
•
P: Pneumothorax
•
E: Equipment failure (eg, ventilator malfunction, oxygen disconnected or off)
■
Recurrent shock – After fluid resuscitation in a child, circulatory instability may recur as the
result of ongoing fluid loss, decreased cardiac function, and/or harmful alterations in systemic vascular resistance. Goal-directed therapy emphasizes the need to assess clinical findings of
perfusion (eg, capillary refill, urine output), measure central venous pressure, and measure
central venous oxygen saturation, to determine the best course of action. Vasopressors (eg, epinephrine infusion (table 6 and table 7)) may be appropriate therapies for children with
documented or suspected cardiovascular dysfunction in the post-resuscitation setting. (See
"Initial management of shock in children", section on 'Early goal-directed therapy'.)
■
Glucose – The clinician should monitor blood glucose levels and promptly treat hypoglycemia.
(See "Approach to hypoglycemia in infants and children", section on 'Immediate management'.)
Sustained hyperglycemia (blood glucose greater than 180 mg/dL [10 mmol/L]) is associated
with higher mortality in critically ill children and should be avoided [13,14]. Evidence indicates that blood glucose should be maintained below this threshold but the role of "tight control" that
uses insulin to achieve a specified blood glucose range is of uncertain value in children after
cardiac arrest [5]. If performed, tight glucose control requires close monitoring of blood glucose and avoidance of hypoglycemia. Intensive insulin therapy in adults to maintain a blood glucose
range of 80 to 110 mg/dL (4.4 to 6.1 mmol/L) increases the risk of hypoglycemia without
demonstrated benefit. (See "Glycemic control and intensive insulin therapy in critical illness", section on 'General approach'.)
■
Therapeutic hypothermia – Lowering of core body temperature to 32 to 34 C
during the first
few hours after cardiac arrest is associated with improved neurologic outcomes and decreased
mortality in adults with an out-of-hospital cardiac arrest and either ventricular fibrillation or
pulseless ventricular tachycardia. (See "Post-cardiac arrest management in adults", section on 'Evidence in ventricular fibrillation and pulseless ventricular tachycardia'.)
In children, evidence is very limited [5]. The use of therapeutic hypothermia (core body temperature 32 to 34°C) may be appropriate for children with out-of-hospital arrest and
persistent coma or those with ventricular fibrillation or pulseless ventricular tachycardia. The
timing, duration, and methods for therapeutic hypothermia are discussed in detail separately.
Guidelines for pediatric advanced life support
(See "Post-cardiac arrest management in adults", section on 'Temperature management and
therapeutic hypothermia (TH)'.)
If the child is not being treated in a center with pediatric emergency and critical care expertise, the child should be stabilized and rapidly transferred for definitive care at a regional pediatric center.
Critically ill or injured children typically benefit from transport by a team with pediatric expertise and
advanced pediatric treatment capability, although in some isolated cases (eg, expanding epidural hematoma) more rapid transport by an immediately available non-pediatric team may be
advantageous. (See "Prehospital pediatrics and emergency medical services (EMS)", section on 'Inter
-facility transport'.)
Prior to transfer the physician responsible for the child's care at the transferring hospital should speak directly to the physician who will be taking charge of the patient at the receiving hospital. All
documentation of care (eg, medical chart, medication administration record, laboratory results, copies
of ancillary studies [radiographs, ECGs]) should be sent with the patient. (See "Prehospital pediatrics and emergency medical services (EMS)", section on 'Inter-facility transport'.)
RAPID RESPONSE TEAMS — A rapid response team (RRT), also known as a medical emergency
team, consists of personnel from medical, nursing and/or respiratory therapy who have critical care
training and are available 24 hours per day, seven days a week for evaluation and treatment of patients who show signs of clinical deterioration and are located in nonacute care settings (eg,
medical or surgical inpatient wards). Implementation of a RRT has been promoted as a major strategy
for improving patient safety in hospitals [15].
The quality and generalizability of the evidence describing the effectiveness of implementing RRTs is limited by features such as before and after observation design, selection of primary and secondary
outcome measures, and varied indications for RRT activation. In addition, because the mortality
following PICU admission is typically low, its utility as an outcome measure may be limited. Finally, the systems being studied are complex, making it difficult to identify confounding factors such as changes
in secular trends or indirect benefits derived from the RRT implementation.
We suggest that pediatric tertiary care centers develop and maintain rapid response teams for the
prompt assessment and management of hospitalized infants and children who are showing signs of clinical deterioration and are located in nonacute care settings. This recommendation is based in part
upon the following information. However, the benefit of an RRT is not consistent across all settings,
and it is possible that explanations other than the RRT may be responsible for at least part of the benefit.
A meta-analysis of five pediatric prospective observational studies with a total of 347,618 patient
admissions found that implementation of a RRT was associated with a significant reduction in deaths
from cardiac arrest when compared to historical control periods (0.05 versus 0.17 percent, RR 0.6, 95% CI 0.5-0.8) [16]. However, decreased mortality after implementation of a RRT was not found in all
A cohort study of 29,294 patient admissions (7257 admissions after institution of a RRT) that was
included in the meta-analysis compared hospital-wide mortality rates and rates of respiratory and cardiopulmonary arrests outside of the intensive care unit before and after implementation of an RRT
in a 264-bed freestanding children's hospital [17]. Major findings included:
■ The mean monthly mortality rate decreased from 1.0 to 0.8 deaths per 100 discharges (18
percent decrease, 95% CI 5-30 percent).
■ The mean monthly code rate (respiratory or cardiopulmonary arrest) decreased from 2.5 to 0.7
codes per 1000 patient admissions (RR 0.3, 95% CI 0.1-0.7). A possible explanation for this
finding is that early activation of the RRT in a critically ill patient might have prevented codes.
Guidelines for pediatric advanced life support
■ Over 18 months, the RRT was activated 143 times, most commonly for respiratory distress,
hypotension, hypoxemia, altered mental status, and tachycardia. The most common actions by the RRT were respiratory support, fluid resuscitation, airway management, and transfer to the
intensive care unit.
■ A multicenter, prospective observational study of the implementation of a physician-led
pediatric RRT in four pediatric academic centers found that initiation of an RRT was associated
with a significant reduction in pediatric intensive care unit mortality rate after readmission from a medical or surgical unit (0.3 to 0.1 deaths per 1000 hospital admissions) but no significant
decline in the rate of cardiopulmonary arrests [18].
However, these observations do not prove that the RRT was responsible for the improvement in outcomes. Support for this concern comes from an observational study in a children's hospital that did
not implement an RRT but also found a significant reduction in mortality over the same time period in
which other pediatric centers reported decreased mortality in association with RRT implementation [19].
FAMILY PRESENCE DURING RESUSCITATION — Observational studies indicate that caretakers
should be given the option of being present during the in-hospital resuscitation of their child [5]. Key
findings include:
■ Most parents want the opportunity to remain with their child during resuscitation [5] and believe
it is their right [20].
■ Caretakers present during the resuscitation of a family member frequently reported that their
presence during the resuscitation was beneficial to the patient [20-22].
■ Two-thirds of caretakers present during the resuscitation of a child who died reported that their
presence helped with their adjustment to the death and the grieving process [22].
■ Studies of hospital personnel suggest that the presence of a family member, in most instances,
was not stressful to staff and did not negatively impact staff performance [20,21,23].
When family members are present during a pediatric resuscitation, a staff member with clinical
knowledge, empathy, and strong interpersonal skills should be present with them to provide support
and answer questions.
In the rare instance that family presence is disruptive to team resuscitation efforts, the family members should be respectfully asked to leave.
SUMMARY — The principle aim for pediatric advanced life support (PALS) is to prevent
cardiopulmonary failure through early recognition and management of respiratory distress, respiratory
failure, and shock. (See 'Overview of assessment' above and "Initial assessment and stabilization of children with respiratory or circulatory compromise".)
Respiratory distress and failure
■ A major goal of pediatric advanced life support is to recognize and treat respiratory conditions
amenable to simple measures (eg, supplemental oxygen, inhaled albuterol) (table 2). The clinician may also have to treat rapidly progressive conditions and intervene with advanced
therapies to avoid cardiopulmonary arrest in patients with respiratory failure. Early detection
and treatment improve overall outcome. (See 'Respiratory distress and failure' above.)
■ Airway – Key steps in basic airway management include (see "Basic airway management in
Guidelines for pediatric advanced life support
• Provide 100 percent inspired oxygen
• Allow child to assume position of comfort or manually open airway
• Clear airway (suction)
• Insert oropharyngeal airway or nasopharyngeal airway if consciousness impaired
■ Breathing – The clinician should assist ventilation manually in patients not responding to basic
airway maneuvers, monitor oxygenation by pulse oximetry, monitor ventilation by end-tidal CO2 if available, and administer medications as needed (albuterol, epinephrine). In preparation for
intubation, the patient should receive 100 percent oxygen via a high-concentration mask, or if indicated, positive pressure ventilation with a bag-valve-mask to preoxygenate and improve
ventilation. (See "Basic airway management in children" and "Carbon dioxide monitoring
■ Children who cannot maintain an effective airway, oxygenation, or ventilation should undergo
endotracheal intubation. A rapid overview provides the steps in performing rapid sequence
intubation (table 3). (See "Emergent endotracheal intubation in children" and "Rapid sequence intubation (RSI) in children".)
Shock and cardiac arrhythmias
■ Proper treatment of shock in children requires the clinician to recognize and eventually
categorize the type of shock in order to prioritize treatment options (algorithm 1). Early
treatment of shock may prevent the progression to cardiopulmonary failure (algorithm 2). (See "Initial evaluation of shock in children" and "Initial management of shock in children" and
■ Algorithms provide the pediatric advanced life support guidelines for treatment of children with
tachyarrhythmias (algorithm 4), pulseless arrest (algorithm 5), and symptomatic bradycardia
(algorithm 3). (See 'Bradyarrhythmias' above and 'Tachyarrhythmias' above and 'Pulseless
■ The early postresuscitation period includes the time soon after return of spontaneous
circulation or recovery from circulatory or respiratory failure. During this time, the clinician must
continue to treat the underlying cause for the life-threatening event and monitor for common
respiratory or circulatory problems that may cause secondary morbidity or death.
■ If the child is not being treated in a center with pediatric emergency and critical care expertise,
the child should be stabilized and rapidly transferred for definitive care at a regional pediatric
Rapid response teams
■ We suggest that pediatric tertiary care centers develop and maintain multidisciplinary rapid
response teams for the prompt assessment and management of hospitalized infants and children who are showing signs of clinical deterioration and are located in nonacute care units
(eg, medical or surgical inpatient wards) (
Grade 2C). (See 'Rapid response teams' above.)
Use of UpToDate is subject to the Subscription and License Agreement.
Guidelines for pediatric advanced life support
1. ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005
American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112:IV1.
2. Ralston, M, Hazinski, MF, Zaritsky, AL, et al. PALS Provider Manual. American Academy of
Pediatrics, American Heart Association, Dallas, Texas, 2006.
3. Kleinman ME, de Caen AR, Chameides L, et al. Pediatric basic and advanced life support: 2010
International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 2010; 126:e1261.
4. Kleinman ME, de Caen AR, Chameides L, et al. Part 10: Pediatric basic and advanced life
support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010; 122:S466.
5. Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support:
2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:S876.
6. Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital
cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation 2009; 119:1484.
7. Gerein RB, Osmond MH, Stiell IG, et al. What are the etiology and epidemiology of out-of-
hospital pediatric cardiopulmonary arrest in Ontario, Canada? Acad Emerg Med 2006; 13:653.
8. Smith BT, Rea TD, Eisenberg MS. Ventricular fibrillation in pediatric cardiac arrest. Acad Emerg
Med 2006; 13:525.
9. Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in
children. N Engl J Med 2006; 354:2328.
10. Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an
analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes 2013; 6:42.
11. Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension
and outcome after resuscitation from cardiac arrest. Circulation 2011; 123:2717.
12. Ferguson LP, Durward A, Tibby SM. Relationship between arterial partial oxygen pressure after
resuscitation from cardiac arrest and mortality in children. Circulation 2012; 126:335.
13. Srinivasan V, Spinella PC, Drott HR, et al. Association of timing, duration, and intensity of
hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med 2004; 5:329.
14. Kong MY, Alten J, Tofil N. Is hyperglycemia really harmful? A critical appraisal of "Persistent
hyperglycemia in critically ill children" by Faustino and Apkon (J Pediatr 2005; 146:30-34). Pediatr Crit Care Med 2007; 8:482.
15. Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a
goal and a deadline for improving health care quality. JAMA 2006; 295:324.
16. Chan PS, Jain R, Nallmothu BK, et al. Rapid Response Teams: A Systematic Review and Meta-
analysis. Arch Intern Med 2010; 170:18.
17. Sharek PJ, Parast LM, Leong K, et al. Effect of a rapid response team on hospital-wide mortality
and code rates outside the ICU in a Children's Hospital. JAMA 2007; 298:2267.
18. Kotsakis A, Lobos AT, Parshuram C, et al. Implementation of a multicenter rapid response
system in pediatric academic hospitals is effective. Pediatrics 2011; 128:72.
19. Joffe AR, Anton NR, Burkholder SC. Reduction in hospital mortality over time in a hospital
without a pediatric medical emergency team: limitations of before-and-after study designs. Arch Pediatr Adolesc Med 2011; 165:419.
Guidelines for pediatric advanced life support
20. Mangurten J, Scott SH, Guzzetta CE, et al. Effects of family presence during resuscitation and
invasive procedures in a pediatric emergency department. J Emerg Nurs 2006; 32:225.
21. Dudley NC, Hansen KW, Furnival RA, et al. The effect of family presence on the efficiency of
pediatric trauma resuscitations. Ann Emerg Med 2009; 53:777.
22. Tinsley C, Hill JB, Shah J, et al. Experience of families during cardiopulmonary resuscitation in a
pediatric intensive care unit. Pediatrics 2008; 122:e799.
23. Curley MA, Meyer EC, Scoppettuolo LA, et al. Parent presence during invasive procedures and
resuscitation: evaluating a clinical practice change. Am J Respir Crit Care Med 2012; 186:1133.
Topic 6392 Version 19.0
Guidelines for pediatric advanced life support
Glasgow coma scale and pediatric Glasgow coma scale
Pediatric Glasgow Coma
Age-appropriate vocalization, smile, or
orientation to sound, interacts (coos,
babbles), follows objects
Confused, disoriented
Inappropriate words
Incomprehensible
Spontaneous movements (obeys verbal
Withdraws to touch (localizes pain)
Withdraws to pain
Abnormal flexion to
Abnormal flexion to pain (decorticate
Abnormal extension
Abnormal extension to pain
(decerebrate posture)
The Glasgow coma scale (GCS) is scored between 3 and 15, 3 being the worst, and 15
the best. It is composed of three parameters: best eye response (E), best verbal
response (V), and best motor response (M). The components of the GCS should be
recorded individually; for example, E2V3M4 results in a GCS of 9. A score of 13 or
higher correlates with mild brain injury; a score of 9 to 12 correlates with moderate
injury; and a score of 8 or less represents severe brain injury. The pediatric Glasgow
coma scale (PGCS) was validated in children 2 years of age or younger.
1. Teasdale G and Jennett B. Assessment of coma and impaired consciousness. A
practical scale. Lancet 1974; 2:81.
2. Holmes JF, Palchak MJ, MacFarlane T, Kuppermann N. Performance of the pediatric
Glasgow coma scale in children with blunt head trauma. Acad Emerg Med 2005;
Graphic 59662 Version 8.0
Guidelines for pediatric advanced life support
Causes of acute respiratory compromise in children
Respiratory tract
Retropharyngeal abscess
Peritonsillar abscess
Foreign body (upper airway, lower airway, esophagus)
Airway anomalies (eg, laryngomalacia, laryngospasm, tracheoesophageal fistula,
tracheal stenosis, tracheal ring or sling)
Biologic or chemical weapons (eg, anthrax, tularemia, phosgene, nitrogen mustard,
nerve agents, ricin)
Chest wall trauma or abnormalities (eg, flail chest, open pneumothorax, thoracic
Thoracic cavity trauma or conditions (eg, pneumothorax, hemothorax, pleural effusion,
empyema, mediastinal mass)
Pulmonary trauma or conditions (contusion, embolism, hemorrhage)
Chemical agent exposures (eg, phosgene, chlorine, cyanide)
Submersion injury (near-drowning)
Congenital heart disease
Acute decompensated heart failure
Cardiac tamponade
Myocardial infarction
Depressed ventilation (eg, ingestion, CNS trauma, seizures, or CNS infection)
Guidelines for pediatric advanced life support
Hypotonia (conditions causing poor airway or respiratory muscle tone and ineffective
respiratory effort)
Pulmonary aspiration due to loss of airway protective reflexes
Hypoventilation due to abdominal pain or distention (eg, intraabdominal trauma, small
bowel obstruction, bowel perforation)
Gastroesophageal reflux with pulmonary aspiration
Metabolic and endocrine diseases
Metabolic acidosis (eg, diabetic ketoacidosis, severe dehydration, sepsis, toxic
ingestions, inborn errors of metabolism)
Decreased O2 carrying capacity (eg, acute severe anemia from hemolysis,
methemoglobinemia, carbon monoxide poisoning)
Acute chest syndrome (patients with sickle cell disease)
Conditions listed in red are immediately life threatening.
CNS: central nervous system; O2: oxygen.
Graphic 61637 Version 9.0
Guidelines for pediatric advanced life support
Rapid overview of rapid sequence intubation in children
Begin preoxygenation as soon as the decision to intubate is considered.
Administer oxygen at the highest concentration available.
Identify conditions that will affect choice of medications.
Identify conditions that will predict difficult intubation or bag-mask ventilation.
Assemble equipment and check for function.
Develop contingency plan for failed intubation.
Atropine: All children ≤1 year, children <5 years receiving succinycholine, and older
children receiving a second dose of succinylcholine. Dose: 0.02 mg/kg IV (maximum
single dose 0.5 mg, minimum 0.1 mg; if no IV access, can be given IM).
Lidocaine: Optional for increased intracranial pressure. Dose: 1.5 mg/kg IV (maximum
dose 100 mg). Give 2 to 3 minutes before intubation.
Etomidate: Safe with hemodynamic instability, neuroprotective, transient adrenal
corticosuppression. Do not use routinely in patients with septic shock. Dose: 0.3 mg/kg
Ketamine: Safe with hemodynamic instability if patient is not catecholamine depleted.
Use in patients with bronchospasm and septic shock. Use with caution in patients with
increased intracranial pressure. Dose: 1 to 2 mg/kg IV. (If no IV access, can be given
IM dose: 3 to 7 mg/kg).
Midazolam: Time to clinical effect is longer, inconsistently induces unconsciousness.
May cause hemodynamic instability at doses required for sedation. Dose: 0.2 to 0.3
mg/kg IV (maximum dose 2 mg, onset of effect requires 2 to 3 minutes).
Thiopental: Neuroprotective. Do not use with hemodynamic instability. Dose: 3 to 5
Succinycholine: Do not use with chronic myopathy or denervating neuromuscular
disease; 48 to 72 hours after burn, crush, or denervating injury; malignant
hyperthermia; or pre-exisiting hyperkalemia. Dose: infants and young children: 2
mg/kg IV, older children: 1 to 1.5 mg/kg IV. (If IV access unobtainable, can be given
IM, dose: 3 to 5 mg/kg).
Rocuronium: Use for children with contraindication for succinylcholine. Suggested dose:
1 mg/kg IV (range 0.6 to 1.2 mg/kg). •
Protection and positioning
Maintain manual cervical spine immobilization during intubation in the trauma patient.
If cervical spine injury is not potentially present, put the patient in the "sniffing
position" (ie, head forward so that the external auditory canal is anterior to the
shoulder and the nose and mouth point to the ceiling). Apply cricoid pressure when the
Guidelines for pediatric advanced life support
child is unconscious. Remove cricoid pressure if it causes airway obstruction or difficulty
viewing the larynx.
If used, maintain cricoid pressure until tracheal tube position is verified.
Positioning, with placement
Confirm tracheal tube placement with end-tidal CO2 detection and auscultation.
Chest radiograph for tracheal tube placement; provide ongoing sedation (eg,
midazolam), analgesia (eg, fentanyl 1 mcg per kilogram), and, if indicated, paralysis. ∆
If IV access unobtainable, intraosseous administration of drugs listed is feasible (no
data for ketamine).
* Not available in the United States and Canada.
• Vecuronium may be used in children with contraindications to succinylcholine and when
rocuronium is not available. Suggested dose for RSI: 0.15 to 0.2 mg/kg. Patients may
experience prolonged and unpredictable duration of paralysis at this dose.
∆ If decompensation after successful intubation use DOPE mnemonic to find cause:
- D: Dislodgement of the tube (right mainstem or esophageal)
- O: Obstruction of tube
- P: Pneumothorax
- E: Equipment failure (ventilator malfunction, oxygen disconnected or not on).
Graphic 51456 Version 17.0
Guidelines for pediatric advanced life support
Approach to the classification of undifferentiated shock in children
Graphic 81356 Version 2.0
Guidelines for pediatric advanced life support
Initial management of shock in children
* For possible cardiogenic shock with hypovolemia, give 5 to 10 mL/kg of isotonic fluids (eg,
normal saline or Ringers lactate), infused over 10 to 20 minutes. Evaluate target end points and
slowly give another 5 to 10 cc/kg if there has been improvement or no change. For patients with
diabetic ketoacidosis, give 10 mL/kg of isotonic fluids over one hour.
• Such as inotropes or vasodilators. For newborns, prostaglandin E1.
∆ For patients with DKA who do not improve with 20 mL/kg, look for another cause of shock
before administering additional crystalloid. For possible cardiogenic shock, slowly give another 5
to 10 mL/kg if there has been improvement or no change.
Guidelines for pediatric advanced life support
◊ Dopamine if normotensive, norepinephrine if hypotensive and vasodilated, and epinephrine if
hypotensive and vasoconstricted.
Adapted from: Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of
pediatric and neonatal patients in septic shock. Crit Care Med 2002; 30:1365.
Graphic 77079 Version 2.0
Guidelines for pediatric advanced life support
Pediatric respiratory rate and heart rate by age*
(1st-99th percentile)
(1st-99th percentile)
143 (107-181); term newborn at
birth: 127 (90-164)
* The respiratory and heart rates provided are based upon measurements in awake, healthy
infants and children at rest. Many clinical findings besides the actual vital sign measurement
must be taken into account when determining whether a specific vital sign is normal in an
individual patient. Values for heart rate or respiratory rate that fall within normal limits for
age may still represent abnormal findings that are caused by underlying disease in a
particular infant or child.
Data from: Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and
respiratory rate in children from birth to 18 years of age: a systematic review of observational
studies. Lancet 2011; 377:1011.
Graphic 78097 Version 6.0
Guidelines for pediatric advanced life support
Single-lead electrocardiogram (ECG) showing first degree atrioventricular (AV) block I
Electrocardiogram of lead II showing normal sinus rhythm, first degree
atrioventricular block with a prolonged PR interval of 0.30 sec, and a
QRS complex of normal duration. The tall P waves and P wave duration
of approximately 0.12 sec suggest concurrent right atrial enlargement.
Courtesy of Morton Arnsdorf, MD.
Graphic 67882 Version 3.0
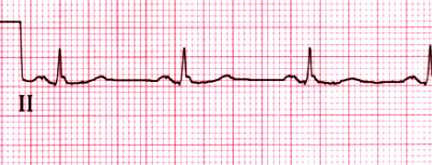
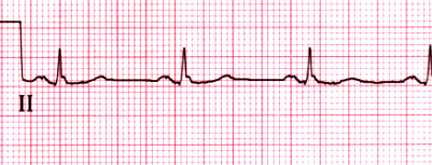
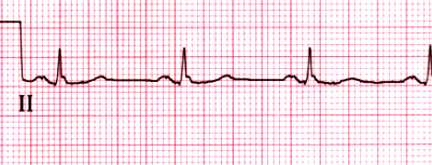
Normal rhythm strip
Normal rhythm strip in lead II. The PR interval is 0.15 sec and the
QRS duration is 0.08 sec. Both the P and T waves are upright.
Courtesy of Morton F Arnsdorf, MD.
Graphic 59022 Version 3.0
Guidelines for pediatric advanced life support
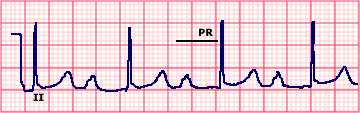
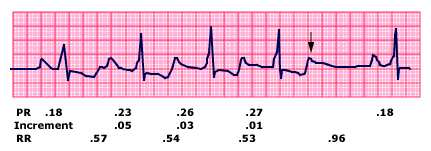
Electrocardiogram (ECG) showing Mobitz type I (Wenckebach) Atrioventricular (AV) block
Single lead electrocardiogram (ECG) showing Mobitz type I
(Wenckebach) second degree AV block with 5:4 conduction. The
characteristics of this arrhythmia include: a progressively increasing PR
interval until a P wave is not conducted (arrow); a progressive decrease
in the increment in the PR interval; a progressive decrease in the RR
interval; and the RR interval that includes the dropped beat (0.96 sec)
is less than twice the RR interval between conducted beats (0.53 to
Courtesy of Morton Arnsdorf, MD.
Graphic 73051 Version 4.0
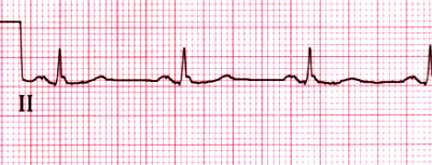
Normal rhythm strip
Normal rhythm strip in lead II. The PR interval is 0.15 sec and the
QRS duration is 0.08 sec. Both the P and T waves are upright.
Courtesy of Morton F Arnsdorf, MD.
Graphic 59022 Version 3.0
Guidelines for pediatric advanced life support
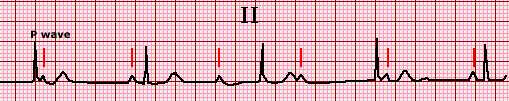
II ECG of mobitz II second degree heart block
The lead II rhythm strip shows four sinus beats with P wave followed by a QRS
complex; the fifth P wave is not followed by a QRS complex and represents second
degree heart block. There is no change in the PR interval prior to or after the blocked
P wave and thus this is Mobitz II second degree heart block. A second episode of
second degree heart block can be seen after the seventh QRS complex.
Reproduced with permission by Samuel Levy, MD.
Graphic 51492 Version 2.0
Guidelines for pediatric advanced life support
Third degree (complete) atrioventricular block with narrow QRS escape rhythm
The P waves are completely dissociated from the QRS complexes. The QRS
complexes are narrow, indicating a junctional escape rhythm. The atrial and
ventricular rates are stable; the former is faster than the latter.
Graphic 65545 Version 5.0
Normal rhythm strip
Normal rhythm strip in lead II. The PR interval is 0.15 sec and the
QRS duration is 0.08 sec. Both the P and T waves are upright.
Courtesy of Morton F Arnsdorf, MD.
Graphic 59022 Version 3.0
Guidelines for pediatric advanced life support
Pediatric bradycardia algorithm (with a pulse and poor perfusion): 2010 PALS guidelines
PALS: pediatric advanced life support; CPR: cardiopulmonary resuscitation; IO:
intraosseous; IV: intravenous; HR: heart rate; AV: atrioventricular; ABCs: airway,
Reprinted with permission. Pediatric Advanced Life Support: 2010. American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular
Care. 2010 American Heart Association, Inc.
Graphic 52446 Version 7.0
Guidelines for pediatric advanced life support
AV reentrant tachycardia
AV reentrant tachycardia breaking to sinus rhythm with Wolff-Parkinson-
White syndrome.
Graphic 77867 Version 2.0
Guidelines for pediatric advanced life support
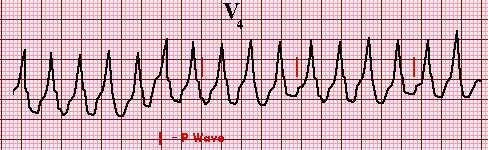
Single lead electrocardiogram (ECG) showing monomorphic ventricular tachycardia
Three or more successive ventricular beats are defined as ventricular
tachycardia (VT). This VT is monomorphic since all of the QRS complexes
have an identical appearance. Although the P waves are not distinct, they can
be seen altering the QRS complex and ST-T waves in an irregular fashion,
indicating the absence of a relationship between the P waves and the QRS
complexes, ie, AV dissociation is present.
Graphic 63176 Version 5.0
Normal rhythm strip
Normal rhythm strip in lead II. The PR interval is 0.15 sec and the
QRS duration is 0.08 sec. Both the P and T waves are upright.
Courtesy of Morton F Arnsdorf, MD.
Graphic 59022 Version 3.0
Guidelines for pediatric advanced life support
Drug- and toxin-induced electrocardiographic abnormalities
Supraventricular
Cardiac glycosides
Digitalis purpurea
Alpha-adrenergic
Cardiac glycosides
Cellular asphyxiants
Graphic 66773 Version 7.0
Guidelines for pediatric advanced life support
Pediatric tachycardia algorithm (with a pulse and poor perfusion): 2010 PALS guidelines
PALS: pediatric advanced life support; IO: intraosseous; IV: intravenous; ECG: electrocardiogram.
* Vagal manuevers: In infants or young children, place a plastic bag filled with ice and cold water over
the face for 15 to 30 seconds or stimulate the rectum with a thermometer. In older children, encourage
bearing down (Valsalva maneuver) for 15 to 20 seconds. Carotid massage and orbital pressure should
not be performed in children.
Guidelines for pediatric advanced life support
Reprinted with permission. Pediatric Advanced Life Support: 2010. American Heart Association
Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. 2010 American
Heart Association, Inc.
Graphic 67438 Version 14.0
Guidelines for pediatric advanced life support
Pediatric cardiac arrest algorithm: 2010 PALS guidelines
PALS: pediatric advanced life support; VF: ventricular fibrillation; VT: ventricular tachycardia; PEA:
pulseless electrical activity; IO: intraosseous; IV: intravenous; CPR: cardiopulmonary resuscitation;
ROSC: return of spontaneous circulation.
Reprinted with permission. Pediatric Advanced Life Support: 2010. American Heart Association Guidelines
for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. 2010 American Heart
Association, Inc.
Guidelines for pediatric advanced life support
Graphic 80003 Version 10.0
Guidelines for pediatric advanced life support
Continuous electrocardigraphic (ECG) strip during an episode of ventricular fibrillation (VF) that progresses to fine VF and then asystole
At the onset of ventricular fibrillation (VF), the QRS complexes are regular,
widened, and of tall amplitude, suggesting a more organized ventricular
tachyarrhythmia. Over a brief period of time, the rhythm becomes more
disorganized with high amplitude fibrillatory waves; this is coarse VF. After a longer
period of time, the fibrillatory waves become fine, culminating in asystole.
Graphic 67777 Version 3.0
Guidelines for pediatric advanced life support
Single lead electrocardiogram (ECG) showing torsades de pointes
This is an atypical, rapid, and bizarre form of ventricular tachycardia
that is characterized by a continuously changing axis of polymorphic
QRS morphologies.
Graphic 53891 Version 4.0
Guidelines for pediatric advanced life support
Example of epinephrine infusion - Pediatric 10 kg
Example of preparation of epinephrine infusion for refractory
symptoms of anaphylaxis for pediatric patient of 10 kg body
weight for emergency/critical care units
Epinephrine 10 micrograms/mL (0.01 mg/mL)
Add 1 mg (1000 micrograms) of epinephrine to 100 mL of 5 percent dextrose
Resulting concentration is 10 micrograms per milliliter (mL).
1. CHECK vial strength.
2. To prepare epinephrine infusion for a final concentration of 10 micrograms per
mL, dilute 10 mL of 0.1 mg/mL epinephrine (also labeled 1:10,000) in 100 mL of
D5W OR 1 mL of 1 mg/mL epinephrine (also labeled 1:1000) in 100 mL of D5W.*
Infuse an initial dose of 0.1 micrograms per kg per minute using a programmable
infusion pump and titrate as needed while continuously monitoring the patient's
cardiac rhythm and blood pressure.
Administration rate
Pediatric dose for 10 kg child
1 milligram epinephrine diluted in 100
10 micrograms per milliliter
■ To reduce the risk of making a medication error, we suggest that centers have
available an institutionally approved protocol for epinephrine infusion that includes
steps on how to prepare and administer the infusion and standard concentration(s)
Guidelines for pediatric advanced life support
■ The above table is provided as an example; there are other acceptable
■ Intravenous epinephrine, like all vasopressors, can cause life-threatening
hypertension, cardiac ischemia, and ventricular arrhythmias. It should be
administered ONLY by clinicians trained and experienced in dose titration of
intravenous epinephrine using continuous non-invasive electronic
monitoring of heart rate and blood pressure.
■ Epinephrine is an ischemia causing agent and peripheral venous irritant. Monitor
infusion site for extravasation. See Lexicomp drug reference for information on
managing extravasation including infiltration of phentolamine. Central line
administration is preferred when available.
* Unused diluted solutions should be discarded within 24 hours or less of preparation
depending on local standards.
1. Gahart BL, Nazareno AR; Intravenous Medications 28th ed. Elsevier-Mosby 2012 St.
2. Lieberman P, et al. J Allergy Clin Immunol 2010; 126(3):477.
Graphic 56049 Version 5.0
Guidelines for pediatric advanced life support
Example of epinephrine infusion - Pediatric 20 kg
Example of preparation of epinephrine infusion for refractory
symptoms of anaphylaxis for pediatric patient of 20 kg body
weight for emergency/critical care units
Epinephrine 10 micrograms/mL (0.01 mg/mL)
Add 1 mg (1000 micrograms) of epinephrine to 100 mL of 5 percent dextrose
Resulting concentration is 10 micrograms per milliliter (mL).
1. CHECK vial strength.
2. To prepare epinephrine infusion for a final concentration of 10 micrograms per
mL, dilute 10 mL of 0.1 mg/mL epinephrine (also labeled 1:10,000) in 100 mL of
D5W OR 1 mL of 1 mg/mL epinephrine (also labeled 1:1000) in 100 mL of D5W.*
Infuse an initial dose of 0.1 micrograms per kg per minute using a programmable
infusion pump and titrate as needed while continuously monitoring the patient's
cardiac rhythm and blood pressure.
Administration rate
Pediatric dose for 20 kg child
1 milligram epinephrine diluted in 100
10 micrograms per milliliter
■ To reduce the risk of making a medication error, we suggest that centers have
available an institutionally approved protocol for epinephrine infusion that includes
steps on how to prepare and administer the infusion and standard concentration(s)
■ The above table is provided as an example; there are other acceptable
■ Intravenous epinephrine, like all vasopressors, can cause life-threatening
hypertension, cardiac ischemia, and ventricular arrhythmias. It should be
Guidelines for pediatric advanced life support
administered ONLY by clinicians trained and experienced in dose titration of
intravenous epinephrine using continuous non-invasive electronic
monitoring of heart rate and blood pressure.
■ Epinephrine is an ischemia causing agent and peripheral venous irritant. Monitor
infusion site for extravasation. See Lexicomp drug reference for information on
managing extravasation including infiltration of phentolamine. Central line
administration is preferred when available.
* Unused diluted solutions should be discarded within 24 hours or less of preparation
depending on local standards.
1. Gahart BL, Nazareno AR; Intravenous Medications 28th ed. Elsevier-Mosby 2012 St.
2. Lieberman P, et al. J Allergy Clin Immunol 2010; 126(3):477.
Graphic 66791 Version 6.0
Guidelines for pediatric advanced life support
Disclosures: Eric Fleegler, MD, MPH Nothing to disclose. Monica Kleinman, MD Nothing to disclose. Susan B Torrey,
MD Nothing to disclose. James F Wiley, II, MD, MPH Employee of UpToDate, Inc.
Contributor disclosures are reviewed for conflicts of interest by the editorial group. When found, these are addressed by
vetting through a multi-level review process, and through requirements for references to be provided to support the content.
Appropriately referenced content is required of all authors and must conform to UpToDate standards of evidence.
Conflict of interest policy
Source: http://www.segwfg.nl/wp-content/uploads/2014/06/APLS-Up2Date-2014.pdf
Brain Research 1010 (2004) 151 – 155 Stimulation of the superior cerebellar peduncle during the development of amygdaloid kindling in rats Carmen Rubioa, Vero´nica Custodioa, Francisco Jua´rezb, Carlos Paza,* a Departamento de Neurofisiologı´a, Instituto Nacional de Neurologı´a y Neurocirugı´a M.V.S., Insurgentes Sur 3877, Mexico 14269 D.F., Mexico b Instituto Nacional de Psiquiatrı´a R.F., Mexico
Journal of Oral Rehabilitation 2008 35; 509–523 Review ArticlePrinciples for the management of bruxism* F . L O B B E Z O O * , J . VAN DER Z A A G * , M . K . A . VAN S E L M S * , H . L . H A M B U R G E R † &M . N A E I J E * *Department of Oral Function, Academic Centre for Dentistry Amsterdam (ACTA) and †Departments of Neurology andClinical Neurophysiology, and Amsterdam Center for Sleep-Wake Disorders, Slotervaart General Hospital, Amsterdam, The Netherlands











