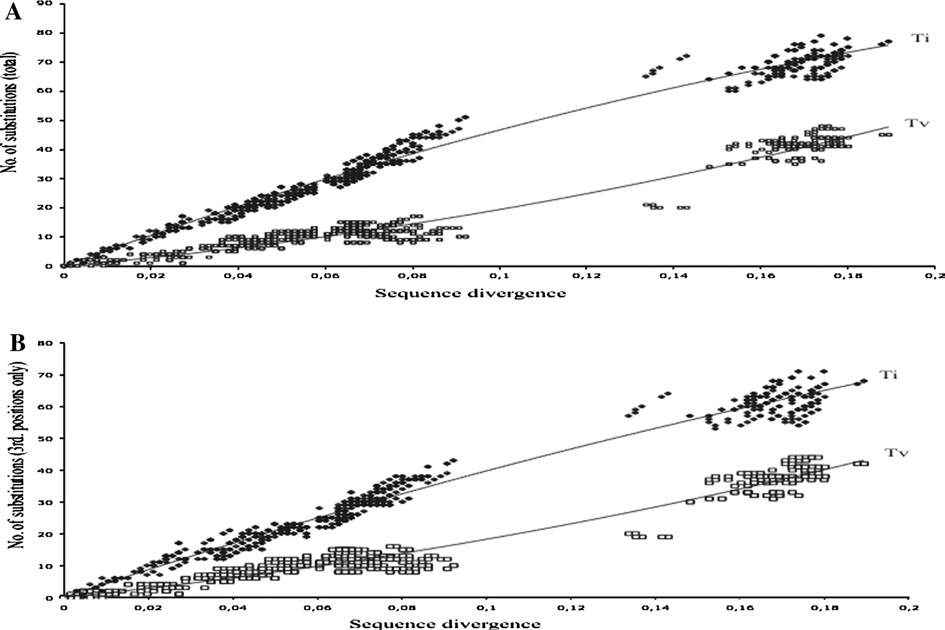
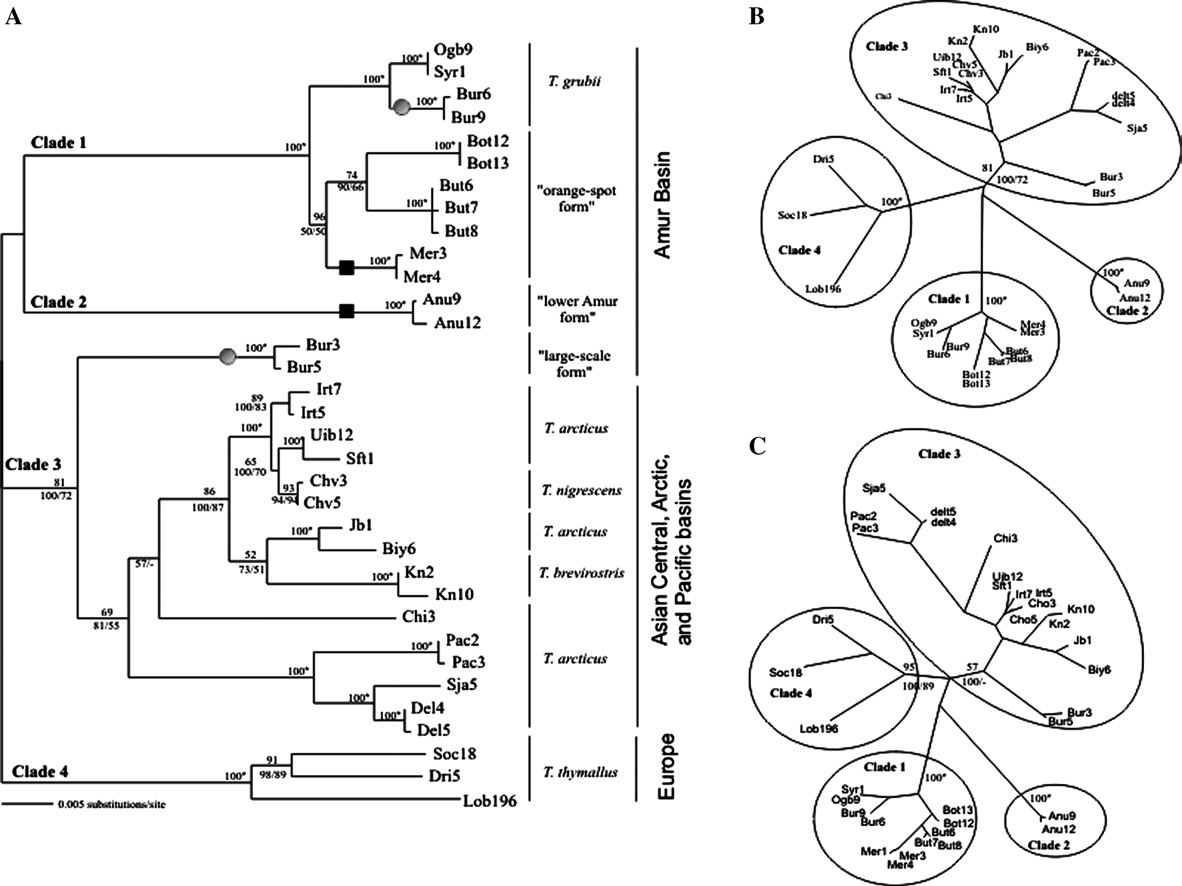
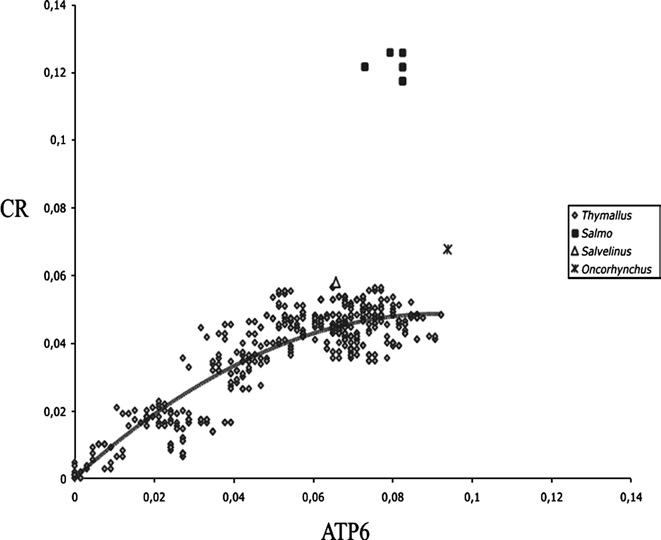
El honorable concejo municipal de la ciudad de santa fe de la vera cruz, sanciona la siguiente
ORDENANZA Nº 11829 EL HONORABLE CONCEJO MUNICIPAL DE LA CIUDAD DE SANTA FE DE LA VERA CRUZ, SANCIONA LA SIGUIENTE Art. 1º: El despacho de los asuntos del Departamento Ejecutivo Municipal estará a cargo de las siguientes Secretarías: Obras Públicas y Recursos Hídricos; Desarrollo Social; Planeamiento Urbano;