Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Keralamarinelife.in

Journal of Aquatic Biology and Fisheries Vol. 2/2014/ pp. 133 to 140
ANTIDIABETIC AND ANTIOXIDANT ACTIVITY OF PADINA
TETRASTROMATICA IN HIGH CALORIE FED/STREPTOZOTOCIN
TREATED RATS
Divya S. Mohan, Mini Saraswathy, Muraleedhara Kurup and Gopala Kurup*
Department of Biochemistry, University of Kerala, Kariavattom Campus,
Thiruvananthapuram-695581, Kerala, India.
Received on: 10 October 2013, accepted on: 12 December 2013
Abstract: Type 2 diabetes mel itus is a complex, heterogeneous, and polygenic disease and the major sources of morbidity
and mortality in diabetes mellitus can be attributed to the direct and indirect effects of chronic hyperglycemia on
human vasculature. Chronic hyperglycemia condition produces multiple biochemical sequels and diabetes induced
oxidative stress could play an important role in the onset and progression of the disease. The present study was designed
to simulate the natural history and metabolic characteristics of human T2DM in male Wistar rats and its treatment
with 80 % aqueous methanolic extract of Padina tetrastromatica (PME). The rats, except the controls were fed with
high calorie/energy diet for two months and then intraperitoneally injected with streptozotocin (STZ) at a dose of 15
mg/kg body weight. Effect of oral administration of graded doses of PME viz, 150, 300, 450 and 600 mg/kg body weight
for 45 days on body weight, fasting blood glucose, glycated haemoglobin, serum toxicity markers, hepatic and renal
antioxidant enzymes and concentration of lipid peroxidation products in serum and tissues were evaluated using
standard protocols. Treatment with the extract significantly decreased the blood glucose and glycated haemoglobin in
a dose dependent manner. However a more significant effect was produced by treatment with 450 mg/kg, suggesting
that it is the optimum dose for acquiring good glycemic control. Activities of various toxicity markers demonstrated the
non toxic nature of the extract at these doses. PME restored the diabetes induced alterations in the activities of
antioxidant enzymes as well as the levels of lipid peroxidation products. The present findings clearly demonstrated the
invivo antidiabetic and antioxidant activities of PME. Since no work is available in this regard, further clinical studies
are warranted to recommend P. tetrastromatica as a source of drug for the management of type II diabetes mellitus.
Key words: Type 2 diabetes mellitus, Insulin resistance, Oxidative stress, Antioxidant defence system, Lipid
peroxidation, Edible brown seaweed
INTRODUCTION
Type 2 Diabetes mellitus accounts for 90 to 95% rised by resistance to the action of insulin (in
of all the diagnosed cases, affecting 10 to 20% of liver, muscle and adipose tissue) that results in
adults in many developed countries (Engelgau an impairment of glucose uptake, leading to
et al., 2004; Bell and Polonsky, 2001). T2DM insulin hyperstimulation and subsequently to a
results from a complex interaction of multiple dysfunction of pancreatic -cells (decreasing
factors, namely genetic and environmental secretion of insulin) (Yki, 1995; Cheng and
components, such as high-energy diets and Fantus, 2005).
sedentary lifestyle (Conget, 2002). Diabetic
abnormalities include post prandial glucose When high plasma glucose concentration
production by the liver (in an unregulated persists for a long time, the physiological
fashion), diminished glucose uptake by the pathways of glucose (glycolysis and oxidative
skeletal muscle and liver, increased fatty acid phosphorylation) saturate and glucose is
generation (lipolysis) by the adipose tissue and shunted to other pathways such as polyol
increased levels of digestive enzymes and pathway, diacylglycerol pathway and
absorptive cells in the intestine (Bell and intracellular advanced glycation end-products
Polonsky, 2001). The risk to develop macro and (AGE) pathway (Nishikawa et al., 2007;
microvascular complications in T2DM is very Robertson and Harmon, 2006). All of these
high, since in addition to hyperglycaemia, there pathways lead to the increase in the production
is an abnormal lipid profile, (Smith, 2007; Boden of reactive species (reactive oxygen species –
and Pearson, 2000). Type 2 diabetes is characte- ROS and reactive nitrogen species – RNS) which
in turn increase oxidative stress and oxidative invivo antidiabetic and antioxidant activities of
damage in proteins, lipids and DNA of the cells, PME in experimental diabetes mellitus.
particularly damaging pancreatic -cells and
endothelial cells (Robertson and Harmon, 2006; MATERIALS AND METHODS
Jay et al., 2006). The control of postprandial Seaweed material: Padina tetrastromatica was
hyperglycemia and inhibition of oxidative stress collected freshly from the coastal rocks of
have often been suggested as important Vizhinjam, Thiruvananthapuram, India (Lat. 8°
measures for the treatment of diabetes.
22' N; Long. 76°59' E) during the month of
One therapeutic approach to decrease January-February. Immediately after collection,
postprandial hyperglycemia is to retard the the algae were thoroughly washed in tap water
absorption of glucose via inhibition of to remove the epiphytes, sand and other
extraneous matter. The specimen was identified
carbohydrate-hydrolyzing enzymes, such as
by Dr. M.V.N Panikkar, (Department of Botany,
-amylase and -glucosidase, in the digestive S.N.College, Kollam, Kerala, India). A voucher
organs (Yki 1990; Holman et al., 1999; Tewari et specimen (KUBH 5804) was deposited in the
al., 2003). The negative effects of oxidative stress Department of Botany, University of Kerala.
may be mitigated by antioxidants (Larson, 1995;
Halliwell et al., 1995). Due to concerns on the Chemicals: All the chemicals used were
toxic and carcinogenic effects of synthetic analytical grade reagents purchased from Sisco
chemicals, the search for alternatives from Research Laboratories Ltd, India.
natural sources has received much interest. Preparation of seaweed extracts: The
Traditionally, due to ease of access terrestrial extract was prepared according to the
sources have provided humans with these procedure of Kim et al. (2008). Briefly, the
much-needed cures. However, the oceans cover algal sample (1 kg) was soaked in MeOH: H O
70% of the earth's surface and are the habitat of
(8:2, v/v, 1l) and extracted under conditions
an extremely rich and varied fauna and flora. where the solvent was heated until reflux
Marine algae are exposed to an extreme began, this being maintained for 3 h. Each
environment in a combination of light and resulting residue was dissolved in MeOH:
oxygen, but the absence of oxidative damage in H O (8:2, v/v, 500 ml) and extracted twice as
their structural components (Matsukawa et al.,
above, with the extracts individually f iltered
1997) and their stability to oxidation during using Whatman No. 1 paper. Each combined
storage (Ramarathnam et al., 1995) suggest they supernatant was then concentrated under
have good antioxidant defence system.
reduced pressure at a temperature of 40°C,
Padina tetrastromatica Hauck is a striped, defatted with hexane and again concentrated.
yellowish brown, fan shaped alga, which grows The extract thus obtained was kept at -20°C
in the coastal rocks of tropical areas. It is used and used for present study.
as seasoning in dried flake form and as table Experimental animals: Male Wistar rats
salt replacement for high blood pressure patients from the same breed with a mean body weight
(Novaczek and Athy, 2001). Studies from our of about 100 g were selected for the study. The
laboratory reported that sulphated fucan from animals were bred and kept in the animal
P.tetrastromatica possesses anti-oxidative and house, Department of Biochemistry, University
anti-inflammatory activities against carrageenan of Kerala. They were housed in polypropylene
induced paw edema in rats (Mohsin et al., 2011). cages under standard environmental
However, to the best of our knowledge, no conditions (25±2°C; 12/12h light/ dark cycle).
reports are available regarding the antidiabetic Prior to the beginning of the experiment, all
and antioxidant activities of 80 % aqueous rats were fed with basal diet at least for one
methanolic extract of P.tetrastromatica (PME). week. Body weights of rats were recorded once
Moreover we found that PME possessed in every two weeks. All studies were conducted
disaccharidase inhibitory as well as antioxidant after obtaining prior approval f rom the
activities invitro (unpublished data). So the institutional ethics committee [IEAC-KU-22/
present study was designed to evaluate the 2010-11 BC GMK (10)].
Experimental design: Rats were divided into Diagnostics Pvt. Ltd. India. Serum urea was
six groups with six animals each. Normal determined by enzymatic method using urease
control was fed with the standard laboratory according to the procedure of Webster, (1977)
diet throughout the experimental period. All using the kit from Accurex Biomedical Pvt. Ltd.,
other groups were fed with high calorie/ India. Serum creatinine was determined by
energy diet (HCD) prepared with a slight modif ied Jeffe's method using the kit from
modif ication of Wang et al. (2007), by adding Agappe diagonostics, India (Allen et al., 1982).
20% sucrose (w/w) and 10% groundnut oil (w/ The activities of superoxide dismutase (SOD)
w) into the basal diet in order to develop and catalase (CAT) in the liver and kidney were
insulin resistance. After two months of HCD assayed as described by Kakkar et al. (1984) and
feeding, streptozotocin (STZ) at a dose of 15 Maehly and Chance, (1954) respectively. The
mg/kg (Zhang et al., 2003) in 0.1 M citrate activity of Glutathione peroxidise (GPx) was
buffer (pH4.6) was injected intraperitoneally determined by the method of Lawrence and
to induce pancreatic -cell damage. The Burk, (1976). Glutathione reductase (GRd)
diabetic rats were treated with graded doses activity was determined by the method David and
of PME viz, 150, 300, 450 and 600 mg/kg body Richard, (1983). Thiobarbituric Acid Reactive
weight for 45 days. The groupings are as Substances (TBARS) were estimated according
to the procedures of Ohkawa et al. (1979).
Statistical Analysis: The SPSS 17 statistical
: HCD + STZ (Diabetic control)
programme was employed for analysis, and the
: HCD + STZ + PME (150mg/kg
results were evaluated using one way analysis
of variance (ANOVA). The results were
: HCD + STZ + PME (300mg/kg presented as mean value ± SD. Difference
among the mean were assessed using Duncan's
: HCD + STZ + PME (450mg/kg Multiple range test. P < 0.05 was considered
signif icant.
: HCD + STZ + PME (600mg/kg
RESULTS AND DISCUSSION
At the end of the experimental period the rats An ideal experimental model for human T2DM
were fasted overnight and sacrificed. Blood and was developed following the procedures of Wang
tissues were removed to ice cold containers for et al. (2007) and Zhang et al. (2003). In this
the estimation of various biochemical model system, insulin resistance was created by
feeding HCD for two months and the pancreatic
Biochemical estimations: Blood glucose was
-cells were damaged by the intraperitoneal
determined by glucometer (One Touch injection of streptozot.
Horizon). Glycated hemoglobin (HbA ) was
Changes in body weight: In the present study
estimated according to the procedure of Trivelli we found that the high-energy feeding for two
et al. (1971) using kit purchased from Beacon months increased the body weight of animals
Diagonostics Pvt. Ltd. India. Serum glutamate (Table 1). But after the intra peritoneal injection
pyruvate transaminase (SGPT) and serum of STZ, the body weight decreased significantly.
glutamate oxaloacetate transaminase (SGOT) This was similar to the observations of Zhang et
were assayed using commercial kits purchased al. (2003). Diabetic rats showed marked reduction
from Agappe diagonostics, India according IFCC in their body weights when compared to normal
recommended procedure (Bergmeyer et al., rats, which could be due to poor glycemic control.
1976). Serum alkaline phosphatase (ALP) was The excessive catabolism of proteins to provide
estimated by DGKC-SCE procedure using the aminoacids for gluconeogenesis during insulin
kit from Agappe diagonostics, India. Serum deficiency resulted in muscle wasting and weight
gamma glutamyl transferase (GGT) was assayed loss in diabetic untreated rats (Babu et al., 2010).
according to the procedure of Szasz, (1976) Treatment with graded doses of PME
using commercial kits purchased from Reckon significantly increased the body weight of rats.
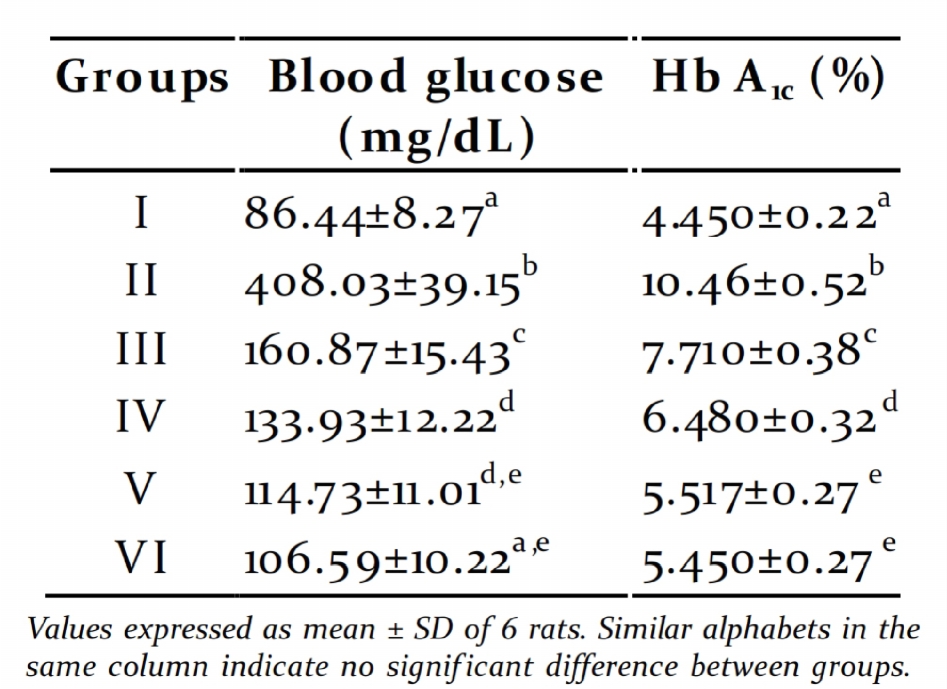
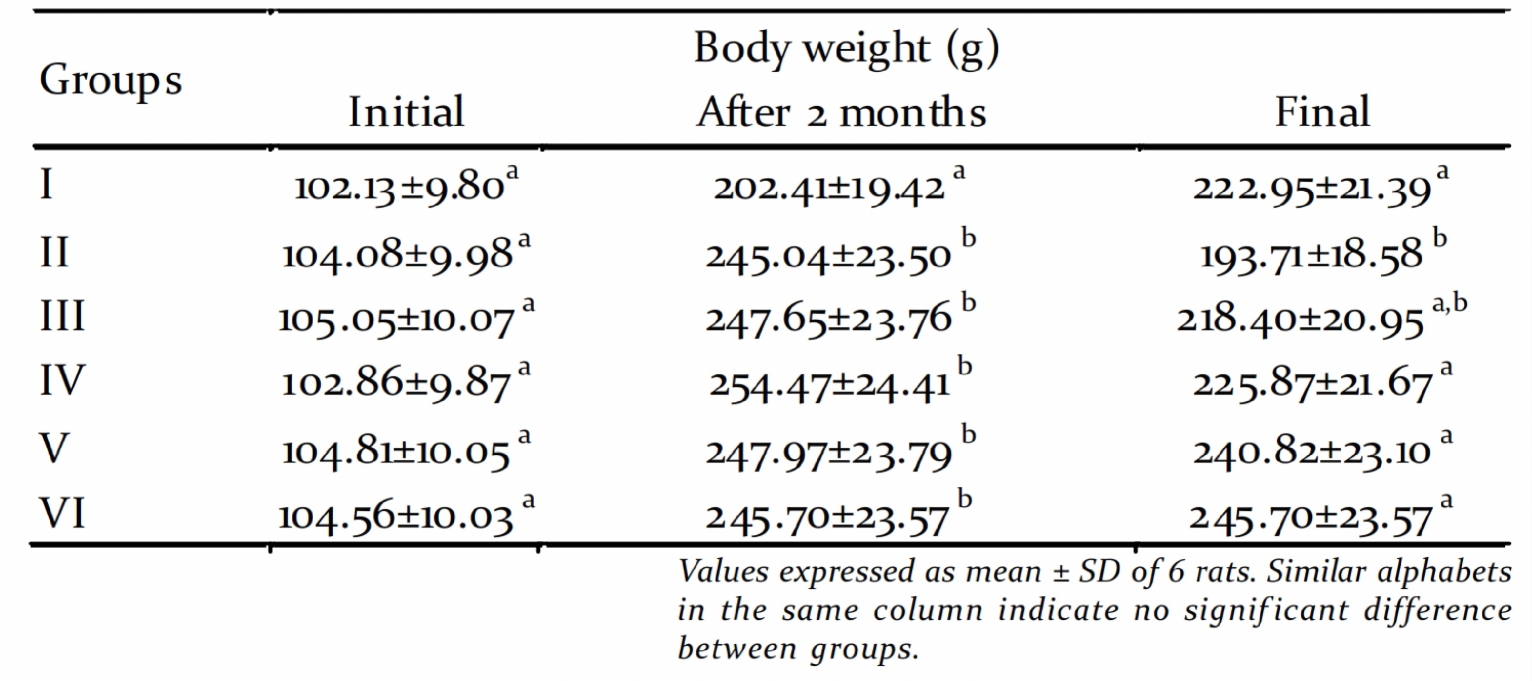
Table 1. Changes in body weight
The ability of the extract to restore body weight for acquiring good glycemic control. The
seems to be a result of its ability to reduce antihyperglycemic effect of PME can be
attributed to the presence of phenolic
compounds present in the extract. The phenolic
Fasting blood glucose and glycated compounds stimulates insulin secretion by the
haemoglobin: Two months of high energy closure of K+-ATP channels, membrane
feeding resulted fasting blood glucose around depolarization and stimulation of Ca2+ influx,
150mg/dL and upon STZ injection, the values an initial key step in insulin secretion from the
shoot up to 300-400mg/dL. This may be due to remanent -cells or from regenerated -cells
the concerted action of insulin resistance and (Sunil et al., 2012).
pancreatic -cell damage. It was observed that
treatment with graded doses of PME for 45 days Serum toxicity markers: The activities of
reduced the fasting glucose to normal level hepatic toxicity markers like GOT, GPT, ALP and
(Table 2). The percentage of glycated GGT were increased in serum of diabetic groups
haemoglobin also showed a similar pattern compared to control group (Table 3). The
(Table 2). There was no statistically significant activities decreased signif icantly in PME treated
difference in the fasting blood glucose and groups when compared to diabetic group in a
glycated haemoglobin between the groups dose dependent manner. The concentration of
treated with 450mg/kg and 600 mg/kg PME, renal toxicity markers urea and creatinine were
suggesting that 450mg/kg is the optimum dose increased in serum of diabetic groups as
compared to control group (Table 4). Their
Table 2. Fasting blood glucose and glycated
concentration decreased signif icantly in PME
treated groups when compared to diabetic
group in a dose dependent manner.
Liver is the vital organ of metabolism, detoxif i-
cation, storage and excretion of xenobiotics and
their metabolites (Rej, 1978). Serum GOT, GPT,
ALP and GGT are reliable markers of liver func-
tion. In STZ induced diabetic rats the liver was
necrotized. An increase in the activities of these
toxicity markers in serum might be mainly due
to the leakage of these enzymes from the liver
cytosol into the blood stream (Navarro et al.,
1993) which gives an indication of the hepato-
toxic effect of STZ. Treatment of the diabetic
rats with graded doses of PME reduced the
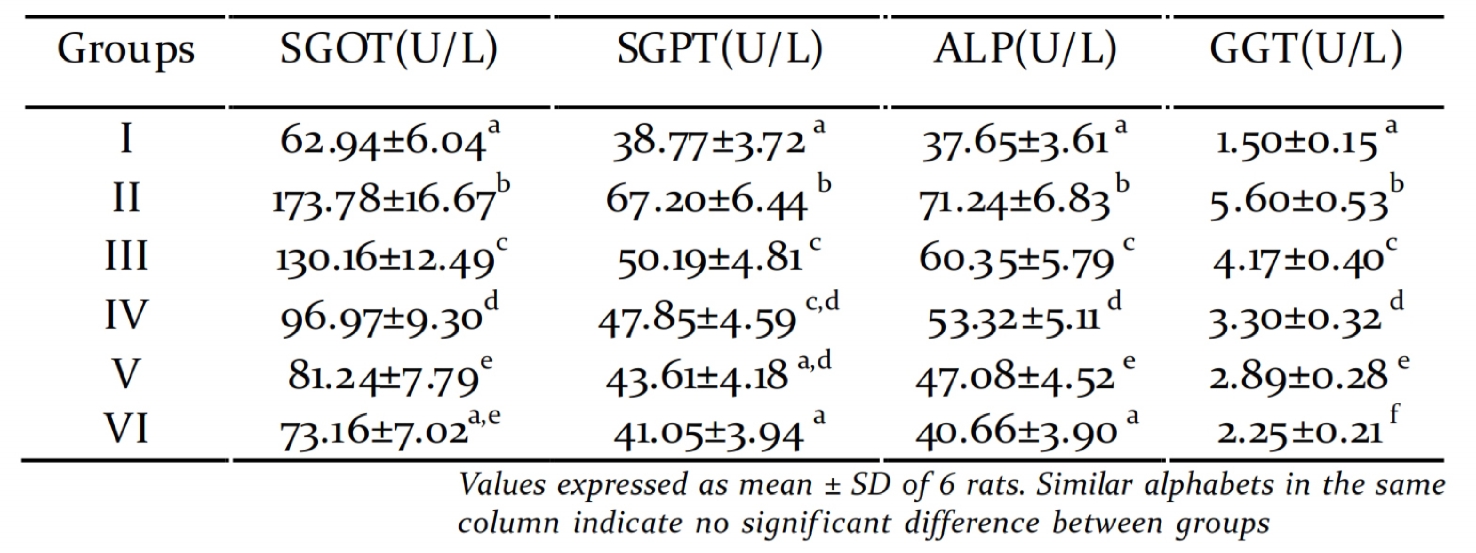
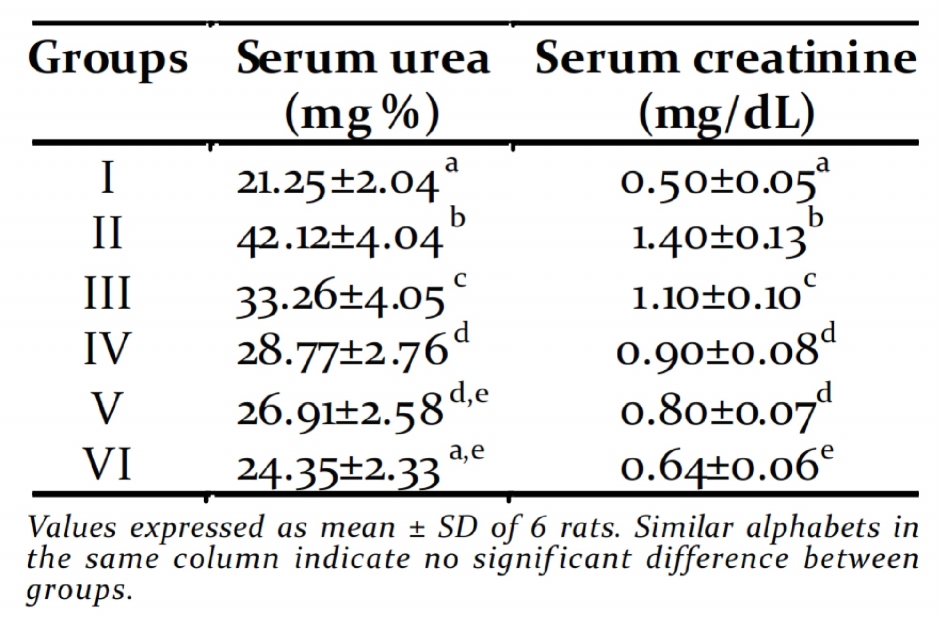
Table 3. Activity of hepatic toxicity markers
Table 4. Concentration of renal toxicity markers production of reactive oxygen species (ROS),
which can lead to increased lipid peroxidation,
alter antioxidant defense and further impair
glucose metabolism in biological system
(Balasubashini et al., 2004). SOD has been
postulated as the one of the most important
enzymes in the enzymatic antioxidant defense
system which catalyses the dismutation of
superoxide radicals to produce H O and
molecular oxygen. Catalase is a hemoprotein
which catalyses the reduction of hydrogen
peroxides and protects the tissues from highly
reactive hydroxyl radicals. Previous studies have
reported that the activity of SOD is low in
activity of these enzymes in serum when com- diabetes mellitus (Coudary et al., 1999). This is
pared to the diabetic untreated group and con- in agreement with our results of reduced
sequently alleviated liver damage caused by STZ- activities of SOD and CAT in the liver and kidney
induced diabetes. Diabetic rats showed signif i- of diabetic rats. Whereas, treatment with PME
cantly increased levels of urea and creatinine in showed a significant increase in the hepatic
the serum, which are considered as significant renal SOD and CAT. GPx plays a primary role in
markers of renal dysfunction (Bethesda, 2001). minimizing oxidative damage. Glutathione
In the present study, the significant reduction peroxidase (GPx) is an enzyme which
in the levels of serum urea and creatinine in decomposes H O or other organic
the treated diabetic rats indicated that PME
hydroperoxides to non toxic products at the
prevented the progression of renal damage in expense of reduced glutathione. Glutathione
diabetic rats.
reductase is the major enzyme responsible for
Activity of Antioxidant Enzymes
Administration of PME signif icantly restored
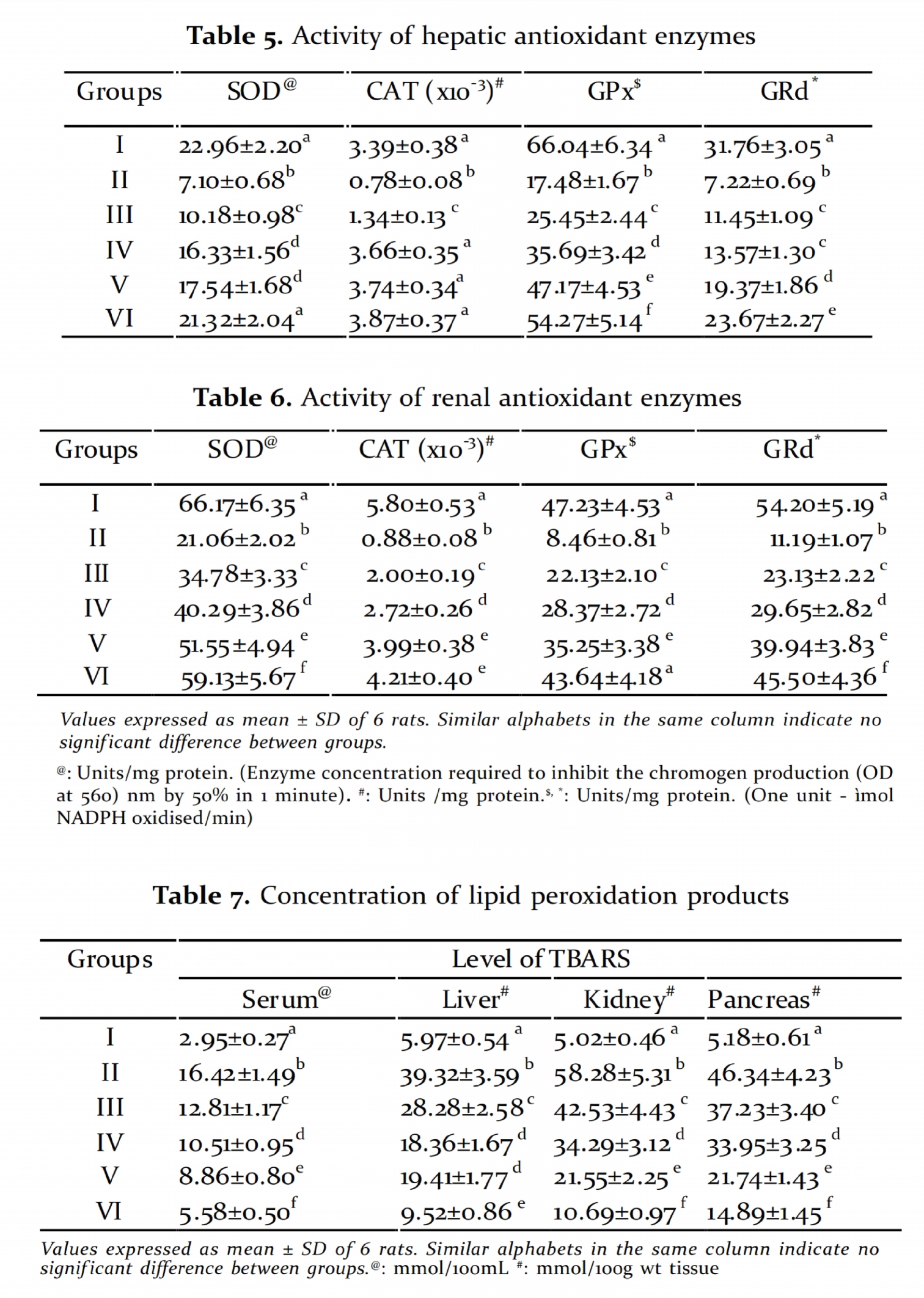
Table 5 and Table 6 show the activity of various the activities of GPx and GRd in the liver and
antioxidant enzymes in liver and kidney kidney of diabetic rats when compared to
respectively. Activity of SOD, CAT, GPx and GRd diabetic control. Thus PME improved the
showed a similar trend with decreased activity activities of all the antioxidant enzymes in
in diabetic group. Treatment with PME diabetic rats which may be due to the free radical
signif icantly increased the activity of the scavenging property of the extract.
scavenging enzymes in all groups in a dose
dependent manner.
Effect of PME on Lipid peroxidation: The level
of lipid peroxidation products is measured as thio
Hyperglycemia is a well-known cause for barbituric acid reactive substances (TBARS). The
elevated free radical levels, followed by concentration of lipid peroxidation products in
serum, liver, kidney and pancreas were increased lipid peroxidation marker, TBARS, in the diabetic
in the diabetic control group. However there is a control rats is a reflection of insuff iciency of
marked decrease in TBARS level in PME treated antioxidant defenses in combating ROS-
groups in a dose dependent manner (Table 7).
mediated damage. The present data also
demonstrated that the treatment with PME
Lipid peroxidation of unsaturated fatty acids is reduced the concentration of TBARS in the liver,
a frequently used indicator of increased kidney, pancreas and serum of diabetic animals.
oxidative stress and subsequent oxidative This activity may be due to the free radical
damage (Hauggard, 1968). The high level of scavenging effect of PME.
in the United States: National Institutes of
Health. National Institute of Diabetes and
From the present observations it was evident that
Digestive and Kidney Diseases.
PME possess significant anti hyperglycaemic as
well as antioxidant effects in a dose dependent Boden, W.E. and Pearson, T.A. 2000. Raising low
manner. However a more signif icant effect was
levels of high-density lipoprotein
produced by treatment with 450mg/kg. Activities
cholesterol is an important target of
therapy. Am. J. Cardiol., 85: 645-650.
of various toxicity markers demonstrate the non
toxic nature of the extract at these doses. In short Cheng, A.Y. and Fantus, I.G. 2005. Oral
the effect of PME was dose dependent and the
antihyperglycemic therapy for type 2
optimum dose which produced significant effect
diabetes. Can. Med. Asso. J., 172: 213-226.
was 450mg/kg body weight. The molecular Conget, I. 2002.Diagnosis, Classif ication and
mechanisms behind the observed effect and the
pathogenesis of diabetes mellitus. Rev Esp
compound responsible for this require further
Cardiol., 55: 528-535.
investigations, which are in progress.
Coudary, C.F., Rock, E. and Coudary, K. 1999.
Lipid peroxidation and antioxidant status
in experimental diabetes. Clin Chim Acta.,
This research was supported by funding from
the Kerala State Council for Science Technology David, M. and Richard, J.S. 1983. Glutathione
and Environment (KSCSTE), Government of
reductase. In: Bermeyer, Hans, Ulrich, Jr.
Kerala, India in the form of Junior Research
(Eds.), Methods of enzymatic analysis. Vol
Fellowship to Divya S. Mohan.
III 3rd edn. pp. 258–265
Engelgau, M.M., Geiss, L.S., Saaddine, J.B., Boyle,
J.P., Benjamin, S.M., Gregg, E.W., Tierney,
E.F., Rios-Burrows, N., Mokdad, A.H., Ford,
Allen, L.C., Michalko, K. and Coons, C. 1982. More
E.S., Imperatore, G. and Narayan, K.M. 2004.
on cephalosporin interference with creatinine
The Evolving Diabetes Burden in the United
States. Ann. Intern. Med., 140: 945-950.
Babu, R.K., Dilip, M.R., Kumar, V.K., Sameena, S.F., Halliwell, B., Aeschbach, R., Loliger, J. and Aruoma,
Kumar, T.E.G., Swapna, S., Ramesh, B. and
O.I. 1995. The characterization of antioxidants.
Rao, C.A. 2010. Antihyperglycemic and
Food Chem. Toxicol., 33: 601–617
antihyperlipidemic activities of methanol: Hauggard, N. 1968. Cellular mechanism of
water (4:1) fraction isolated from aqueous
oxygen toxicity. Physiol. Rev., 48: 311-373.
extract of Syzygium alternifolium seeds in Holman, R.R., Cull, C.A. and Turner, R.C. 1999.
streptozotocin induced diabetic rats. Food
A randomized double-blind trial of
Chem. Toxicol., 48: 1078–1084.
acarbose in type 2 diabetes shows improved
Balasubashini, M.S., Rukkumani, R., Viswanathan,
glycemic control over 3 years. Diabetes
P. and Venugopal, P.M. 2004. Ferulic acid
Care., 22: 960–964.
alleviates lipid peroxidation in diabetic rats. Jay, D., Hitomi, H. and Griendling, K.K. 2006.
Phytother. Res., 18: 310–314.
cardiovascular complications. Free Radic.
Bell, G.I. and Polonsky, K.S. 2001. Diabetes
Biol. Med., 40: 183-192.
mellitus and genetically programmed defects Kakkar, P., Das, B. and Viswanathan, P.N. 1984. A
in beta-cell function. Nature, 414: 788-791.
modified spectrophotometric assay of SOD.
Bergmeyer, H.U., Bowers, G.N., Horder, M. and
Indian J Biochem and Biophy., 2: 130–132.
Moss, D.W. 1976. Provisional recommendation Kim, K.Y., Nama, K.A., Kurihara, H. and Kim,
on IFCC methods for the measurement of
S.M. 2008. Potent á-glucosidase inhibitors
catalytic concentrations of enzymes. Part 2.
purif ied from the red alga Grateloupia
IFCC method for aspartate aminotransferase.
elliptica. Phytochem., 69: 2820-2825.
Clin. Chim. Acta., 70: 19-42.
Larson, R.A. 1995 Plant defenses against
Bethesda, M.D. 2001. US Renal Data System. Annual
oxidative stress. Arch Insect. Biochem.
Data Report: Atlas of End Stage Renal Disease
Physiol., 29: 175–186
Lawerence, R.A. and Burk, R.F. 1976. Glutath-
double jeopardy for the pancreatic islet beta
ione peroxidase activity in selenium
cell. Free Radic. Biol. Med., 41: 177-184.
def icient rat liver. Biochem Biophy Res Smith, S.C. Jr. 2007. Multiple risk factors for
Commun., 71: 952-958.
cardiovascular disease and diabetes
Maehly, A.C. and Chance, B. 1954. The assay of
mellitus. Am. J. Med., 120: 3-11.
CAT and peroxides. Methods Biochem. Sunil, C., Duraipandiyan, V., Agastian, P. and
Anal., 1: 357–424.
Ignacimuthu, S. 2012. Antidiabetic effect of
Matsukawa, R., Dubinsky, Z., Kishimoto, E.,
plumbagin isolated from Plumbago zeylanica
Masaki, K., Matsuda, Y., Takeuchi, T.,
L. root and its effect on GLUT4 translocation
Chihara, M., Yamamoto, Y., Niki, E. and
in streptozotocin-induced diabetic rats. Food
Karube, I. 1997. A comparison of screening
and Chem. Toxicol., 50: 4356–4363.
methods for antioxidant activity in Szasz, G. 1976. Reaction rate method for gamma-
seaweeds. J. Appl. Phycol., 9:29–35
glutamyl transferase activity in serum. Clin.
Mohsin, S., Kurup, G.M. and Rauf, A.A. 2011.
Chem., 22: 2051-2055.
Anti inflammatory and antioxidant effect Tewari, N., Tiwari, V.K., Mishra, R.C., Tripathi,
of sulphated polysaccharide isolated from
R.P., Srivastava, A.K., Ahmad, R., Srivastava,
marine algae Padina tetrastromatica from
R. and Srivastava, B.S. 2003. Synthesis and
Kerala coast. Journal of Pharm. Res.,
bioevaluation glycosyl ureas as alpha-
4(3): 784-788.
glucosidase inhibitors and their effect on
Navarro, M.C., Montilla, M.P., Martin, A.,
mycobacterium. Bioorg Med. Chem.,
Jimenez, J. and Utrilla, M.P. 1993. Free
11: 2911–2922.
radicals scavenger and antihepatotoxic Trivelli, L.A., Ranney, H.M. and Lai, H.T. 1971.
activity of Rosmarinus tomentosus. Plant
Hemoglobin components in patients with
Med., 59: 312–314.
diabetes mellitus. N. Engl. J. Med.,
Nishikawa, T., Kukidome, D., Sonoda, K.,
284: 353-357.
Fujisawa, K., Matsuhisa, T., Motoshima, H., Wang, H.J., Jin, Y.X., Shen, W., Neng, J., Wu,
Matsumura, T. and Araki, E. 2007. Impact
T., Li, Y.J. and Fu, Z.W. 2007. Low dose
of mitochondrial ROS production in the
streptozotocin (STZ) combined with high
pathogenesis of insulin resistance. Diabetes
energy intake can effectively induce type 2
Res. Clin. Pract., 77: 161-164.
diabetes through altering the related
Novaczek, I. and Athy, A. 2001. Sea vegetable
gene expression. Asia Pac. J. Clin. Nutr., 16
recipes for the Pacific Islands. USP Marine
(Suppl 1): 412-417.
Studies Programme/SPC Coastal Fisheries Webster, D. 1977. The immediate reaction
Programme: Training Materials for Pacific
between bromcresol green and serum as a
measure of albumin content. Clin. Chem.,
Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay
of lipid peroxides in animal tissue by thio Yki, J.H. 1990. Acute and chronic effects of
barbituric acid reaction. Anal. Biochem.,
hyperglycemia on glucose metabolism.
95: 351–358.
Diabetologia., 33: 579–85.
Ramarathnam, N., Osawa, T., Ochi, H. and Yki, J.H. 1995. Role of insulin resistance in the
Kawakashi, S. 1995. The contribution of
pathogenesis of NIDDM. Diabetologia,
plant food antioxidants to human health.
38: 1378-1388.
Trends Food Sci. Technol., 6:75–82.
Zhang, F., Ye, C., Li, G., Ding, W., Zhou, W.,
Rej, R. 1978. Aspartate aminotransferase activity
Chen, G., Luo, T., Guang, M., Liu, Y., Zhang,
and isoenzyme proportions in human liver
D., Zheng, S., Yang, J., Gu, Y., Xie, X. and
tissues. Clin. Chem., 24: 1971–1979.
Luo, M. 2003. The rat model of type 2
Robertson, R.P. and Harmon, J.S. 2006. Diabetes,
diabetes mellitus and its glycometabolism
glucose, and oxidative stress: A case of
characters. Exp Anim., 52: 401-407.
Source: http://keralamarinelife.in/Journals/Vol2-2/22.pdf
2014 Fall Newsletter Friends of IWIRC NY, In This Edition Fall is here and along with the seasons, things are changing over at IWIRC NY! It is election time, when half of our board will roll off and make way for new members and fresh ideas. While we are sad to see our parting board members go, we are excited to work with what looks to be an impressive field of candidates!
Journal of Neuroscience Research 87:1484–1499 (2009) The Action of Pulse-Modulated GSMRadiation Increases Regional Changesin Brain Activity and c-Fos Expressionin Cortical and Subcortical Areas in aRat Model of Picrotoxin-InducedSeizure Proneness E. Lo´pez-Martı´n,1* J. Bregains,2 J.L. Relova-Quinteiro,3 C. Cadarso-Sua´rez,4F.J. Jorge-Barreiro,1 and F.J. Ares-Pena21Morphological Sciences Department, University of Santiago de Compostela, Santiago de Compostela,Spain









