Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Optics.sgu.ru
Estimation of melanin content in iris of human eye
Ekaterina V. Koblova1, Alexey N. Bashkatov2, Elina A. Genina2, Valery V. Tuchin2, and
Valery V. Bakutkin1
1Ophthalmology Department of Saratov State Medical University
2Institute of Optics and Biophotonics, Optics Department of Saratov State University
ABSTRACT
Based on experimental data, obtained
in vitro from reflectance measurements and
in vivo from digital analysis of color images of human irises, melanin content in human and bovine eye irises has been estimated. Reflectance measurements have been performed using commercially available optical multichannel spectrometer LESA-5 (BioSpec, Russia). For registration of color images digital camera Olympus C-5060 has been used. Analysis of the reflectance spectra has been performed by the method used for determination of melanin content in skin. For digital analysis of iris color images, decomposition of the images in RGB-color-coordinate system has been performed. The images have been obtained both from irises of health volunteers as from irises of patients with glaucoma. Original computer program based on Mathcad software has been developed for the analysis. The results obtained from spectral and color measurements have a good agreement each to other. In eye irises of patients with glaucoma, smaller melanin content has been obtained, and the result has been useful for development of novel and optimization of already existing methods of glaucoma diagnostics.
Keywords: iris, melanin content, glaucoma, spectroscopy, digital image analysis
1. INTRODUCTION
Knowledge of tissue optical properties is important for development of theoretical models describing the light propagation within tissues (including a human eye iris). These models can be used when designing laser therapy and diagnostic techniques, or interpretation of for the data of spectrophotometric measurements. There are numerous papers describing the methods of determination of optical properties of many types of tissues.1-6
Recently some diagnostic criteria of dystrophic, degenerative and inflammatory diseases of an eye iris have been based on descriptive, relative and, in many respects, subjective criteria to which the discoloration of an iris of the eye is also refered.7-12 Now the iris eye classification is based on the data of iridochromoscopy, iridochromophotography, and biomicroscopy in polarized light.7-13 Objective criteria of inflammatory and degenerative changes can be obtained from data of the fluorescent angiography. Unfortunately, the method is invasive and cannot be applied to all patients. Iris coloration depends mostly on the amount and depth of location of a melanin, located in the frontal mesodermal part of the iris.9,10,12,13 The quantitative assessment of melanin content in an iris can be used as objective criterion in investigating the series of pathological conditions.7,9,12,13Besides, the objective data could be supplement to the existing classifications of an iris types.
In this study, we present technique for estimation of melanin content in eye iris with digital analysis of color images of the eye iris.
2. STRUCTURE AND PROPERTIES OF THE HUMAN EYE IRIS
The iris of the eye is forefront of a choroid, located in frontal in relation to a limbal plane. The iris has the form of a plate or a screen with lightly elliptical shape. The iris is attached by its root at the angle (iridocorneal) of the anterior chamber where it merges with the ciliary body and trabecular meshwork. The iris is 12 mm in horizontal diameter with a circumference of 37 mm. It is a cone shaped with the pupil margin located more anterioly than the root.9,14,15
Ophthalmic Technologies XV, edited by Fabrice Manns, Per G. Söderberg,
Arthur Ho, Bruce E. Stuck, Michael Belkin, Proceedings of SPIE Vol. 5688
(SPIE, Bellingham, WA, 2005) · 1605-7422/05/$15 · doi: 10.1117/12.593651
The anterior surface is divided into two zones: the ciliary zone with a breadth about 3-4 mm and pupil zone over 1-2 mm. The thickened region known as the collarette is located between these two zones. It corresponds to a plexus of the thin arteries amounting a small arterial circle.7,9,16
On a surface recesses (crypts, lacunas) around which vessels placed more densely are almost always seen. The anterior surface is characterized by radial streaks (straight when the pupil is contracted and wavy when dilated), and contraction furrows (more noticeable in dilated iridis).16-18
Iris thickness is not everywhere equal. Most of all it is expressed in the range of a small arterial circle: up to 480-550 microns, and the thinnest part - in ciliary zone - up to 350 microns.16 Iris structure includes two mesodermal layers (superficial and deep), located in front and forming its stroma, and two epithelial pigmented layers, relating to a cerebral ectoderm and covering an iris behind. Mesodermal part includes the front epithelium, derived from flat cells, a front boundary layer, introduced by a narrow band of collagen fibers, the vascular layer formed by a connective tissue which is condensed around the radial vessels. The front boundary layer, which is called the front stromal leaf Krückmann, represents the changed iris stroma with pigment cells, that is more consistent in the structure.9-11,17,18
Accoding to M. Zaltsmann9 this layer is poor for large vessels, and between pigmentary cells collagen fibrils are located with large amount of nervous terminations. The forward boundary layer thickness changes in various sites of an iris and, probably, exceeds the crypts depth. According to electronic microscopy, two basic cellular elements are located in this layer such as fibroblasts and melanocytes and a network of collagen fibrils. This layer also contains a capillary vessels network. Thus, pigment-containing melanocytes alongside with fibroblasts lied as one layer. In the same layer there are melanocyte congestions, towering above a forward boundary layer surface forming the pigmentary spots, so-called nevoses.9-11,17
Melanin synthesis occurs in special organoids - melanosomes, then a transformation into enzymatic-inert pigmentary granules takes place. On the chemical compound the pigment is a combination of sulfur-containing pheomelanin and sulfur-not-containing eumelanin, and the content of the pheomelanin produced by melanocytes at early and mature cells age is dominating (up to 99%). At cells ageing there is a eumelanin contents dominating.19-24
Melanin is put aside with granules in which bounded to protein (melanoproteides). The melanin precursor is the amino asid thyrosinum, which oxidation to dioxiphenylalanin (DOPA), and then to DOPA – quinone is catalyzed with enzyme tyrosinase. The further metamorphosises proceed without fermentative participation and result in formation of melanin which chemical structure is not established (gross formula C77H98O33N14S).25-28
The peak absorption of human melanin pigment occurs around 335 nm, and the absorption is almost completely reduced for wavelengths longer than 700 nm. The reflectance of the iris is quite constant over the range from 700 to 900 nm, although the iris looks much brighter in video images acquired in the longer wavelengths simply because the sclera and skin are much darker (less reflective) in those wavelengths. In Fig. 1 the extinction coefficient spectra29 of pheomelanin and eumelanin are presented.
The melanocyte in submicroscopic level looks like the small oval body with prominent nucleus, nucleolus and numerous branch with a length up to 100 microns.16-18 The branch connect cells to neighboring melanocytes, fibroblasts. The special arrangement around blood vessels is typical for melanocytes. From all vascular system of an organism only the iris has a ring pigmentary coating. Against a melanocyte of a front boundary layer – the stromal melanocyte has more expressed cytoplasm with mitochondria, both smooth and granular endoplasmatic reticular and free ribosomes. The ciliary element penetrating into a stroma of an iris usually arises from a cytoplasm. Melanocytes pigmentary granules are introduced with considerably melanosomes, being carriers of mature melanin. The granules of melanin which are located in a cytoplasm in electronic microscopic level can be traced in different stages of their development: premelanosomes, melanosomes and completely saturated with melanin granular elements which are observed in melanocytes.9-11
Proc. of SPIE Vol. 5688 303
Fig. 1: Extinction coefficient spectra of melanins29
As to lumpy cells they are located basically in a pupillary belt of an iris, especially in a circle of a pupilsphincter. These cells have the spherical form, the cytoplasm is filled in with the melaninous granules masking a cell nucleus. The lumpy cell size is around 100 microns in the diameter. The processes length varies from 1 up to2 microns, their width is about 0.1 microns.16-18 Melaninous granules in a cytoplasm of a lumpy cell in submicroscopy look like the bubbles filled with very small-size melanin particles. The indicated bubbles can beconsidered as the secondary lysosomes which manifest pigment destruction. Lumpy cells can be seen basicallyin irises of older persons, for young man their detection is difficult. Melaninous granules in the residual bodieslaying in stroma's back part are identical in size and form the pigment granules of the pigmentary epitheliumcovering an iris back surface. It should be noted, that melanin's granules size encased in the residual bodieslocated in front of iris stroma are equal to melanin granules size in melanocytes.9-11
Thus, the iris color is determined by structure of a front boundary layer, to be exact, by the concentration ofpigment cells and their types. For thinner boundary layer the light-blue irises are typical saturated with pigmentcells in small concentration with weak pigmentation level. Brown irises differ in thicker boundary layer and more hard pigmented cells.
3. MATERIALS AND METHODS
Estimation of melanin content in human and animal irises using experimental data obtained
in vitro fromreflectance measurements and
in vivo from digital analysis of color images of irises has been carried out.
In this work the method of quantitative estimation of the human iris melanin content by a registration ofreflectance spectra was used. As a material the irises of 2 cadaver human eyes and 10 bovine eyes have beenused. Time interval from post mortem to enucleating does not exceed 24 hours. After the enucleating all sampleswere kept in a normal saline solution (0.9% NaCl) where they were stored up for spectroscopic measurements.
All measurements were performed at room temperature about 20°C.
Eyeballs were prepared as follows: on limbs a cornea was cut off, then an iris was adjoined from choroids of aneye. The bovine irises have dark brown or black color. Color of the human irises was more diverse: from lightblue up to dark brown. Irises thickness and breadth were not measured, as they have constant parameters.
Reflectance measurements have been performed in the spectral range 450-1000 nm using commercially availableoptical multichannel spectrometer LESA-5 (BioSpec, Russia) with the fiber-optic probe. The diameter of the
304 Proc. of SPIE Vol. 5688
lighting and receiving fibers was 200 microns, at distance between centers of the fibers - 290 microns, meanoptical penetration depth was about 150 microns, a numerical aperture of the fibers 0.22. Object illumination was performed through the optical fiber attached to the light source - halogen lamp.
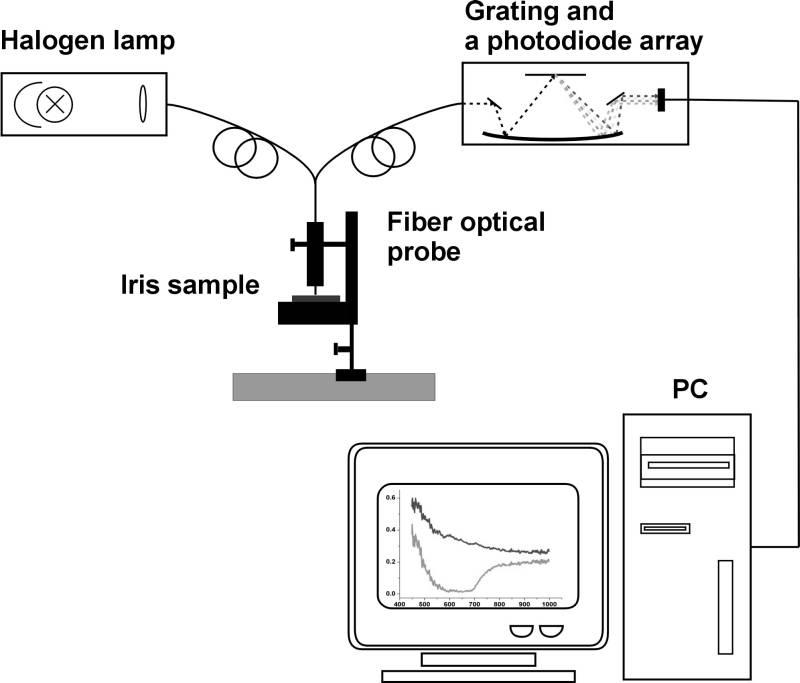
The scheme of the experimental setup is presented in fig. 2. The distance between the surface of the sample (the bovine iris) and the optical fiber was 3 mm. For the measurements the iris samples were fixed in the specialquartz holder having an excavation with a diameter of 12.8 mm that corresponds to the human iris diameter.
Spectra were recorded by shifting of the holder with an iris sample on a meridian 9-3 hours with a walk of 1 mm.
As a spectral reference ȼaSO4 plate was used.
Fig. 2 Experimental setup
For registration of color images the digital camera Olympus C-5060 (Japan) was used. Its parameters: 5,100,000effective pixels, Olympus lens from 5.7 mm to 22.9 mm. During shooting the maximum rating aperture valueequals f3.3, focal length 11.5 was used. The distance between eye and camera objective was 3 cm.
For investigation the special tool stage on which fast facial fixer - for a chin and a forehead has been made, wasused. It included a mobile part which supports a chin and moves up - downwards with manual screw rotation.
Such equipment needed to be enforced by two lighting elements. Lighting elements were fixed on a movablebasis that allowed adjusting the intensity of illumination during the measuring process. Digital camera was attached to the vertical holder fixed to movable basis that could be described as a movable device with ability tomove in all directions according to tool stage. Camera can be shifted with fixed steps. It allowed us to carry outshooting separately each eye in super macro mode with manual focusing. Shooting was done at constant lightexposure which was registered by metering-tool. In fig. 3 digital images of healthy human iris and human eyeswith primary open angle glaucoma are presented.
Proc. of SPIE Vol. 5688 305

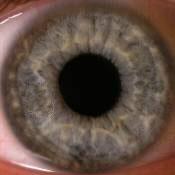

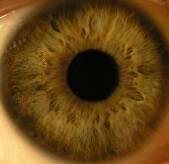
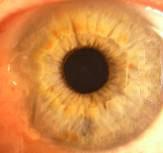
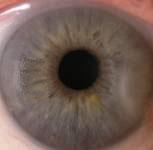
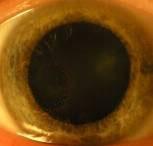
Fig. 3 Digital in vivo images of (a) healthy and (b) primary open angle glaucoma human eyes
To process the images of the iris we have developed the special computer program using Mathcad software(MathSoft Inc., USA). The base image was separated in three color matrixes of red, green, and blue components.
As a result the averaged scans of the iris image for separated color components (red, green, and blue)corresponding to three spectral ranges for reflectance measurements have been obtained. The brightness ofimages of the studied irises and test-objects are expressed as the brightness from 0 to 256.
With the special markers the square form area is cut out from the received image (containing an iris of the eye) in which the circle is entered. Then nearby sclera sites are cut, the markers determine the center pupil areaswhich are deleted. In fig. 4 shows the main steps of image processing. The image decomposed into R, G, B colorcoordinates in each pixel. As a reference a white-test object has been used. For each pixel measured R, G, Bvalues have been normalized to RGB values of the test object. The obtained reflectance values have beenaveraged for the whole investigated area. See fig. 4 step 3.
Fig. 4 The main steps of image processing
4. RESULTS AND DISCUSSION
Quantitative estimation of chromophores content in a scattering medium is based on the analysis of reflectance
and/or transmittance spectra in the selected spectral range. Absorbance of a turbid medium A �O � is defined as
A�O � � ln � R �O �� or A�O � � ln �T �O �� , where R �O � is the reflectance at the wavelength O, and T �O �
is the transmittance at the wavelength O. These relations allow one to measure the relative changes of absorption
306 Proc. of SPIE Vol. 5688
properties of the scattering medium. Spectrum A �O � for melanin can be linearly approximated in the spectralrange from 620 to 1000 nm, and melanin content in tissue is determined as magnitude proportional to the linear
slope of spectral dependence A�O � in this spectral range. Such dependence can be used for an evaluation ofmelanin concentration in an iris by analogy with the evaluation of the melanin content in human skin.
The slopes of such linear approximations were used for estimation of melanin concentration in the human iris in terms of DOPA melanin. Using the absorbance measurements for DOPA melanin solutions of differentconcentrations the following linear approximation in the range 620-720 nm was obtained4
where A is the absorbance of DOPA melanin solution; T is the transmittance of DOPA melanin solution, O is thewavelength in nm;
C 0.0688 � 0.103 C,
C 0.0794 � 0.124 C,
C is the DOPA melanin concentration in mg/ml. From the system of equations (2) we can obtain
C 0.84 C and (
A O) C
(0.84 � O 10 ) C (1 �
From (3) we can obtain:
From the system of equations (2) we can obtain
C � 0.0688
C � 0.0794
Proceeding from the given technique based on measurement of reflectance, it is possible to estimate the melanincontent in an iris of an eye.
In fig. 5 the reflectance spectra and in fig. 6 the optical density of brown human iris measured in vitro are presented.
Proc. of SPIE Vol. 5688 307
Fig.6 The optical density D of brown human iris calculated
Fig. 5 The reflectance spectra (R(O)) of brown human iris
from measured in vitro reflectance spectra in different
measured in vitro in different points of the sample
points of the sample (fig. 5); (D(Ȝ)=–lg(R(Ȝ))
In table 1 the mean melanin concentration values obtained from the spectral dependence optical density of bovine irises measured in vitro are presented.
Table 1. Mean melanin concentration values obtained from the measured in vitro spectral dependencies of optical density ofbovine irises
concentration in an iris,
Basing on the results the average melanin concentration in the bovine iris has been estimated as 39.5 r 6.2mg/cm3.
At examination of human iris samples, the melanin concentration has been estimated as 44.9 r 2.1 and 46.7 r 2.2 mg/cm3, respectively. Thus, the mean melanin concentration obtained from the human irises has been estimatedas 45.8 r 1.2 mg/cm3.
In tables 2 and 3 the results of digital image analysis using designed computer program are respectivelypresented for healthy volunteers and for volunteers with primary open angle glaucoma.
Table 2. Results of digital image analysis of healthy volunteers
308 Proc. of SPIE Vol. 5688
Table 3 Results of digital image analysis of patients with open angle glaucoma
Proc. of SPIE Vol. 5688 309
In tables 4 and 5 data, averaged from tables 2 and 3 are presented. From table 4 it is seen that maximal melanin concentration has been obtained for brown eyes. For blue and green eyes the melanin content is smaller.
Table 4. Mean melanin concentration in healthy human iris measured in vivo (from digital analysis of color images)
Melanin concentration, mg/cm3
Table 5 Mean melanin concentration in human iris with glaucoma measured in vivo (from digital analysis of color images)
Melanin concentration, mg/cm3
From table 5 it is seen that maximal melanin concentration is observed for blue eyes in contrast to data of table 4. So, melanin content in human and animal irises using experimental data obtained in vitro from reflectance measurements and in vivo from digital analysis of color images of irises was estimated. In irises of eyes with glaucoma the mean content of melanin is less in comparison with one in irises of healthy eyes. The most difference was obtained for brown and green color eyes.
The research described in this publication has been made possible, in part, by grant REC-006/SA-006-00, Annex N. 07 "Nonlinear Dynamics and Biophysics" of U.S. Civilian Research and Development Foundation for the Independent States of the Former Soviet Union (CRDF) and the Russian Ministry of Science and Education; the Russian Federation President's grant N 25.2003.2 "Supporting of Scientific Schools" and grant "Leading Research-Educational Teams" N 2.11.03 of the Russian Ministry of Science and Education. The authors thank Dr. S.V. Eremina (Department of English and Intercultural Communication of Saratov State University) for the help in manuscript translation to English.
REFERENCES
1. A.N. Bashkatov, Yu.P. Sinichkin, E.A. Genina, V.V. Tuchin, G.B. Altshuler, "RGB video microscopic
system for in vitro monitoring of optical properties of hair shaft and follicle," Proc. SPIE, 4244, 161-
167, 2001.
2. A.N. Bashkatov, E.A. Genina, A.V. Volokh, S.A. Murikhina, G.B. Altshuler, V.V. Tuchin, "Optical
properties of hair shafts estimated using the digital video microscopic system and inverse Monte Carlo
method," Proc. SPIE, 4609, 1-9, 2002.
3. W.-F. Cheong, S.A. Prahl, A.J. Welch, "A review of the optical properties of biological tissue," IEEE J.
Quantum Electr. 26(12), 2166-2185, 1990.
4. N. Kollias and A. Baqer, "Spectroscopic characteristics of human melanin in vivo," J. Investig.
Dermatol., 85, 38-42, 1985.
310 Proc. of SPIE Vol. 5688
Light transport in tissue, Ph.D. Thesis, Univ. Texas at Austin, 1988.
Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, SPIE
Press, TT38, Bellingham, USA, 2000.
Biomicroscopy of the eye, Moscow, 1972 (in Russian)
Berliner, Biomicroscopy of the eye, in Slit-lamp microscopy of the living eye, New York, p .9-14,
Anatomy and histology of the human eye in normal, its development and withering,
Moscow, 1913 (in Russian).
10. E.S. Velhover, V.F. Ananin, Clinical iridology, Moscow, 1992 (in Russian). 11. E.S. Velhover, Iridology, Moscow, 1992 (in Russian). 12. A.M. Vodovozov, A.A. Ribnikov, Estimation iris of the eye in transformed light, Moscow, 1992 (in
13. V.V. Konovalov, A.A. Antonov, Practical iridology, 1990 (in Russian).
14. A.J. Samoilov, Iris of an eye, in Big Medical Encyclopedia, 27, 842-849, 1962 (in Russian).
15. A.J. Samoilov, Choroid of an eye, in Big Medical Encyclopedia, 30, 953-956, 1963 (in Russian).
16. D.A. Enikeev, S.A. Lobanov, Iridoalloplastic, 1996 (in Russian).
17. E.V. Bobrova, "Ultrastructure of human eye dilator," in Some questions of experimental and clinical
medicine, 22-25, Moscow, 1977 (in Russian).
18. E.V. Bobrova, A.V. Petrov, "Ultrastructure of the front layers and pupill dilator of an human eye iris,"
Bullen of Ophthalmology, 4, 33-36, 1978 (in Russian).
19. T.P. Dryja, M. O'Neil-Dryja, D.M. Albert, "Elemental analysis of melanins from bovine hair, iris,
choroid, and retinal pigment epithelium," Invest. Ophthalmol. Visual Sci., 18, 231–236, 1979.
20. E. Buszman, R. Rozanska, "Interaction of thioridazine with ocular melanin in vitro," Acta Pol Pharm.,
60(4), 257-61, 2003.
21. M. Hammer, D. Schweitzer, E. Thamm, A. Kolb, "Non-invasive measurement of the concentration of
melanin, xanthophyll, and hemoglobin in single fundus layers in vivo by fundus reflectometry," Int.
Ophthalmol., 23 (4-6), 279-89, 2001.
22. S. Peters, U. Schraermeyer, "Characteristics and functions of melanin in retinal pigment epithelium,"
Ophthalmology, 98(12), 1181-5, 2001.
23. M. Braun, A. Kage, K. Heimann, U. Schraermeyer, "Retinal pigment epithelial cells from Royal
College of Surgeons dystrophic rats can take up melanin granules," Graefes Arch Clin Exp
Ophthalmol., 237(1), 67-71, 1999.
24. P. Donatien, G. Jeffery, "Correlation between rod photoreceptor numbers and levels of ocular
pigmentation," Invest Ophthalmol Vis Sci., 43(4), 1198-203, 2002.
25. R.D. Glickman, S.L. Jacques, R.T. Hall, N. Kumar, "Revisiting the internal absorption coefficient of the
retinal pigment epithelium mɟlanosome," Proc. SPIE, 4257, 134–141, 2001.
26. C. Ono, M. Yamada, M. Tanaka, "Absorption, distribution and excretion of 14C-chloroquine after
single oral administration in albino and pigmented rats: binding characteristics of chloroquine-related
radioactivity to melanin in-vivo," J. Pharm. Pharmacol., 55(12), 1647-54, 2003.
27. T. Sarna, "Properties and function of the ocular melanin - a photobiophysical view," J. Photochem.
Photobiol., B, 12, 215-225, 1991.
28. N. Kollias, A. Baqer, "On the assessment of melanin in human skin in vivo," Photochemistry and
Photobiology, 43(1), 49-54, 1986.
29. S. Jacques, Published on the personal web-site: www.omlc.ogi.edu.
Proc. of SPIE Vol. 5688 311
Source: http://optics.sgu.ru/_media/optics/staff/bashkatov/koblova_spie_05_5688_302.pdf
Von Insights zu Wachstum Der Kunde bestimmt die Spielregeln Profitables und anhaltendes Wachstum sichert das Überleben eines Unternehmens und ist der Erfolgsausweis eines Führungsteams. Standardstrategien versagen in den heute sich radikal und schnell ändernden Märkten. Wo soll das Unternehmen ansetzen? Beim sich stetig wandelnden Kunden: Mit der Job-to-be-done-Logik werden Kunden-Insights gewonnen, die unserem Spielplan für Wachstum aktuel e und konkrete Inhalte für die einzelnen Unternehmensstrategien liefern.
Land Rights in a Spatial Environment 1Powertech IST Data, Pretoria, South Africa, [email protected] Abstract In the present day and age, land is scarce and infrastructure expansion is a growing concern. This concern affects any business that needs to either register servitudes for land or manage statutory land regulations. Business Intelligence related to land and right management is essential to maintain, track and enable planning of infrastructure. A system which can capture and visualise these needs electronically, will solve these growing pains. LARIS is a spatially enabled Decision Support System, which has operational, financial and strategic benefits to serve the whole organisation. Organisational challenges which have lead to the current LARIS include:











