Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Production optimization using biochemical profiling
Application Note
Compound Profiling and Toxicity
The Challenge: Understanding a compound's on and off target
activities with associated toxicities and indications
Utilizing a systems approach to drug discovery has generated a multitude of high affinity compounds for various classes of molecular targets with different degrees of disease state
validation. As these compounds are progressed to the clinic, they pass through a number of
defined assays to determine compound efficacy as well as possible toxicities. Although these
assays give substantial information on certain toxicities and mechanism of action, toxic effects as
well as mechanistic understanding can be found further downstream in the pre-clinical to clinical stage.
It is very useful to have new tools for determining a compound's propensity for various toxicities
earlier on in the process as well as tools for understanding a compound's on/off target effects as
they relate to disease states.
The Solution: Global Analysis of Biochemicals
Biochemical homeostasis in the cell and whole organism is fairly well-defined with over 100 years
of study and definition in scientific literature. The association between biochemicals and the pathways in which they reside is comprehensively mapped. Disease states as well as compounds
affect this homeostasis often in defined and understood modes. In fact, biochemical changes are
regularly tracked as indicators of disease state as well as compound efficacy/toxicity.
For example, blood glucose is monitored as an indicator of diabetes and metabolic syndrome. In addition, cholesterol and lipids are markers for arteriosclerosis and coronary disease, whereas
bile acids are used as indicators for liver function. These types of markers have proven successful
in compound selection through to the clinic.
A global analysis of biochemical changes, provides insight into these biochemical markers as well
as hundreds of others. Biochemical profiling (metabolomics) can track changes in numerous pathways and correlate those changes to a variety of biological perturbations including:
• disease state modification
• mechanism of action of a compound
• downstream effects of target inhibition/activation
• off target effects of a compound
In numerous studies, this approach has been able to reproducibly predict compound toxicities
and provide mechanistic insight. Lastly, biochemical pathway modifications have been observed
which have pointed to novel therapeutic uses.
Application of Global Biochemical Information
Case Study: Fenofibrate Toxicity and Pharmacology in Rats
Fenofibrate is a known hepatotoxin in
rats with hepatomeglia as an
endpoint. The purpose of this study
was two fold. First to identify markers
of cell proliferation and liver toxicity for comparison to investigational
drugs incorporated into the study and
secondly to gain mechanistic insight of
PPAR alpha activity. In this study rats
were dosed with fenofibrate (300mg/kg/day) and vehicle control in
groups of 6. Two plasma time points
were taken, 2 days and 14 days with
subsequent 24 hour urine collection. Pathology was performed at day 2 and day 14 on the
respective groups.
Global biochemical profiling revealed profiles consistent with the known pharmacology of the
agonist. There was clear acceleration of fatty acid B oxidation with concomitant reduction of
plasma fatty acids (figure 1 and 2). Interestingly, cholesterol was lowered at day 2 consistent
with the known pharmacology of fenofibrate but elevated at day 14, consistent with liver toxicity.
A novel finding was the strong down regulation of the citric acid cycle which explains the energy
utilization shift to fatty acid B oxidation (Figure
Along with the pathways defining fenofibrate,
pharmacology markers of toxicity were observed. Notably, bile acids were increased at
day 2 and 14. Bile acids are known markers of
liver dysfunction with plasma levels elevating as the liver fails to recycle them. Another pattern of
liver dysfunction is the altered tryptophan pathway. Note the significant shift from conversion to
serotonin to the alternate kynurenine and associated pathways (figure 4). Significant increases in
3-Methyl-L-Histidine were also observed which is a marker of muscle breakdown and indicative of the myopathy observed in the study.
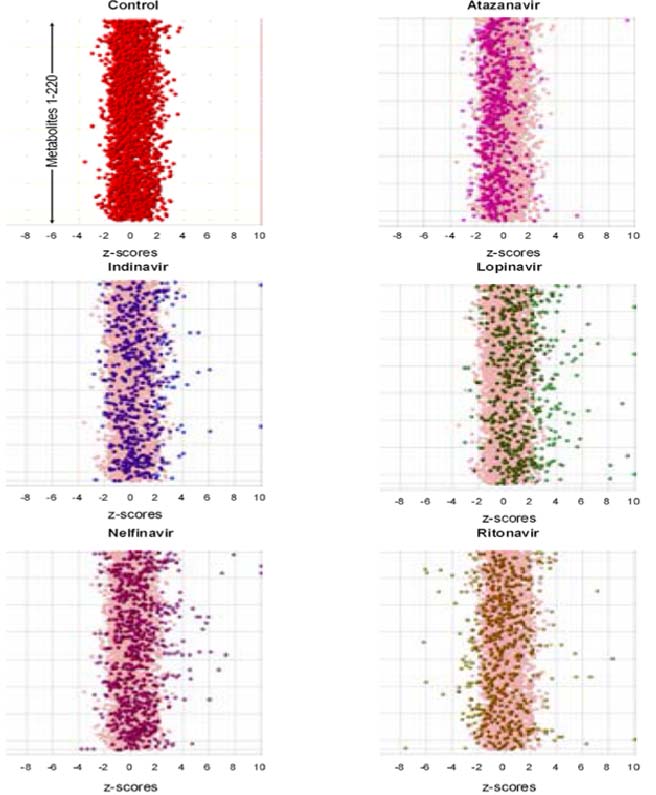
Case Study: Side Effect Profiling of PI Inhibitors
Protease Inhibitors (PI) have been invaluable tools in the battle against HIV infection, although
effective first generation inhibitors have been plagued by side effect profiles specifically in the
area of dislipidemia and fat redistribution. BMS introduced a second generation PI with markedly
fewer side effects. Experiments were designed to track biochemical changes comparing BMS's drug to marketed PI's. The purpose was two fold—to determine the biochemical differences
elicited by the classes of compounds as well as to demonstrate biochemically the "cleanness" of
the BMS compound.
In this study 5 compounds were tested,
Atazanavir (ATZ), Indinavir (IDV), Lopinavir (LPV), Nelfinavir (NFV) and Ritonavir (RTV),
against vehicle control in HEPG2 cells. This
table outlines the number of significantly
altered biochemicals based on comparison to
control. Notably, the study showed that that treatment with ATV induced no significant
biochemical shifts in this experiment. Strength
of biochemical shifts in rank order were:
ATV<IDV = RTV<NFV<LPV.
The scatter plots in figure 5 are a visual representation of these data. The y axis is
the biochemical entities with the x axis
standard deviations from the mean of the
control. Each data point is a biochemical
in an animal replicate. These data
demonstrate visually how little ATZ perturbed the metabolome. Importantly,
these biochemical changes were
associated to energy production and fatty
acid metabolism and correlated with
observed side effect profiles.
Follow on experimentation with LPV and
ATV in HEPG2 cells and Adipocytes
combined with patient plasma samples
followed a similar pattern. In these
experiments LPV showed increased
numbers of significantly altered biochemicals in the HEPG2 system as well
as plasma. The areas impacted that
differentiated these compounds were in
carbohydrate and lipid metabolism which
fit well with the side effect profiles of
these drugs. Lastly, biochemical changes were found that were common to the in
vitro and in vivo systems suggesting that
the cell culture base model could be used
for early drug development.

Conclusion
Biochemistry, the working end of the cell, has proven invaluable in understanding a compounds
action in a biological system. On and off target effects are readily visualized in a variety of model
systems with excellent translatability. Utilizing global biochemical profiling toxicities are more
easily observed with less cost, novel areas of compound impact are visualized leading to new
indications, and drug mechanistic effects can be followed. Tethering these data to DMPK can give
excellent insight into drug and metabolite effects as well.
mVision Analysis
Metabolon's mVision analytical platform is designed and optimized to detect small molecule
biomarkers from numerous biochemical classes. Analysis by three unique analytical platforms
separates and detects the broadest range of biochemicals as well as provides internal QC for
biochemicals common to two or more platforms.
This global, non-targeted approach
offers a unique advantage over other
metabolomics services that provide only
raw data (e.g. mass spec signals) or analyze a limited number of compounds
chosen from a restricted panel of
detectable chemicals (targeted
analysis). With Metabolon's solution,
studies can be undertaken without the
need for a complete understanding of the biology or biochemistry of samples.
The three studies outlined in this application note are also available in greater detail in poster
format. They can be obtained by visiting .
www.metabolon.com │ Metabolon, Inc. │ 919.572.1711
Source: http://www.metabolon.com/media/195858/App-Note-compound-profiling-toxicity.pdf
Appeal Decision Hearing held on 28 October 2014 Site visit made on 28 October 2014 by Susan Ashworth BA (Hons) BPl MRTPI an Inspector appointed by the Secretary of State for Communities and Local Government Decision date: 27 November 2014 Appeal Ref: APP/V2825/A/14/2220834 Land North of Danes Camp Way, Hunsbury Hill, Northampton
RHYTHM IS THE CARRIER OF LIFE A company portrait of WALA Heilmittel GmbH "If one day we understand the rhythms of nature – this will be natural science in itstruest form." Dr. Rudolf Steiner 1 Dr. Ulrich Meyer, Member of the Management Board, and Karl Kossmann, co-founder and member of the WALA Foundation, at the employee information meeting





