Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Pnimnipe_nr56
Mining Science, vol. 23, 2016, 33−42
(previously Prace Naukowe Instytutu Gornictwa
Politechniki Wroclawskiej, ISSN 0370-0798 in polish)
ISSN 2300-9586 (print)
ISSN 2084-4735 (online)
Received: May 18, 2016, reviewed; accepted: June 26, 2016
NEW METHOD OF ELECTROSTATIC SEPARATION
OF THE OXIDIZED IRON ORE
IDRES Abdelaziz1*, BOUHEDJA Ahcène2, BOUNOUALA Mohamed1, BENSELHOUB Aissa1, 3
1 Laboratory of Mining Resources Valorization and Environment, Mining Department, Badji Mokhtar
University, Annaba, Algeria.
2 Laboratory of Physical Metallurgy and Materials Properties, Mining Department, Badji Mokhtar
University, Annaba, Algeria.
3 Department of Ecology and Environment Protection, State Agrarian and Economic University,
Dnipropetrovsk, Ukraine.
Abstract: The complexity of the mineralogical, chemical and exogenous parameters generates a low
efficiency of electrical separation of oxidized iron ore. For this purpose, a study was conducted to provide
a method for electrostatic separation and to select the chemical reagents. And this, for increasing the
contrast of the dielectric separating minerals in the corona discharge field. The reagent-collector used is
the auramine with a specific consumption of 0.3 kg/t. Whereas, the reagent depressants is carboxymethyl
cellulose mixture with a specific consumption of 0.25 kg/t. From the obtained test results electrical treat-
ment of oxidized iron ore are satisfied for proposed process.
Keywords: iron ore, electrostatic separation, reagent-collector reagent – depressant, efficiency
The deposit of Ouenza (Algeria) is the main ore mining iron pole in the country.
This field produces about two million tons of ore per year in which 70% are above the 55% level. The rest of the quantity of contents is generated varying from 42-50%. This is a poor ore stored at the mine and requiring treatment.
Corresponding authors:
[email protected] (I. Abdelaziz)
doi: 10.5277/msc162303
IDRES A., BOUHEDJA A., BOUNOUALA M., BENSELHOUB A.
The development of these minerals will increase a share of industrial reserves of
the deposit, on the other hand safeguarding the environment which is considered a major challenge for the region.
The method of electrical separation is based on the use of the difference in electri-
cal conductivity of minerals to be separated. However, this conventional method has limitations resulting from low separation efficiency. The present study was conducted to provide a method for electrical separation using chemical treatment reagents and reagent -depressants collectors thereby increasing the contrast of the dielectric separat-ing minerals.
STUDY OF THE TECHNOLOGY CONCENTRATION BY ELECTROSTATIC
The study refers to a concentration technology of electric oxidized iron ore at
Ouenza iron ore deposit. It is well known that the electrical isolation is based on the use of the difference in electrical conductivity between the minerals to be separated (Dumitran et al., 2007).
The objective of the study is to increase the separation efficiency of oxidized iron
ore. This can be achieved by increasing the contrast of the dielectric separating miner-als.
The main processes of electric charging of the particles are in the electrical separa-
tion ionization (corona charging, α – β radiation), the electrification by friction, con-tact with the charged electrode, induction heating, combination of raising processes (two or more).
Among these processes, the greatest interest is in the field ionization discharge ring
(Dumitran et al., 2005).
Consider the highest electric charges received by the same particle in the field of
corona discharge due to contact with a rotating cylindrical surface, grounded.
The dependence of the load value is defined by the formula Potenier (Pauthenier et
2 𝑒 𝑛 𝑘𝑛𝜏 𝜋 𝜀0
𝜏 4𝜀0+𝑒 𝑛 𝑘𝑛𝜏
𝑘𝑛 – mobility ion, (
m2/
vs)
ε – relative dielectric permeability of the material;
n– number of elementary charges; 𝜀
0 – dielectric constant,
τ – time, Sec;
e – particle charge, e = 1.6·1012 C;
E – electric field strength, kV/cm
New method of electrostatic separation of the oxidized iron ore
The maximum value is obtained when
𝑄𝑚𝑎𝑥 = 12𝜋𝜀0𝜏2𝐸 (
For a conductive particle, considering where:
𝑄𝑚𝑎𝑥 = 12𝜋𝜀0𝜏2𝐸
Considering that in the electrical load, the field strength and the particle size are
sufficiently high and, thus, it is possible to neglect the small influence of the thermal motion of ions.
The load value of the particle as a function of exposure time in the wrong field is
determined from the formula (1). The maximum load is equal to:
𝑚𝑎𝑥 = 𝜋𝜀0 (1 + 2
Under the condition: 𝑞⁄𝑞𝑚𝑎𝑥 = 0.9 The time of exposure of particle in the field of ring (calculated) is equal to:
where: 𝜀, 𝑑𝑟 −dielectric permeability and particle diameter; 𝐸𝑐 −electric field intensity of the crown;
n,
k – concentration and mobility of ions; 𝜀
0 −dielectric constant,
e – electron charge, 𝑒 = 1.6. 1012𝐶
𝜏 = 𝑐 − exposure of the particles in the field of ring time.
The exposure time of the particle in the field of ring (measured) is equal to:
where: 𝐻𝑐 −crown height field (m/s)
ϑ – velocity, (s)
Note: the values of both exposure times – calculated and measured – are
practically very close (0.12/0.10 seconds).
All particles receive, in the field of the ring, the load:
IDRES A., BOUHEDJA A., BOUNOUALA M., BENSELHOUB A.
0 = 0,9𝜋𝜀0 (1 + 2
In this work, the study is focused on the process of loading a crushed during its
movement by the troughs and metal dielectric material et al., 2011). The results show that the quartzite of diameter 60 μm receives a load of about 10−17 C, the same particle receives current field crowned load 𝑄𝑚𝑎𝑥 = 10−13 C. This represents an increase of 104times.
The value of triboelectric charge of the separated particles reaches a certain
percentage of loading in the region of a normal discharge crowned.
Therefore, there is a possibility to use fillers for the separation of friction of certain
ground materials. The separation corona charging field is an advantage with respect to tribo-charging method (Dumitran et al., 2005et al., 2009).
We evaluate how different the charges received by the quartz and hematite ring
field of the same intensity (Tiberiu et al., 2010).
The calculation of these values of loads is as follows:
where 𝜀ℎ, 𝜀𝑞 − relative dielectric permeability of quartz and hematite.
Knowing that the dielectric permeabilities for hematite and quartz are equal to:
𝜀ℎ = 81, 𝜀𝑞 = 6.5, we obtain 𝑄 = 27.6 %. This value is to be used in the separation of a poly-dispersed material et al., 2011).
To compare the effectiveness of different methods of loading particles "fluidized"
layer, the first value of the specific charge of particle is determined to be evaluated according to the following formula:
where
m, – Mass and density of the particle (Kg, Kg/m3).
From the formula above, it is deduced that the greater particle size decreases, the
specific electrical load increases.
The discharge mechanism of the particles in the field of the corona discharge is
shown in Figure 1. In the presence of sufficiently high voltages applied in the space between the electrodes, with the surface of the corona electrode, is made of the intense shock ionization of gas accompanied by the appearance of the load ring and s '
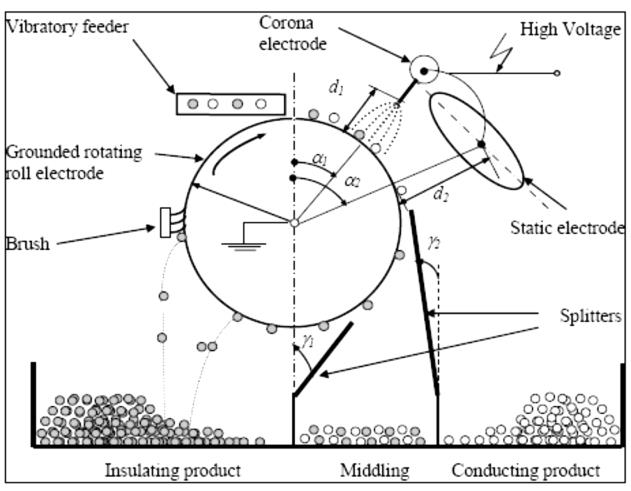
New method of electrostatic separation of the oxidized iron ore
attenuates gradually as the decrease in the intensity of the electric field in the direction of the collecting electrode (Medles et al., 2011, Tabti er al., 2010).
Gaseous ions of different polarities formed in the crown area under the action of an
electric field (Coulomb force), move to the electrodes of opposite polarity. However, in the inter – electrode forms the electric current of said crown. The ore particles receive, due to the absorption of ions onto their surface an electric charge in the inter–electrode (Tiberiu et al., 2010, Bendaoud et al., 2010).
The loading of particle increases until the ions do not couple with each other. By
increasing loading time and the number of ions deposited on the particle, increasing the intensity of the field created by the charged particle is directed towards the outside of the main field intensity. When these intensities become equal, the particle stops receiving new ions. Therefore it will stop charging. At this time, the particle has the maximum possible charge (Younes et al., 2010, Tabti et al., 2009).
Fig. 1.The loading mechanism in the field of ring (Dumitran et al., 2007)
α1et d1 – angular and radial positions of the corona electrode;
α2 et d2 – angular and radial positions of the electrostatic electrode;
γ1 et γ2 – angular positioning of dividers.
The study of the samples mineralogical composition of iron ore is carried out by
metallographic microscopy of polished sections (see Table 1).
Chemical analysis by XRF show the poverty iron ore deposit of the above-
mentioned (see Table 2) (Idres et al., 2005).
The oxidized iron ore is ground and dusted heated to a temperature of 120 to
150°C. The latter is treated with the reagent by aerosol – auramine manifold that is
IDRES A., BOUHEDJA A., BOUNOUALA M., BENSELHOUB A.
heated to a temperature of 80 to 90 °C, and followed by the electrochemical treatment at a voltage of 10 Volts. The fixation-depressant reagent on the surface of the gangue minerals and provides surface protection against moisture and increases the dielectric properties of sterile particles. As reagent – depressant, was used and the mixture of Carboxymethyl cellulose nitrolignine whose ratio corresponding to (20–25)/ (80–75).
Tab. 1. Mineralogical composition of the iron ore to the low levels of Ouenza
Minerals and rocks
H – Hematite, G – goethite, HG – Hydrogoethite, SA – siderite-ankerite, D – Dolomite, Calcite – C, Q – Quartz, A – Apatite, MA – Clay minerals.
Tab. 2. Chemical composition of the iron ore to the low levels of Ouenza
Minerals Fe t. SiO
Goethite 41.7 22.5
0.01 0.022 42.4 42.5 99.56
Sandstone 0.06 94.5
The particle temperature before treatment with aerosol must be 120 to 150°C.
While the reactants, it ranges from 80 to 90°C. In addition, during treatment with rea-gent-collector, the ore is subjected to electrochemical treatment for five minutes with a voltage of 10 Volts.
The auramine C12H25NH2 with a molecular weight of 188.35 and melting tempera-
ture of 28.32 °C., represents a derivative of ammonia, wherein one or more hydrogen atoms are mixed with the aliphatic or heterocyclic radicals.
The ionic micelles have a nonpolar structure whose ends are directed towards the
center, whereas the NH2 groups represent a layer. Following this ionic micelle struc-ture, the amines have an electrical conductivity (Andersen, 1995).
The carboxymethyl cellulose is cellulose ether and containing the carboxyl which
is obtained by means of interaction of the cellulose with the alcohol of sodium salt of the monochloro acid vinegar (Samant et al., 2006).
6 H7 O2 (OH) 2ONa]n + nCl CH2
6H7 O2 (OH)2 OCH2 COONa]n + Na Cl
New method of electrostatic separation of the oxidized iron ore
The nitrolignine is a mixture of calcium salt of lingo sulfonic acid addition sugars
and minerals. It is obtained as industrial waste cellulose paper. The selectivity of bind-ing of the reagent manifold and the reagent depressant on gangue minerals and metal are provided by the physico-chemical nature of the reagent and the mineral. The elec-trical treatment increases the collector reagent physicochemical binding activity on the metal inorganic (Butunoi et al., 2010).
Tab. 3. Indexes of testing electrical separation of oxidized iron ore
Technological Indices
Recovery Efficiency
1. Optimization of reagent consumption collector
2. Optimization of consumption and carboxymethyl cellulose nitrolignine
3. Optimization of the heating temperature of the ore and reagents
4. Optimization of electrochemical reagent depressant treatment
IDRES A., BOUHEDJA A., BOUNOUALA M., BENSELHOUB A.
The greatest effect of increasing the separation efficiency of the electric oxidized
iron ore is achieved during processing of ore heated at a temperature of 120 to 150 °C and 80 to 90 °C reagents. The relationship between Carboxymethyl cellulose and ni-trolignine is obtained on the basis of experimental tests.
The comparative test results of the methods of electrical separation of oxidized
iron ores according to the conventional embodiment and the one proposed are shown in table 3.
RESULTS DISCUSSION
Experimental tests on oxidized iron ores are done at the Laboratory of Physical
Metallurgy and Materials Properties, University of Annaba – Algeria. The sample of iron ore weight 1.380 kg of a size +5 – 0 mm, is grounded in a ball mill with a capaci-ty of 7 liters. The size of the crushed ore is 70% of less than 0.25 mm wafer. The ore is deslimed in a cyclone diameter of 150 mm. Ore processing at the electrical electro-static separator ring drum type PS-1 is made in two patterns: classic and proposed.
According to the conventional scheme, the ore is heated to a temperature of 150 to
170 °C and passed directly to the electric separator. While, for the proposed scheme:
ore is heated to a temperature of 120 to150°C
treating the already heated by itself heated to a temperature of 80–90 °C and
electrochemically reactive ore collector.
This study highlights the importance of the chemical treatment before the opera-
tion of electrostatic separation; this allows us to conclude generally that:
1. As collector reagent, is used and auramine -depressant reagent (mixture of car-
boxymethyl cellulose and nitrolignine). The consumption of the reagent – manifold is 0.25 kg/t, and 0.30 kg/t to the reagent – depressant.
2. The aerosol treatment of heated ore ensures fixation on the surface of minerals
and increases contrast in electrical properties between minerals and gangue. This con-tributes to a considerable increase of the separation efficiency of the electric iron ore.
3. Treatment of experimental tests gives respectively a separation efficiency of
23.30 % of the conventional method and of 42.90 % for the proposed method.
4. Considering the obtained results, the proposed method improves the separation
efficiency of 1.84 times compared to the conventional method.
5. To improve the efficiency of electrostatic separation in the case of depos-
it of iron oxide ores at low levels, it is recommended to use chemical reagents
New method of electrostatic separation of the oxidized iron ore
such as auramine and the mixture of carboxymethyl cellulose and nitrolignine prior to electrostatic separation.
ACKNOWLEDGEMENTS
The work described in this article is the result of a part of the research program in the laboratory of
Physical Metallurgy and properties of materials.
It is our pleasure to express our gratitude to all those who in one way or another, have contributed to
the completion of this work.
ANDERSEN F.A., 1995, Final report on the safety assessment of lauramine and stearamine
Vol. 14, No 3, 196-203.
BENDAOUD A., TILMATINE A., MEDLES K., YOUNES M., BLEJAN, O., DASCALESCU, L.,
2010. Experimental study of corona discharge generated in a modified wire–plate electrode configu-ration for electrostatic process applications. IEEE Transactions on Industry Applications, Vol.46, No 2, 666 – 671.
IDRES A., BOUNOUALA M., 2005. Possibilité d'une nouvelle technologie de traitement des minerais
de fer du gisement de l'ouenza par radiometrie. J. Physique IV. Vol.124, 177 – 181.
KOCHERBITOV V., VERYAZOV V., SÖDERMAN O., 2007. Hydration of trimethylamine-N-oxide
and of dimethyldodecylamine-N-oxide: An ab initio study. Journal of Molecular Structure: HE-OCHEM, 808(1), 111-118.
DUMITRAN L.M., DASCALESCU L., NOTINGHER P.V., ATTEN P., 2007. Modelling of corona
discharge in cylinder-wire-plate electrode configuration. Journal of Electrostatics, 65(12), 758-763.
DUMITRAN L. M., BLEJAN O., NOTINGHER P., SAMUILA A., DASCALESCU L., 2005. Particle
charging in combined corona-electrostatic fields. In Fourtieth IAS Annual Meeting. Conference Rec-ord of the 2005 Industry Applications Conference, 2005. (Vol. 2, pp. 1429-1434). IEEE.
MEDLES K., DASCALESCU L., TILMATINE A., BENDAOUD A., YOUNES M., 2007. Experimental
modeling of the electrostatic separation of granular materials. Particulate Science and Technolo-gy, 25(2), 163-171.
BILICI M., DASCALESCU L., DRAGAN C., FATI O., IUGA A., SAMUILA A., 2011. Tribocharging
and electrostatic separation of mixed granular solids in fluidized bed devices. IEEE Transactions on Dielectrics and Electrical Insulation, 18(5), 1476-1483.
PAUTHENIER M.M.,MOREAU-HANOT M.M., 1932. Le problème de la charge acquise par une
particule dans un champ électrique ionisé. J. Phis. Radium, vol. 3, 590-613.
TABTI B., DASCALESCU L., PLOPEANU M., ANTONIU A., MEKIDECHE M. 2009. Factors that
influence the corona charging of fibrous dielectric materials. Journal of Electrostatics, 67(2), 193-197.
TABTI B., MEKIDECHE M.R., PLOPEANU M.C., DUMITRAN L.M., ANTONIU A., DASCALESCU
L., 2010. Factors that influence the decay rate of the potential at the surface of nonwoven fabrics af-ter negative corona discharge deposition. IEEE Transactions on Industry Applications, 46(4), 1586-1592.
TILMATINE A., BENDIMERAD S., YOUNEs M., & DASCALESCU L., 2009. Experimental analysis
and optimisation of a free-fall triboelectric separator of granular plastic particles. International Jour-nal of Sustainable Engineering, 2(3), 184-191.
IDRES A., BOUHEDJA A., BOUNOUALA M., BENSELHOUB A.
SAMANT B.S., SARAF Y.P., BHAGWAT S.S., 2006. Chlorination of aromatic compounds in micellar
media: Regioselectivity. Journal of Colloid and Interface Science, 302(1), 207-213.
BUTUNOI T., GAGIU G., BILICI M., SAMUILA A., NEAMTU V., MORAR R., IUGA A., 2010.
Electric and electronic equipment of a research-oriented electrostatic separator. In Optimization of Electrical and Electronic Equipment (OPTIM), 2010 12th International Conference on IEEE, 639-645.
Source: http://www.miningscience.pwr.edu.pl/pdf-64025-4956?filename=New%20method%20of.pdf
Visit www.HyponatremiaCME.org for additional cases and activities Clinical Perspectives in As a physician scientist who has been studying and treating hyponatremic patients for the past 30 years, I am pleased to introduce this case-based continuing medical education publication and associated Web-based interactive learning program, Clinical Perspectives
Il nostro scheletro Contrariamente alle apparenze, anche il nostro scheletro, con le sue 203 ossa fra grandi e piccole, è una parte "viva" del nostro corpo. Basta osservare i cambiamenti che esso subisce nel corso della vita. Dalla nascita fino ai vent'anni circa lo scheletro cresce e si sviluppa. Le ossa aumentano di peso e di volume mentre assumono la loro forma adulta




