Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Rccm2011081553oc 48.55
Blood Eosinophils to Direct Corticosteroid Treatment of
Exacerbations of Chronic Obstructive Pulmonary DiseaseA Randomized Placebo-Controlled Trial
Mona Bafadhel1, Susan McKenna1, Sarah Terry1, Vijay Mistry1, Mitesh Pancholi1, Per Venge2,David A. Lomas3, Michael R. Barer1, Sebastian L. Johnston4, Ian D. Pavord1, and Christopher E. Brightling1
1Institute for Lung Health, University of Leicester, Leicester, United Kingdom; 2Department of Medical Sciences, Clinical Chemistry, University ofUppsala, Uppsala, Sweden; 3Cambridge Institute for Medical Research, University of Cambridge, Cambridge, United Kingdom; and 4Department ofRespiratory Medicine, National Heart and Lung Institute, Centre for Respiratory Infections, Imperial College London, London, United Kingdom
Rationale: Exacerbations of chronic obstructive pulmonary disease(COPD) and responses to treatment are heterogeneous.
AT A GLANCE COMMENTARY
Objectives: Investigate the usefulness of blood eosinophils to directcorticosteroid therapy during exacerbations.
Scientific Knowledge on the Subject
Methods: Subjects with COPD exacerbations were entered into a
Current guidelines advocate systemic corticosteroids during
randomized biomarker-directed double-blind corticosteroid versus
exacerbations of COPD, but treatment responses are het-
standard therapy study. Subjects in the standard arm received prednis-
erogeneous, efficacy is marginal, and the treatment is not
olone for 2 weeks, whereas in the biomarker-directed arm, predniso-
without harm. Airway eosinophilia is associated with cor-
lone or matching placebo was given according to the blood eosinophil
ticosteroid responsiveness in COPD, and the peripheral
count biomarker. Both study groups received antibiotics. Blood eosi-
blood eosinophil count is a sensitive and specific biomarker
nophils were measured in the biomarker-directed and standard ther-
for airway eosinophilia during COPD exacerbations.
apy arms to define biomarker-positive and -negative exacerbations(blood eosinophil count . and < 2%, respectively). The primaryoutcome was to determine noninferiority in health status using the
What This Study Adds to the Field
chronic respiratory questionnaire (CRQ) and in the proportion of
A biomarker-directed treatment strategy using the peripheral
exacerbations associated with a treatment failure between subjectsallocated to the biomarker-directed and standard therapy arms.
blood eosinophil count to guide corticosteroid prescription
Measurements and Main Results: There were 86 and 80 exacerba-
can be safely used to treat exacerbations of COPD. Whether
tions in the biomarker-directed and standard treatment groups,
this peripheral blood eosinophil biomarker can be used in
respectively. In the biomarker-directed group, 49% of the exacerba-
severe exacerbations requiring hospitalization warrants fur-
tions were not treated with prednisolone. CRQ improvement after
ther investigation.
treatment in the standard and biomarker-directed therapy groupswas similar (0.8 vs. 1.1; mean difference, 0.3; 95% confidence inter-val, 0.0–0.6; P ¼ 0.05). There was a greater improvement in CRQ in
treatment failures occurred in 15% given prednisolone and 2% of
biomarker-negative exacerbations given placebo compared with
those given placebo (P ¼ 0.04).
those given prednisolone (mean difference, 0.45; 95% confidence
Conclusions: The peripheral blood eosinophil count is a promising
interval, 0.01–0.90; P ¼ 0.04). In biomarker-negative exacerbations,
biomarker to direct corticosteroid therapy during COPD exacerba-tions, but larger studies are required.
Clinical trial registered with www.controlled-trials.com (ISRCTN92422949).
(Received in original form August 27, 2011; accepted in final form March 8, 2012)
Supported by the Medical Research Council (UK) and AstraZeneca jointly as
Keywords: chronic obstructive pulmonary disease; exacerbations; prednis-
a "Biomarker Call Project." C.E.B. is a Wellcome Trust Senior Clinical Fellow,
olone; infection; eosinophils
and the research was performed in laboratories partially funded by the EuropeanRegional Development Fund grant ERDF 05567.
Acute exacerbations of chronic obstructive pulmonary disease
The Medical Research Council, Wellcome Trust, and the European Regional De-
(COPD) are associated with substantial morbidity and mortality
velopment Fund had no involvement in the design of the study, data collection,
(1, 2) and are heterogeneous with respect to inflammation (3, 4)
analysis and interpretation of the data, writing of the manuscript, or the decision
and etiology (5–7). Although primarily associated with asthma,
to submit the manuscript.
eosinophilic airway inflammation is present in some patients
Author Contributions: S.M. and S.T. were involved in the recruitment of volunteers
with COPD (8). Previous studies have shown that a sputum
and in data collection. V.M. and M.P. were involved in data collection and inter-
eosinophilia is associated with a positive response to corticoste-
pretation. M.R.B., D.A.L., S.L.J., P.V., and I.D.P. were involved in the design of the
roid treatment in stable COPD (9–11), and the sputum eosino-
study and data collection and interpretation. M.B. and C.E.B. were involved in
phil count can be used to titrate corticosteroid therapy to reduce
the study design, volunteer recruitment, data collection, data interpretation, anddata analysis, had full access to the data, and are responsible for the integrity of
exacerbations of COPD (12).
the data and final decision to submit. All authors contributed to the writing of the
Current guidelines advocate the use of systemic corticosteroids
manuscript and have approved the final version for submission.
during acute exacerbations of COPD because of improvements in
Correspondence and requests for reprints should be addressed to Mona Bafadhel,
the rate of recovery (13, 14); this is despite being associated with
M.B.Ch.B., Institute for Lung Health, Clinical Sciences Wing, University Hospitals
significant side effects (15) and with limited benefits in reducing
of Leicester, Leicester, LE3 9QP, UK. E-mail:
mortality (14). Increased eosinophilic airway inflammation has
This article has an online supplement, which is accessible from this issue's table of
been shown to occur during exacerbations of COPD, and we have
shown that the peripheral blood eosinophil count is a valid bio-
Am J Respir Crit Care Med
Vol 186, Iss. 1, pp 48–55, Jul 1, 2012
marker of this pattern of inflammation (16). We hypothesized that
Copyright ª 2012 by the American Thoracic Society
the peripheral blood eosinophil count can be used to direct sys-
Originally Published in Press as DOI: 10.1164/rccm.201108-1553OC on March 23, 2012Internet address: www.atsjournals.org
temic corticosteroid treatment during an exacerbation of COPD
Bafadhel, McKenna, Terry, et al.: Biomarker-directed Corticosteroid Therapy in COPD Exacerbations
resulting in reduced total exposure to systemic corticosteroids
arm to have 80% power at the 5% level. This also provided 95% power at
without adversely affecting the outcome of treatment. To test this
the 5% level to show a 50% reduction in exacerbations requiring cortico-
hypothesis we undertook a noninferiority study of patients ran-
steroid therapy, using an exacerbation frequency (SD) of 2.8 (1.7) per year.
domized to biomarker-directed corticosteroid therapy versus stan-
To exclude a change in the proportion of treatment failure of 20%, from10 to 30%, between treatment arms, 60 exacerbations in each arm would
dard care in patients presenting with an exacerbation of COPD.
have a power of 90% at the 5% level. Secondary analysis of health status,symptom scores, lung function, and treatment failures was performed in
(1) blood eosinophil biomarker-positive and biomarker-negative exacer-bations, (2) blood eosinophil biomarker-negative exacerbations prescribed
Participants and Study Design
prednisolone and placebo, and (3) blood eosinophil biomarker-positive
Subjects with COPD were recruited consecutively from general respi-
and -negative exacerbations prescribed prednisolone. Subjects could only
ratory clinics at the Glenfield Hospital, Leicester (UK) to enter a ran-
be randomized into the study once, but multiple captured exacerbations
domized biomarker-directed double-blind corticosteroid therapy versus
were treated as independent events.
standard care study, wherein the peripheral blood eosinophil count at
Further methodology details are available in the online supplement.
exacerbation was used to guide corticosteroid treatment in thebiomarker-directed arm. At exacerbation, subjects were randomized
by minimization (17) for baseline lung function, exacerbation fre-quency, and sputum eosinophil count and followed up at 2 (post-
One hundred sixty-four subjects were recruited to enter the study
therapy) and 6 (recovery) weeks after exacerbation (see Figure E1 in
(107 men, 57 women). One hundred nine consecutive subjects with
the online supplement). Randomization and minimization were per-
166 exacerbation events were captured during the study period; 55
formed by an independent clinical team. Subjects and study personnel
and 54 subjects with 86 and 80 exacerbation events, respectively,
involved in data collection and treatment failure assessment were
were randomized to the biomarker-directed and standard therapy
blinded to randomization, biomarker results, and treatment allocation.
arm, as shown in Figure 1. There were 66, 32, 8, and 3 subjects who
Subjects in the biomarker-directed group received a 30-mg predniso-
subsequently had one, two, three, and four captured exacerbations.
lone capsule once daily or identical-appearing placebo for 14 days
There were no differences in the clinical characteristics between
when the peripheral blood eosinophil count was greater than 2% andless than or equal to 2%, respectively. This cut-off was derived with
subjects who were randomized or not (Table E1) or between sub-
a high sensitivity aimed to ensure prednisolone treatment in all subjects
jects in the biomarker-directed and standard therapy arm (Table 1).
with a sputum eosinophilia (16). Subjects in the standard group re-
There were 10 severe exacerbations requiring hospitalization. A
ceived a 30-mg prednisolone capsule once daily irrespective of the
sputum eosinophil, virus, and bacteria culture positive-associated
blood eosinophil biomarker results. All subjects received open-labeled
exacerbation was identified in 17, 32, and 42% of all exacerbations,
broad-spectrum oral antibiotic therapy (amoxicillin, or doxycycline if
respectively. There were no differences in the proportions of spu-
amoxicillin allergic) for 7 days. Blood eosinophils were measured at ex-
tum eosinophil-associated, virus-associated, and bacteria culture
acerbation to define blood eosinophil biomarker-positive and -negative
positive-associated exacerbations in the biomarker-directed and
subjects in both study groups (peripheral blood eosinophil levels < 2%
standard therapy arm at randomization.
termed biomarker negative; peripheral blood eosinophil levels . 2%termed biomarker positive), but these results were not disclosed. Exacer-bation visits were defined according to the criteria of Anthonisen and
colleagues (18) and healthcare use (19), and all subjects were given daily
The primary outcome of noninferiority of health status in the
diary cards to complete (20). Data sampling and randomization were only
standard therapy and biomarker-directed groups after 2 weeks
obtained in subjects who were confirmed as having COPD exacerbations
of treatment was achieved (CRQ mean score change, 0.8 vs.
and were treatment naive. At all study visits, the following measurements
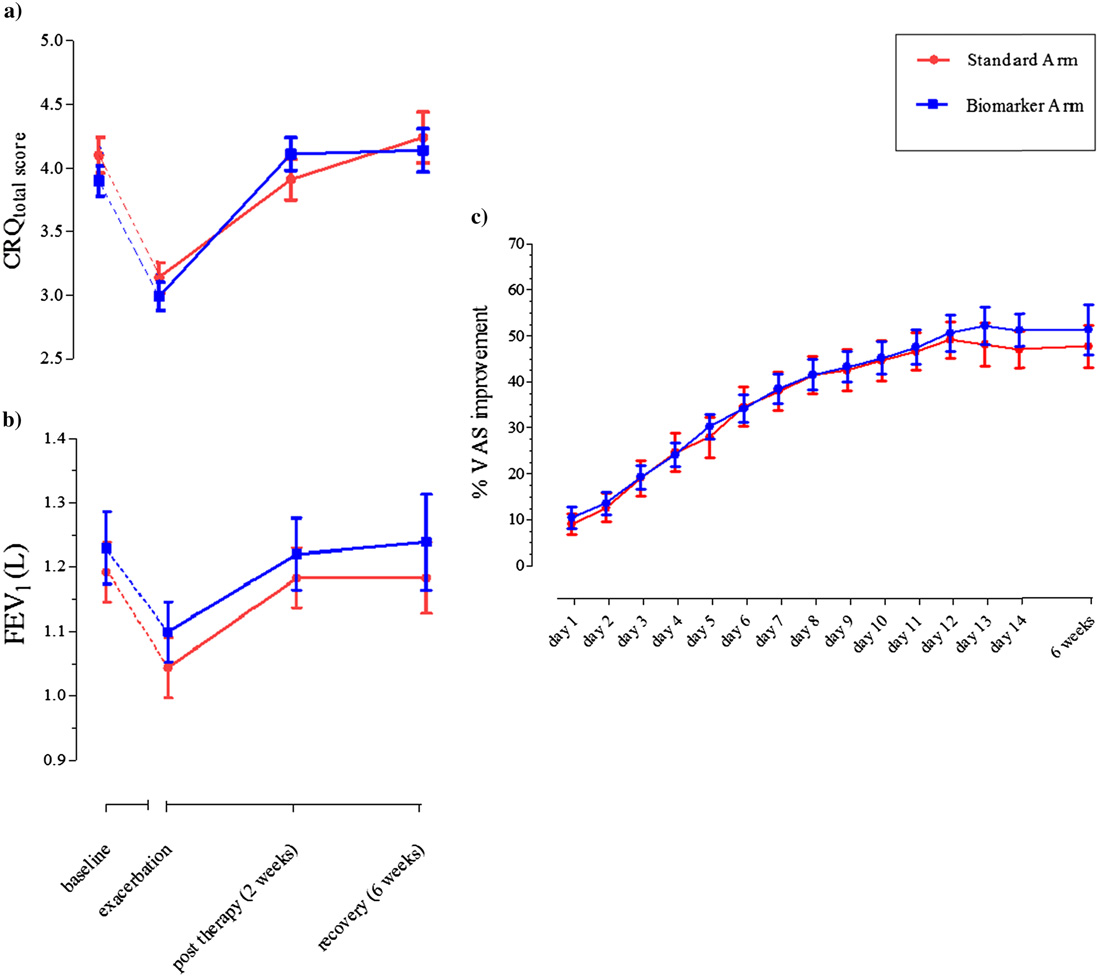
1.1; mean difference, 0.3; 95% CI, 0.0–0.6; P ¼ 0.05; Figure
were undertaken: pre- and post-bronchodilator spirometry; health qualityquestionnaires using the Chronic Respiratory Disease Interviewer-
2a). There was a similar reduction in the CRQ score from base-
Administered Standardized Questionnaire (CRQ) (21) (McMaster Univer-
line to exacerbation in the biomarker-directed and standard
sity, Hamilton, Canada); symptom assessment of cough, breathlessness,
therapy arms (0.9 vs. 0.9; mean difference, 0.0; 95% CI, 20.3
sputum production, and sputum purulence using the visual analog scale
to 0.3; P ¼ 0.97). There was no difference in FEV1 or % VAS
(VAS) (22); blood for measurement of cell differential and C-reactive
improvement between biomarker-directed and standard ther-
protein; and sputum for analysis of bacteria, colony-forming units (CFU),
apy arms after treatment allocation (Figures 2b and 2b). There
virus, and sputum cell differential (23–26). All subjects gave informed
were 14 treatment failures associated with worsening symptoms
written consent, and the study was approved by the local ethics committee
of COPD after treatment during the study; 10 occurred in the
and the Medicines and Healthcare Products Regulatory Agency.
standard arm and 4 in the biomarker-directed arm, demonstrat-ing at least equivalence with a trend favoring the biomarker-
Statistical Analysis
directed arm as there were fewer treatment failures (13 vs. 5%;
Statistical analysis was performed using PRISM version 4 (GraphPad Soft-
95% CI, 21 to 16; P ¼ 0.07). In the biomarker-directed group,
ware, San Diego, CA) and SPSS version 16 (SPSS, Inc., Chicago, IL). Para-
49% of the exacerbations were not treated with prednisolone.
metric and nonparametric data are presented as mean (SEM) and median
There were similar proportions of subjects within the standard
(interquartile range), unless stated otherwise. Log-transformed data are
therapy group and the biomarker-directed therapy group that
presented as geometric mean (95% confidence interval [CI]). The primary
had one exacerbation (35 vs. 31), two exacerbations (13 vs. 19),
objective of the study was to assess whether the blood eosinophil count can
three exacerbations (5 vs. 3), and four exacerbations (1 vs. 2).
be used as a biomarker to direct corticosteroid therapy at the onset of anexacerbation. The primary outcome was to show (1) noninferiority in thehealth status score after treatment between the standard therapy and
Secondary Analysis
biomarker-directed therapy study groups; (2) equivalence in the propor-
There were 85 exacerbations that were blood eosinophil bio-
tions of exacerbations associated with a treatment failure defined as the
marker positive given prednisolone, 39 exacerbations that were
need to start or repeat treatment within 30 days of randomization, hospi-
blood eosinophil biomarker negative given prednisolone, and 42
talization for any cause, or death, between the standard therapy andbiomarker-directed therapy study groups; and (3) demonstration of a re-
exacerbations that were blood eosinophil biomarker negative
duction in corticosteroid therapy prescription in the biomarker-directed
given placebo. Changes in clinical characteristics for biomarker-
therapy study group. To demonstrate noninferiority in health reported
positive and -negative exacerbations in the biomarker-directed
outcomes after 14 days of treatment, using the minimally clinical important
and standard treatment arms at stable, exacerbation, post-
CRQ mean change of 0.5 (SD, 0.91), 53 subjects were required in each
therapy, and recovery visits are presented in Table E2.
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Figure 1. CONSORT diagram for patient enrollment and randomization. Biomarker blood eosinophil levels were measured at exacerbation in bothstudy groups, but only in the biomarker-directed arm were biomarker levels used to direct placebo or matching prednisolone treatment in additionto antibiotic therapy. In the standard arm, all subjects received prednisolone and antibiotic therapy. Four subjects in the biomarker-directed treatmentarm switched from placebo to prednisolone treatment, and two subjects switched from prednisolone to placebo. Subjects and study personnel involvedin data collection and assessment of treatment failure were blinded to study group allocation, biomarker results, and treatment allocation.
Blood eosinophil biomarker-negative and -positive exacerba-
eosinophil count (>3% nonsquamous cells) at exacerbation. For
tions. Baseline and exacerbation health status, lung function,
all exacerbation events captured, the cutoff of 2% blood eosino-
and airway inflammation characteristics in blood eosinophil
phil count had a positive predictive value of 91% for identifying
biomarker-positive and biomarker-negative exacerbations are
a sputum eosinophilia of greater than or equal to 3%.
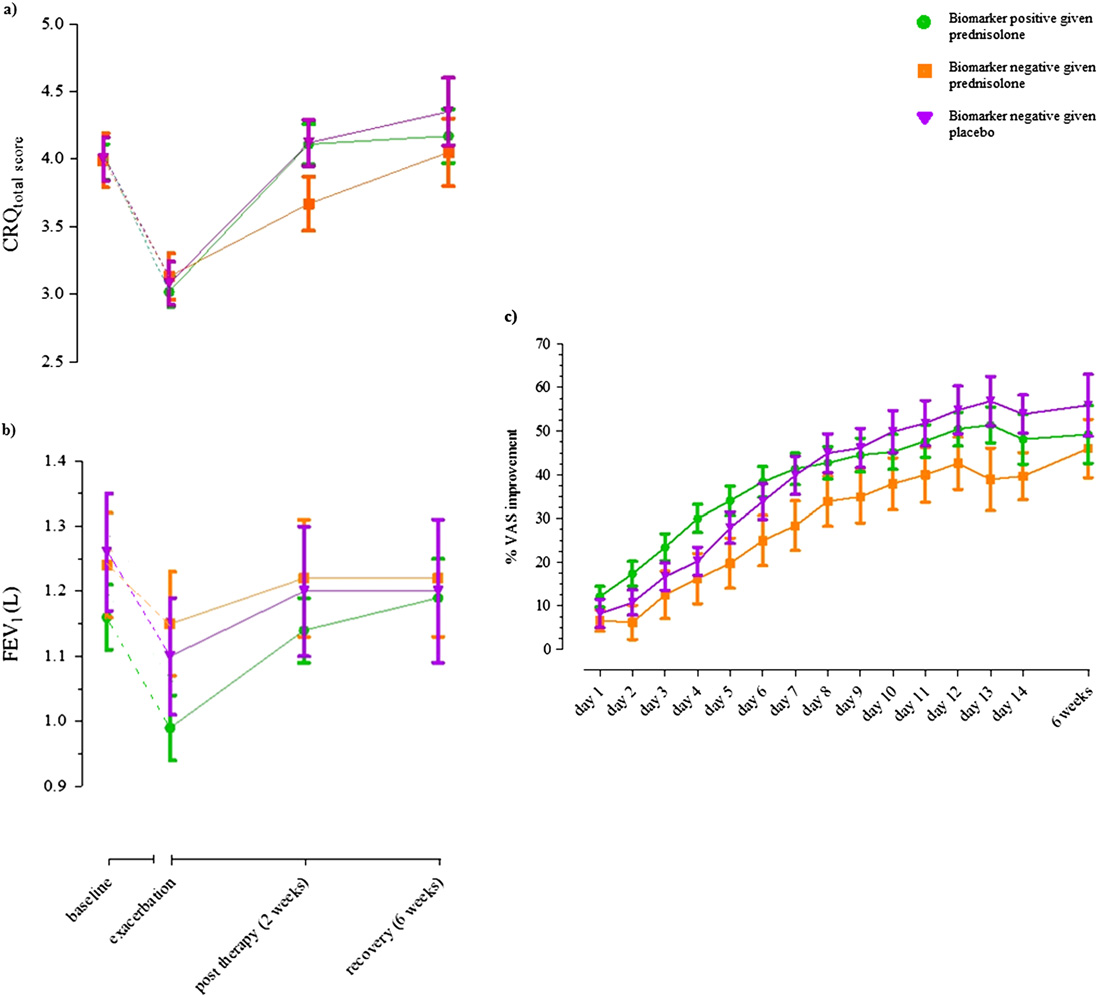
presented in Table 2. The mean reduction in CRQ from base-
Blood eosinophil biomarker-negative exacerbations prescribed
line to exacerbation was similar between biomarker-positive
prednisolone and placebo. Biomarker-negative exacerbations
and -negative exacerbations (CRQ units, 1.0 vs. 0.9; mean dif-
given placebo compared with those given prednisolone had
ference, 0.1; 95% CI, 20.2 to 0.3; P ¼ 0.54). At exacerbation,
greater improvements in CRQ score after 14 days of treatment
blood eosinophil biomarker-negative exacerbations had higher
(mean change in CRQ [units], 1.01 vs. 0.56; mean difference,
sputum neutrophils, sputum total cell counts, serum CRP, and
0.45; 95% CI, 0.01–0.90; P ¼ 0.045; Figure 3a). There were
FEV1% predicted compared with blood eosinophil biomarker-
positive exacerbations (mean [SEM] sputum neutrophils, 86 [2]
biomarker-negative exacerbations given prednisolone than pla-
vs. 78% [3], P ¼ 0.03; geometric mean [95% CI] sputum
cebo (15 vs. 2% [95% CI, 1–25], P ¼ 0.04). There was no differ-
total cell counts 3 106 cells/g, 9.2 [6.5–13.0] vs. 5.4 [3.9–7.5],
ence in FEV1 for these groups (Figure 3b). The proportion of
P ¼ 0.03; median [interquartile range] CRP mg/L, 20 [49] vs.
exacerbations with no improvement in symptoms after 7 days of
9 [22], P , 0.01; mean [SEM] FEV1% predicted, 46 [2] vs. 39
treatment was higher in biomarker-negative treated with prednis-
[2]; P ¼ 0.03). There was a significant difference in absolute and
olone compared with biomarker-negative treated with placebo
percentage blood eosinophil counts at baseline, exacerbation,
(21 vs. 4% [95% CI, 0–31], P ¼ 0.03). In biomarker-negative
post-therapy, and recovery between biomarker-positive and -neg-
exacerbations treated with prednisolone or placebo, there were
ative exacerbations (for each visit between groups, P , 0.01;
no differences in the proportions of those associated with bacteria
Table 3 and Table E2). There were similar proportions of
(44 vs. 49%, P ¼ 0.70) or virus (36 vs. 38%, P ¼ 0.87).
bacteria-associated biomarker-positive and biomarker-negative
Blood eosinophil biomarker-positive and -negative exacerbations
exacerbations (38 vs. 46%, P ¼ 0.31) and virus-associated
prescribed prednisolone. There was a statistical and clinically signif-
biomarker-positive and -negative exacerbations (26 vs. 37%, P ¼
icant difference in the CRQ improvement after prednisolone therapy
0.16). The colony forming units (CFU) at exacerbation were sig-
in blood eosinophil biomarker-positive compared with biomarker-
nificantly higher in biomarker-negative exacerbations compared
negative exacerbations (mean improvement in CRQ [units], 1.11
with biomarker-positive exacerbations (CFU cells/ml geometric
vs. 0.56; mean difference, 0.56; 95% CI, 0.15–0.96; P , 0.01). There
mean [95% CI], 1.1 3 107 [6.2 3 106 to 1.9 3 107] vs. 2.9 3 106
was no difference in treatment failure rates between the biomarker-
[1.6 3 106 to 5.3 3 106]; P ¼ 0.002). A sputum eosinophil–asso-
positive and -negative exacerbations treated with prednisolone (8
ciated exacerbation was found in more biomarker-positive than
vs. 15%; 95% CI, 210 to 43; P ¼ 0.23). There was a greater recov-
biomarker-negative exacerbations (31 vs. 2%, P , 0.001),
ery over 14 days in biomarker-positive exacerbations treated with
whereas only one patient treated with placebo had a sputum
prednisolone compared with biomarker-negative exacerbations
Bafadhel, McKenna, Terry, et al.: Biomarker-directed Corticosteroid Therapy in COPD Exacerbations
TABLE 1. CLINICAL CHARACTERISTICS OF PATIENTS IN RANDOMIZED PLACEBO CONTROLLED-TRIAL
Current smoker, n (%)
Pack-year history†
Exacerbation frequency in previous yr†
Body mass index, kg/m2
Inhaled corticosteroid usage, n (%)
Inhaled corticosteroid dose, mg‡
FEV1/FVC ratio, %k
Reversibility, ml
Sputum total cell count, x 106/g¶
Sputum neutrophils, %
Sputum eosinophils, %¶
Sputum eosinophil–associated exacerbation, %
Virus-associated exacerbation, %
Bacteria-associated exacerbation, %
Definition of abbreviations: CI ¼ confidence interval; CRQ ¼ Chronic Respiratory Disease Questionnaire, scores range
between 1 and 7 with higher score representing better health quality; VAS ¼ Visual Analog Scale, performed on 100-mmline from "no symptoms" to "worst symptoms." Higher scores represent worse symptoms (total score addition of measureddomains: cough, dyspnea, sputum production, and sputum purulence).
Data presented as mean (SD), unless otherwise stated.
* t Test or Mann-Whitney for continuous variables or x2 for proportions.
y Mean (range).
x Median (interquartile range).
z Beclomethasone dipropionate equivalent.
k Post-bronchodilator.
¶ Geometric mean (95% CI).
treated with prednisolone (area under the % change in VAS curve
treatment with corticosteroids, was not associated with an in-
[95% CI], 516 [449–583] vs. 350 [241–458]; P , 0.01) (Figure 3c).
crease in treatment failure or worsening of symptoms compared
Biomarker phenotype stability. The blood eosinophil biomarker
with standard conventional therapy. More important, we have
status at baseline had an odds ratio (OR) (95% CI) of 5.5 (2.7–11.0)
shown that a biomarker-directed strategy using the peripheral
for predicting the blood eosinophil biomarker status at exacerba-
blood eosinophil count can safely reduce prednisolone prescrip-
tion; specifically, blood eosinophil biomarker negative at baseline
tion at exacerbations. There was a trend for outcomes to be bet-
had an OR of 2.9 (1.6–5.0) for a blood eosinophil biomarker-
ter in the group randomized to biomarker-directed treatment
negative exacerbation, and blood eosinophil biomarker-positive
versus standard care. Critically, in the subgroup of patients
at baseline had an OR 2.2 (1.5–3.2, P , 0.01) for a blood eosinophil
who were blood eosinophil biomarker negative, corticosteroid
biomarker-positive exacerbation. A blood eosinophil biomarker-
treatment resulted in worse outcomes compared with placebo.
negative status at baseline was identified in 59% of all subjects
These findings make it very unlikely that we have missed an im-
randomized. In the biomarker-directed group, 80% of patients
portant difference in outcome in favor of standard, non–
who were initially assigned prednisolone therapy would have
been assigned prednisolone from the baseline blood eosinophil
A peripheral blood eosinophilia has been previously shown to
count. Similarly, 59% of patients assigned to placebo at exac-
be associated with an increase in all-cause mortality in patients
erbation would have been assigned this treatment from the
with airways disease (27–29), and we have previously shown that
baseline blood eosinophil count. In subjects with repeated ex-
the peripheral blood eosinophils are a highly sensitive and spe-
acerbation events, comparison of the first and second exacer-
cific marker of a sputum eosinophilia during exacerbations of
bation event demonstrated that 22% switched biomarker
COPD (16). It is an attractive biomarker to use in clinical prac-
status (from blood eosinophil biomarker negative to bio-
tice as it is simple to measure, widely available at the time of an
marker positive or vice versa), whereas the remainder stayed
exacerbation, and reliable. Current guidelines advocate the use
in the same blood eosinophil biomarker group.
of corticosteroids during exacerbations in patients who haveincreasing symptoms of breathlessness (14). Although studies
have shown that corticosteroids can improve lung function
In this study we have shown that a biomarker-directed strategy,
and dyspnea scores in the short term (13), these improvements
which used the peripheral blood eosinophil count to guide
are marginal (30) and need to be weighed against the potential
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Figure 2. Standard
group (red) and biomarker-directed therapy group (blue).
(a) Chronic Respiratory DiseaseQuestionnaire total score atbaseline, exacerbation, after14-day placebo or prednisolonetreatment (2 wk after exacerba-tion) and recovery (6 wk afterexacerbation) in standard ther-apy arm (n ¼ 80) and biomarker-directed therapy arm (n ¼ 86).
Data presented as mean (SEM).
(b) FEV1 at baseline, exacerba-tion, after 14 days of placebo orprednisolone treatment (2 wkafter exacerbation) and recov-ery (6 wk after exacerbation)in standard therapy arm (n ¼80) and biomarker-directed ther-apy arm (n ¼ 86). (c) Percentimprovement in visual analogscale total score from exacerba-tion and for duration of treat-ment period in exacerbations instandard therapy arm (n ¼ 80)and biomarker-directed therapyarm (n ¼ 86). Data points pre-sented as mean (SEM). CRQ ¼Chronic
Questionnaire; VAS ¼ visual an-alog scale.
for harm in a population who often have significant comorbid-
(14). Our findings suggest that a biomarker-directed strategy for
ities (14, 15). This, together with evidence in stable COPD that
initiating corticosteroid therapy would result in maintenance of
patients with eosinophilic airway inflammation respond better
the benefits of therapy with a simultaneous reduction in the
to corticosteroid treatment (9–11), provides a strong rationale
number harmed by this treatment. Using the peripheral blood
for a study investigating biomarker-directed therapy. Pooled
eosinophil count as a surrogate marker of eosinophilic airway
data analysis has shown that the number needed to harm using
inflammation, we have shown similar findings of corticosteroid
corticosteroid therapy in COPD exacerbations is 5, whereas for
responsiveness in a COPD eosinophilic phenotype but impor-
every 13 patients treated, 1 will develop significant hyperglycemia
tantly demonstrated this during exacerbations.
TABLE 2. LUNG FUNCTION AND INFLAMMATION AT BASELINE AND EXACERBATION IN ALL EXACERBATIONS CAPTURED CATEGORIZEDAS BLOOD EOSINOPHIL BIOMARKER POSITIVE AND BIOMARKER NEGATIVE
Biomarker Negative (n ¼ 56, nE ¼ 81)
Biomarker Positive (n ¼ 53, nE ¼ 85)
Mean Difference (95% CI)*
Mean Difference (95% CI)*
20.13 (20.19 to 20.07)
,0.01 1.16 (0.42)
20.17 (20.22 to 20.12)
FEV1, % predicted†
20.88 (21.06 to 20.70)
,0.01 3.99 (1.20)
20.96 (21.16 to 20.77)
Sputum total cell count, 3106/g‡
Sputum neutrophils, %
Sputum eosinophils, %‡
Blood total cell count, 3109 cells/L‡
10.3 (9.5–11.1)
Blood neutrophil count, 3109 cells/L‡
Blood eosinophil count, 3109 cells/L‡ 0.15 (0.13–0.17) 0.11 (0.10–0.13)
,0.01 0.30 (0.26–0.34) 0.34 (0.31–0.38)
Blood eosinophil %
20.9 (21.1 to 20.7)
Definition of abbreviations: CI ¼ confidence interval; CRQ ¼ Chronic Respiratory Disease Questionnaire score; CRP ¼ C-reactive protein; n ¼ number of patients; nE ¼
number of exacerbation events.
Statistical analysis performed using a paired t test analysis or Wilcoxon signed rank test. Differences between exacerbation and baseline presented as mean difference
(95% CI of difference), fold difference (95% CI of fold difference), and median (interquartile range) of differences as appropriate. Data presented as mean (SD) unlessotherwise stated.
* Mean, median, or fold difference as appropriate.
z Geometric mean (95% CI).
Bafadhel, McKenna, Terry, et al.: Biomarker-directed Corticosteroid Therapy in COPD Exacerbations
TABLE 3. LUNG FUNCTION AND INFLAMMATION (ABSOLUTE DATA) AT BASELINE, EXACERBATION, 2WEEKS AFTER EXACERBATION (POST-THERAPY) AND 6 WEEKS AFTER EXACERBATION (RECOVERY), FORALL EXACERBATIONS CATEGORIZED INTO BIOMARKER POSITIVE GIVEN PREDNISOLONE, BIOMARKERNEGATIVE GIVEN PREDNISOLONE, AND BIOMARKER NEGATIVE GIVEN PLACEBO
Biomarker Positive Given Prednisolone
FEV1, % predicted*
Sputum total cell count, 3106/g†
Sputum neutrophils, %
Sputum eosinophils, %†
Blood total cell count, 3109 cells/L†
11.6 (10.9–12.4)
Blood neutrophil count, 3109 cells/L†
Blood eosinophil count, 3109 cells/L†
0.30 (0.26–0.34)
0.34 (0.31–0.38)
0.19 (0.15–0.23)
0.26 (0.19–0.34)
Blood eosinophil %
Biomarker Negative Given Prednisolone
FEV1, % predicted*
Sputum total cell count, 3106/g†
10.6 (7.0–16.1)
Sputum neutrophils, %
Sputum eosinophils, %†
Blood total cell count, 3109 cells/L†
10.8 (9.8–12.0)
11.9 (10.4–13.7)
Blood neutrophil count, 3109 cells/L†
Blood eosinophil count, 3109 cells/L†
0.15 (0.12–0.18)
0.10 (0.09–0.12)
0.11 (0.09–0.14)
0.12 (0.09–0.15)
Blood eosinophil %
Biomarker Negative Given Placebo
FEV1, % predicted*
Sputum total cell count, 3106/g†
Sputum neutrophils, %
Sputum eosinophils, %†
Blood total cell count, 3109 cells/L†
Blood neutrophil count, 3109 cells/L†
Blood eosinophil count, 3109 cells/L†
0.15 (0.12–0.17)
0.12 (0.10–0.13)
0.14 (0.11–0.15)
0.17 (0.13–0.18)
Blood eosinophil %
Definition of abbreviation: CI ¼ confidence interval; CRP ¼ C-reactive protein; n ¼ number of patients; nE ¼ number of
Data presented as mean (SD) unless otherwise stated.
* Post-bronchodilator.
y Geometric mean (95% CI).
z Median (interquartile range).
We identified that patients who were biomarker positive had
a reduced and possibly detrimental response. We also found that
higher peripheral blood and sputum eosinophil counts and recovered
biomarker-positive exacerbations were more likely to have
more quickly with prednisolone than patients who were biomarker
higher blood eosinophils during stable state compared with
negative. In contrast, prednisolone treatment in biomarker-negative
biomarker-negative exacerbations. Further interrogation of the
patients was associated with more treatment failures and less im-
data also showed that subjects who were biomarker negative at
provement of health status or symptoms compared with placebo.
stable state were also more likely to be biomarker negative at
This finding was unexpected and may have arisen by chance.
the exacerbation event and that repeated exacerbation events
However, it raises the possibility that the absence of the blood
remained in the same blood eosinophil biomarker subgroup.
eosinophil biomarker identifies a COPD population whose re-
Previous work investigating the heterogeneity of COPD exacer-
covery is adversely affected by corticosteroid therapy, indepen-
bations has shown that the presence of airway eosinophilic inflam-
dent of the presence of bacteria or virus at exacerbation. There is
mation or bacterial pathogen at stable state could predict the
increasing evidence that inhaled corticosteroids are associated
exacerbation phenotype (16). In this study, we have determined
with an increased risk of pneumonia in COPD (31–33). These
that a blood eosinophil biomarker status in stable state can predict
findings would suggest that in blood eosinophil biomarker-
the exacerbation blood eosinophil biomarker status, highlighting
negative COPD exacerbations, infection may be a primary
a blood biomarker that has repeatability, has a high predictive
driver, and thus treatment with corticosteroids is associated with
value, and is indicative of treatment responsiveness. Whether
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Figure 3. (a) Chronic Respira-tory
total score at baseline, exacer-bation, after 14 days of treat-ment (2 wk after exacerbation)and recovery (6 wk after exac-erbation) in exacerbations thatwere biomarker-positive trea-ted with prednisolone (green),biomarker
and biomarker negative trea-ted with placebo (purple). Datapresented as mean (SEM). (b)FEV1 at baseline, exacerbation,after 14 days of placebo orprednisolone treatment (2 wkafter exacerbation) and recov-ery (6 wk after exacerbation)in exacerbations that werebiomarker
with prednisolone (green), bio-marker negative treated withprednisolone
biomarker negative treated withplacebo (purple). Data pre-sented as mean (SEM). (c) Per-cent improvement in visualanalog scale total score from ex-acerbation and for duration oftreatment period in exacerba-tions that were biomarker posi-tive treated with prednisolone(green),
treated with prednisolone (or-ange), and biomarker negativetreated with placebo (purple).
Data points presented as mean(SEM). CRQ ¼ Chronic Respi-ratory Disease Questionnaire;VAS ¼ visual analog scale.
these patients represent a specific phenotype that can be identified
in our study were low, probably reflecting the moderate severity
a priori and whether baseline knowledge of blood eosinophil bio-
of the exacerbations. It is therefore important that our hypoth-
marker status could direct treatment at the onset of an exacerba-
esis is tested in larger studies including patients hospitalized
tion requires further study in larger randomized controlled trials.
with severe exacerbations of COPD. These studies should also
A limitation of this study is that the majority of the exacerba-
investigate whether outcomes of biomarker-directed therapy
tions studied were moderate and did not require hospitalization.
differ by the presence of features such as tapered prednisolone
We would be cautious in extrapolating our findings beyond this
treatment; duration of treatment; and the presence of infection,
group. However, the population we studied reflects a population
emphysema, and chronic bronchitis. This study was not pow-
of patients who exacerbate and present to clinics and primary care,
ered to study health economic impact of biomarker-directed
and our findings are likely to be relevant and applicable in this
corticosteroid therapy, and this important potential benefit
setting (34). Furthermore, our study population had to have a
requires further study. A final concern is that our population
prior history of exacerbations, and therefore they are likely to
may have included patients who had fixed airflow obstruction as
reflect predominately a frequent exacerbator group. Whether
a result of asthma and may not be relevant to settings where
differences in response to therapy exist between infrequent and
diagnostic abilities are greater. We acknowledge that this is
frequent exacerbator groups requires future study.
possible but maintain that we made stringent efforts to reduce
Although bacteria are believed to play a role in up to 50% of
a population with characteristics of asthma and were careful to
exacerbations (7), evidence on the benefits of antibiotics is con-
ensure that our population met current diagnostic criteria for
flicting (35–37). In our study, we have concentrated on targeting
COPD (1). It is notable that, as we have shown before (16),
corticosteroid therapy and thereby standardized the effects of
features such as atopy and bronchodilator responsiveness were
any bacterial etiology by prescribing open-labeled antibiotic
not related to eosinophilic airway inflammation.
therapy in an aim to eliminate any confounding effects of bac-
In conclusion, a biomarker-directed strategy using the periph-
teria within exacerbations. We found no difference in bacteria
eral blood eosinophil count can be used to direct corticosteroid
culture-positive rates in the biomarker-directed and standard
therapy during acute exacerbations of COPD and allows the iden-
therapy arms, so this variable is unlikely to have confounded
tification of subgroups that have benefit and detriment from the
our comparison between these groups. Treatment failure rates
use of prednisolone treatment. This simple stratification allows for
Bafadhel, McKenna, Terry, et al.: Biomarker-directed Corticosteroid Therapy in COPD Exacerbations
the identification of clinically important phenotypes of COPD and
17. Treasure T, MacRae KD. Minimisation: the platinum standard for trials?
may identify groups for whom modified therapy is needed. Our
Randomisation doesn't guarantee similarity of groups; minimisation
data suggest that in the outpatient treatment of exacerbations
does. BMJ 1998;317:362–363.
of COPD, systemic corticosteroids should be only be given to
18. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK,
those who have a peripheral blood eosinophil count greater than
Nelson NA. Antibiotic therapy in exacerbations of chronic obstruc-tive pulmonary disease. Ann Intern Med 1987;106:196–204.
2%, but a larger confirmatory study is required. Whether this ap-
19. Rodriguez-Roisin R. Toward a consensus definition for COPD exacer-
proach can also be used for patients with severe COPD exacerba-
bations. Chest 2000;117:398S–401S.
tions who require hospitalization warrants further investigation.
20. Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA.
Time course and recovery of exacerbations in patients with chronic
are available with the text of this article at .
obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:
Acknowledgment: The authors thank all the research volunteers who participated in
the study. They also thank the following people for their contributions during the study:
21. Guyatt G. Measuring health status in chronic airflow limitation. Eur
J. Agbetile, M. Bourne, D. Desai, P. Dodson, B. Hargadon, T. Kebadze, M. McCormick,
Respir J 1988;1:560–564.
P. Newbold, H. Patel, A. Riding, P. Rugman, M. Saunders, M. Shelley, and A. Singapuri.
22. Brightling CE, Monterio W, Green RH, Parker D, Morgan MD, Wardlaw
AJ, Pavord ID. Induced sputum and other outcome measures in
chronic obstructive pulmonary disease: safety and repeatability. RespirMed 2001;95:999–1002.
1. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi
23. Health Protection Agency. Investigation of bronchoalveolar lavage, sputum
Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy
and associated specimens [Internet]. National Standard Method BSOP
for the diagnosis, management, and prevention of chronic obstructive
57 Issue 2.3. c2009 [accessed 2010 Aug]. Available from:
pulmonary disease: GOLD executive summary. Am J Respir Crit Care
24. Pye A, Stockley RA, Hill SL. Simple method for quantifying viable
2. Halpin D. NICE guidance for COPD. Thorax 2004;59:181–182.
bacterial numbers in sputum. J Clin Pathol 1995;48:719–724.
3. Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of
25. Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten
sputum inflammatory markers to symptoms and lung function changes
J, Tozzi CA, Polos P. Montelukast reduces asthma exacerbations in
in COPD exacerbations. Thorax 2000;55:114–120.
2- to 5-year-old children with intermittent asthma. Am J Respir Crit
4. Saetta M, Di SA, Maestrelli P, Turato G, Ruggieri MP, Roggeri A,
Care Med 2005;171:315–322.
Calcagni P, Mapp CE, Ciaccia A, Fabbri LM. Airway eosinophilia in
26. Pizzichini MM, Popov TA, Efthimiadis A, Hussack P, Evans S,
chronic bronchitis during exacerbations. Am J Respir Crit Care Med
Pizzichini E, Dolovich J, Hargreave FE. Spontaneous and induced
sputum to measure indices of airway inflammation in asthma. Am J
5. Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori
Respir Crit Care Med 1996;154:866–869.
G, Fabbri LM, Johnston SL. Infections and airway inflammation in
27. Hospers JJ, Schouten JP, Weiss ST, Postma DS, Rijcken B. Eosinophilia
chronic obstructive pulmonary disease severe exacerbations. Am J
is associated with increased all-cause mortality after a follow-up of 30
Respir Crit Care Med 2006;173:1114–1121.
6. Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G,
years in a general population sample. Epidemiology 2000;11:261–268.
Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, et al.
28. Hospers JJ, Schouten JP, Weiss ST, Rijcken B, Postma DS. Asthma
Respiratory viruses, symptoms, and inflammatory markers in acute
attacks with eosinophilia predict mortality from chronic obstructive
exacerbations and stable chronic obstructive pulmonary disease. Am J
pulmonary disease in a general population sample. Am J Respir Crit
Respir Crit Care Med 2001;164:1618–1623.
Care Med 1999;160:1869–1874.
7. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic
29. Hospers JJ, Rijcken B, Schouten JP, Postma DS, Weiss ST. Eosinophilia and
obstructive pulmonary disease. N Engl J Med 2008;359:2355–2365.
positive skin tests predict cardiovascular mortality in a general population
8. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J
sample followed for 30 years. Am J Epidemiol 1999;150:482–491.
Chron Obstruct Pulmon Dis 2006;1:39–47.
30. Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J,
9. Shim C, Stover DE, Williams MH Jr. Response to corticosteroids in
Dickinson G, Brison R, Rowe BH, Dreyer J, et al. Outpatient oral
chronic bronchitis. J Allergy Clin Immunol 1978;62:363–367.
prednisone after emergency treatment of chronic obstructive pulmo-
10. Pizzichini E, Pizzichini MM, Gibson P, Parameswaran K, Gleich GJ,
nary disease. N Engl J Med 2003;348:2618–2625.
Berman L, Dolovich J, Hargreave FE. Sputum eosinophilia predicts
31. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW,
benefit from prednisone in smokers with chronic obstructive bron-
Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in
chitis. Am J Respir Crit Care Med 1998;158:1511–1517.
chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–789.
11. Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw
32. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use
AJ, Pavord ID. Sputum eosinophilia and short-term response to
in chronic obstructive pulmonary disease and the risk of hospitaliza-
prednisolone in chronic obstructive pulmonary disease: a randomised
tion for pneumonia. Am J Respir Crit Care Med 2007;176:162–166.
controlled trial. Lancet 2000;356:1480–1485.
33. Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/
12. Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S,
fluticasone propionate versus salmeterol on exacerbations in severe
Monteiro W, Berry M, Parker D, Wardlaw AJ, et al. Eosinophilic
chronic obstructive pulmonary disease. Am J Respir Crit Care Med
airway inflammation and exacerbations of COPD: a randomised
controlled trial. Eur Respir J 2007;29:906–913.
34. National Clinical Guideline Centre. Chronic obstructive pulmonary dis-
13. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients
ease: management of chronic obstructive pulmonary disease in adults
admitted to hospital with exacerbations of chronic obstructive pul-
in primary and secondary care [Internet]. London: National Clinical
monary disease: a prospective randomised controlled trial. Lancet
Guideline Centre; c2010 [accessed 2010 Aug]. Available from: http://
14. Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Sys-
35. Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK.
temic corticosteroids for acute exacerbations of chronic obstructive
Antibiotic therapy and treatment failure in patients hospitalized for
pulmonary disease. Cochrane Database Syst Rev 2009;CD001288.
acute exacerbations of chronic obstructive pulmonary disease. JAMA
15. Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW,
Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacer-
36. Puhan MA, Vollenweider D, Steurer J, Bossuyt PM, Ter RG. Where is
bations of chronic obstructive pulmonary disease. Department of Veterans
the supporting evidence for treating mild to moderate chronic ob-
Affairs Cooperative Study Group. N Engl J Med 1999;340:1941–1947.
structive pulmonary disease exacerbations with antibiotics? A sys-
16. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick
tematic review. BMC Med 2008;6:28.
M, Haldar K, Kebadze T, Duvoix A, et al. Acute exacerbations of
37. Sethi S. The problems of meta-analysis for antibiotic treatment of chronic
COPD: identification of biological clusters and their biomarkers. Am J
obstructive pulmonary disease, a heterogeneous disease: a commentary
Respir Crit Care Med 2011;184:662–671.
on Puhan et al. BMC Med 2008;6:29.
Source: http://healthylungs.com.au/resources/Blood-Eosinophils-to-Direct-Corticosteroids-in-COPD_AJRCCM-2012-1.pdf
Cheryl Lopate, MS, DVMDiplomate, American College of Theriogenologists Pyometra in the Bitch Pyometra is a condition that affects intact bitches, causing a variety of clinical signs and symptoms. Pyometra is typically pre-empted by pathologic changes in the uterus. The Greekderivation of pyometra is: pyo = pus and metra = uterus, so pyometra = an accumulation of pus inthe uterus.
DAROU PAKHSH PHARMACEUTICAL MFG. CO. SITE MASTER FILE Issued Date: 09/05/2015 CODE : DPSMF422 Validation Date: 09/05/2016 Rev. No:3 This Site Master File was prepared on the basis of the PIC/S document "Explanatory notes for industry on the preparation of a Site Master File" PE 008-4. Contents: Chapter 1: General information 1.1





