Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Healthcare-bulletin.com
ISCHEMIC HEART DISEASE ORIGINAL RESEARCH
Frequency of Clopidogrel Resistance
in Patients of Ischemic Heart Disease
Syed Khizar Abbas Rizvi1, Shahida Mohsin1, Tahir Saeed2, Saeed Ahmad3,
Shabbir Hussain1 Muhammad Ikram Ullah2
Received: 14/3/13, Reviewed 10/4/13, Accepted: 16/4/13
Key words: Clopidogrel, Ischemic Heart Disease, platelet Aggregation, Platelet Aggregation Inhibitors
DOI: 10.5083/ejcm.20424884.93
1. Department of Haematology,
University of Health Sciences
Clopidogrel and Aspirin are widely used antiplatelet agents in the prevention and treatment of isch-
2. Department of Biochemistry,
emic heart disease (IHD). Many patients have been noticed with recurrence of major ischemic events,
University of Health Sciences
due to resistance of these drugs. Different platelet function tests can be used to evaluate the de-
gree of achieved platelet inhibition in patients treated with clopidogrel. The objective of this study
3. Department of Pathology,
was to determine frequency of clopidogrel resistance in patients of ischemic heart disease. Seventy
Sharif Medical and Dental College
one patients of IHD were selected from out-patient department of Punjab Institute of Cardiology
Lahore. Platelet aggregation studies were performed on Diamed Impact R. Clopidogrel response as-
4. Hussani Blood Transfusion
Centre Karachi
say was performed with DiaAdin(ADP 110µmol/L). Chi-square test was applied to measure statistical
significance. Resistance to Clopidogrel was observed in 17% (12 out of 71). Clopidogrel resistance
was significantly associated with female gender (p=0.046). In our study no statistically significant as-
sociation was observed between clopidogrel resistance and risk factors like diabetes mellitus, family
history ischemic heart disease, hypertension and smoking. We concluded that resistance to Clopido-
Syed Khizar Abbas Rizvi,
grel therapy is seen in significant number of patients and female patients are at high risk of develop-
ing the resistance to clopidogrel therapy. These patients can be identified by performing platelet
Department of Haematology,
aggregation studies on Impact R.
University of Health Sciences
Contact No. 0092-322-6686637
A number of studies have shown that aspirin
or clopidogrel resistance is associated with in-
[email protected]
Ischemic heart disease is a major public health
creased risk of recurrent cardiovascular events
problem and a leading cause of death in the
[6,7].The term resistance is used to indicate fail-
world. Its risk increases with age, smoking, hy-
LIMITATIONS OF STUDY
ure of an agent to prevent the clinical condi-
Our study was a descriptive
percholesterolemia, diabetes and hypertension
tion for which it is used or failure of the agent
study with its limitations. The
[1]. Currently, cardiovascular diseases accounts
to achieve the biochemical (pharmacokinetic
exact prevalence of clopidogrel
for 16.7 million deaths worldwide each year and
and/or pharmacodynamic) effect
[8]. Different
resistance may be different from
strategies for improving prevention and man-
platelet function tests have been used to mea-
the one which is observed in the
agement of thrombotic conditions are required
sure the degree of platelet inhibition in patients
present study.
[2]. The most commonly used antiplatelet drugs
treated with clopidogrel. Light transmission ag-
are Acetylsalicylic acid (Aspirin) and thienopyri-
gregometry with ADP as an agonist, is the most
We acknowledge University
used method, but the test is time consuming
of Health Sciences, Lahore,
and not practical for routine use.
Pakistan for technically
Clopidogrel is a prodrug, which needs to be me-
supporting this research project.
tabolized in the liver to active metabolites. Clop-
New point of care devices like PFA100, Veri-
We also extend our gratitude to
idogrel inhibits the ADP receptor P2Y12, and
fyNow and The cone and plate(let) analyser
the participants of the study.
thereby inhibiting the ADP mediated platelet
are being utilised for the determination of an-
activation. In vivo transformation of clopidogrel
CONFLICTS OF INTEREST
tiplatelet therapy effectiveness. The PFA-100®
to active metabolites is an important and criti-
We are grateful to
measures the fall in flow rate as platelets within
cal step for the drug effect. This metabolisation
University of Health Sciences,
citrated whole blood are aspirated through a
Lahore, Pakistan for providing
is dependent on the hepatic cytochrome P450
capillary and begin to seal a 150μm aperture
financial support to pursue this
isoenzymes like CYP2C19, CYP1A2, CYP2B6, CY-
within a collagen coated membrane. The disad-
research project. There are no
P2C9 and CYP3A4
[3]. One of the key enzymes
vantages of PFA 100 are: This is inflexible, VWF
conflicts of interest.
in clopidogrel metabolism is CYP2C19, which is
dependent, Haematocrit dependent and insen-
involved in both stages of clopidogrel biotrans-
sitive to clopidogrel
[9].
EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL II ISSUE III
FREQUENCY OF CLOPIDOGREL RESISTANCE IN PATIENTS OF ISCHEMIC HEART DISEASE
VerifyNow is fully automated platelet aggregometer which can be
used to monitor the therapy of aspirin, clopidogrel and GpIIbIIIa in-
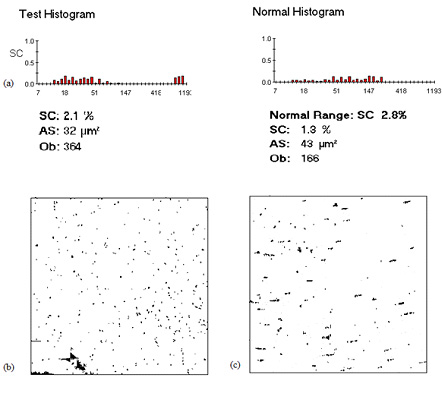
Figure 1: Illustration of test report of clopidogrel resistance in a
hibitors but this assay is expensive and Cartridges can only be used
patient. (Processed on Diamed Impact-R, Software Version: 1.28).
for single purpose [9]. The cone and plate(let) analyser, originally
developed by Varon and Savion, monitors platelet adhesion and
aggregation to a plate coated with collagen or extracellular matrix
(ECM) under high shear conditions of 1800 s− 1[10]. In the Com-
mercial version of the device, the Impact® (DiaMed), a plastic plate
is utilized instead of a collagen or an ECM-coated surface.
In this study we used Diamed Impact R to determine the frequency
of resistance of clopidogrel in patients of IHD. Diamed Impact R
is the instrument which provides the physiological condition for
platelet adhesion and aggregation. Platelet adhesion and aggrega-
tion on the polystyrene surface is evaluated using an image analy-
sis system. The results are expressed as the percentage of surface
covered (%SC) by platelets and the average particle size (AS; micron
m2). Normal value of % SC is >7.5 and AS is >25 micron m2 for
physiological platelet function.
PATIENTS AND METHODS
It was a descriptive cross sectional study conducted at the depart-
ment of Haematology, University of Health Sciences Lahore in col-
*SC: Surface Covered; *AS: Average Size; *Ob: Object Number
laboration with Punjab Institute of Cardiology, Lahore Pakistan. The
study was approved by ethical committee of University of health
sciences Lahore. Patients were enrolled after informed written
consent from April 2011- March 2012. Diagnosed patients of IHD
who were more than 21 years of age and on Clopidogrel 75 mg
for at least more than 07 days were included in study and patients
who have Family or personal history of bleeding disorders, Platelet
All data was entered and analysed with the help of SPSS version
count <150 or >450 × 109/L, Hemoglobin <8 g/dl, Major surgical
18.0. The quantitative variables were expressed as mean+SD. Chi-
procedure within one week before enrollment and Administration
square test was applied to measure the statistical significance of
of un-fractionated or low molecular weight heparin within 24 hours
frequency of drug resistance between groups e.g. Diabetics vs.
before enrollment were excluded from study.
Non-diabetics, Males vs. Females. A p value of less than 0.05 was
considered for statistical significance.
Laboratory Techniques
5ml of venous blood was collected using aseptic methods. Citrated
whole blood (3ml) was used for platelet studies (platelet adhe-
sion and aggregation) using Impact R (Diamed, Israel). DiaAdin
Out of 71 patients 37 patients had IHD without PCI (Percutaneous
(ADP110µmol/L) was used as platelet agonist. 2ml blood (in EDTA)
Coronay Intervention) or CABG (Coronary Arterial Bypass Graft-
was used to determine Haemoglobin, Haematocrit and platelet
ing). Nineteen patients had IHD with Angioplasty and 15 patients
count using Sysmex XI-1800 (manufact). Resistance to Clopidogrel
were treated with CABG. Mean Age in the study population in years
therapy was assessed by Impact R on the basis of software generat-
was 52.85+ 1.14 (95% CI 50.56-55.13) Median duration of illness in
ed results: Surface covered (SC) > 2.8% was considered as response
the study population was 24+ 39 (Tukeys's Hinges: 9-48) months.
to clopidogrel and SC < 2.8 % was considered as no response (or
Demographic Characteristics of study population are shown in
Resistance) to Clopidogrel. Resistance to clopidogrel therapy in a
Table 1.
patient is illustrated in Figure 1.
Hemoglobin, Hematocrit and platelet count were measured for
each subject before performing the platelet aggregation studies to
fulfill exclusion criteria for Hb, HCT% and platelet count
Data about patient's demographic features (age, sex, and address),
(Table 2).
clinical diagnosis, duration of illness, drug intake history, history
of recurrent ischemic events and relevant clinical history was ob-
tained on a specially designed proforma. The data about platelet
aggregation studies was entered after performing the laboratory
tests in the specified columns.
EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL II ISSUE III
HEALTHCARE BULLETIN ISCHEMIC HEART DISEASE
Table 1: Demographic Data of the Subjects included in the Study
Family H/o IHD
Rec. ischemia
*DM: Diabetes Mellitus *HTN: Hypertension *IHD: Ischemic Heart Disease *Rec: Recurrent
Table 2: Demographic Data of the Subjects included in the Study
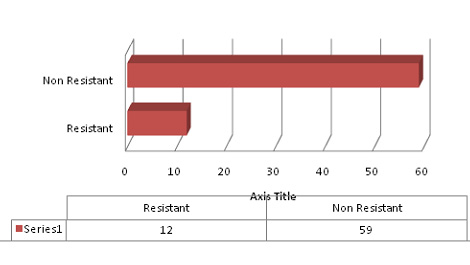
Figure 2: Frequency of Clopidpgrel Resistance
Hb of the patients (g/dl)
Hct. of the patients (%)
Platelets count of the patient (109/L)
*Hb: Haemoglobin *Hct: Hematocrit *SD:Standard Deviation
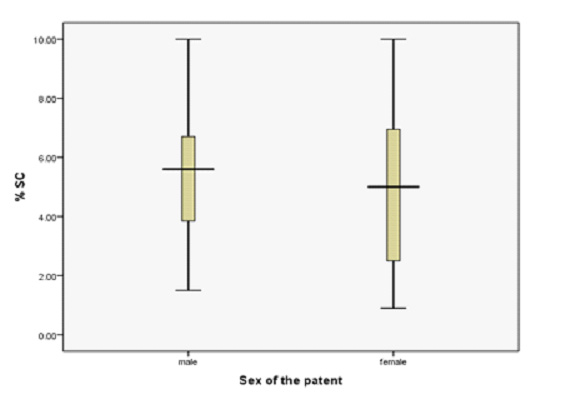
Figure 3: Comparison of % SC (Surface Covered) of
Clopidogrel Response Assay between male and female patients
Clopidogrel Response Assay
Out of 71 patients12 (17%) were found resistant to clopidogrel. The
frequency of resistance to clopidogrel is shown in Figure 2. Samples
of resistant patients were retested, without DiaAdin to assess any
baseline platelet functional aggregation defect. No case was found
to have any platelet functional defect.
There was significant association (P value of 0.046) between resis-
tance to Clopidogrel and patients with female gender. It was also
observed that variance in % SC (surface covered) was higher in fe-
male patients as compare to male patients. Variance of %SC of male
and female patients is shown in Figure 3. Significant association of
Clopidogrel resistance and other risk factors like hypertension, dia-
betes mellitus, smoking and family history of ischemic heart dis-
ease was not observed (p value of >0.05) (Table 1).
EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL II ISSUE III
FREQUENCY OF CLOPIDOGREL RESISTANCE IN PATIENTS OF ISCHEMIC HEART DISEASE
In our study no significant association was observed between clop-
idogrel resistance and risk factors like diabetes mellitus, family H/O
Antiplatelet therapy is effective in primary and secondary preven-
ischemic heart disease, hypertension and smoking. However re-
tion of atherothrombotic events in patients of ischemic heart dis-
sistance to clopidogrel therapy was noted more in female patients
ease[11].The concept of clopidogrel resistance has emerged in the
with a statistically significant P value (P = 0.046). In a study by Boris
medical literature to reflect the failure to inhibit platelet function
et al. (2006) it was reported that female are predisposed to clopi-
in vitro, although its existence and definition remain to be estab-
dogrel resistance (P = 0.0002) This marked sex-related difference in
lished. It has been proposed that the term resistance encompasses
clopidogrel responsiveness needs to be confirmed in a larger num-
patients for whom clopidogrel does not achieve its pharmacologi-
ber of patients matched for other potential confounders. Similarly
cal effect, and failure of therapy reflects patients who have recurrent
various risk factors like age, smoking, diabetes, hypertension, obe-
events on therapy [12]. Despite intensified antiplatelet treatment,
sity, cholesterol, did not show any statistically significant difference
up to 4.7% of the patients undergoing coronary stenting develop
among the groups in a study by Kumar et al. (2007).
thrombotic stent occlusion, suggesting incomplete platelet inhibi-
tion due to clopidogrel resistance [13].
Class IIB recommendations from the American College of Cardi-
ology (ACC)/American Heart Association (AHA) have stated that
The prevalence of clopidogrel non-response in patients is estimat-
platelet aggregation studies are warranted in patients undergoing
ed 4% to 30%[14].The reported rates vary between studies because
PCI who are at risk of sub-acute stent thrombosis, with the option
of the technique used to measure the extent of platelet aggrega-
of increasing their maintenance dose of clopidogrel from 75 to
tion and the presence of factors contributing to platelet reactivity
150mg/day in order to suppress platelet reactivity below 50% [18].
[15]. In our study non responders to clopidogrel were 12 (17%) and
Recent studies have evaluated the effect of modifying therapy on
all patients were on standard dose of 75mg OD. Clopidogrel assay
clinical outcome for patients deemed non-responsive as measured
was measured with DiaAdin (ADP110 µmol/L) on Diamed Impact
by platelet function tests.
R. In another study 16% of Coronary arterial disease (CAD) patients
were classified as non-responders and the electrical impedance ag-
Clopidogrel response can also vary because of inter-individual dif-
gregometry was performed in diluted whole blood in the presence
ferences in drug absorption, resulting in lower levels of the active
of 5 and 20 μmol/L ADP [16].
metabolite. Medications such as statins and certain proton pump
inhibitors have been proposed to affect the metabolisation of
In a study by Mobley et al. (2004) the prevalence of clopidogrel re-
clopidogrel by the CYP isozyme 3A4, although data is controver-
sistance was estimated 30%. These patients were on 75 mg daily
sial [19,20]. Individuals with low baseline metabolic activity of the
maintenance dose and the method used was Optical aggregome-
CYP3A4 enzyme have also been shown to have poor clopidogrel
try 1 µmol/L ADP. A study conducted in India, 2.54% patients were
responsiveness and this aspect should be evaluated on large ran-
found to be clopidogrel resistant, 12.7% were clopidogrel semi-
domised trials [21].
responders and 84.7% were clopidogrelresponders and the opti-
cal aggregometry was used to define clopidogrel resistance, semi-
It is concluded that resistance to clopidogrel therapy is seen in sig-
responders and responders [17]. Estimated frequency of clopidogrel
nificant proportion of patients. These patients can be identified by
resistance in different studies is summarised in Table 3.
performing platelet aggregation studies on Diamed Impact R and
female patients are at high risk for developing the resistance to
Table 3: Clopidogrel resistance in different studies
Müller et al.
Optical aggregometry
Jaremo et al.
Optical Aggregometry
Mobley et al.
Optical Aggregometry
Boris et al.
Optical Aggregometry
Kumar et al.
Optical aggregometry
Boris et al.
Impedence aggregometry
Our study
EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL II ISSUE III
HEALTHCARE BULLETIN ISCHEMIC HEART DISEASE
Gum,PA., Kottke-Marchant, K., Poggio, ED., Gurm, H.,Welsh, PA.,
Brooks, L., et al. Profile and prevalence of aspirin resistance in patients
Ruggeri, ZM. Platelets in atherothrombosis. Nat Med 2004; 8:
with cardiovascular disease. Am.J. Cardiol2001;88: 230-235.
Wiviott, SD. And Antman, EM.Clopidogrel resistance:
Frans,Van de . and Werf, J. Dual antiplatelet therapy in high-risk
A new chapter in a fast-moving story. Circulation 2004;109: 3064–7.
patients. Eur Heart J.Suppl2007;(suppl D): D3-D9.
Muller, I., Besta, F., Schulz, C. (2003).Prevalence of clopidogrel
Pettersen,AA., Arnesen,H., Opstad TB, Seljeflot,I. The influence of CYP
non-responders among patients with stable angina pectoris
2C19*2 polymorphism on platelet function testing during single
scheduled for elective coronary stent placement.
antiplatelet treatment with clopidogrel. Thrombosis Journal 2011;9:4
Hulot, J.S., Bura, A., Villard, E. Cytochrome P450 2C19 loss of function
Nguyen,T.A., Diodati,J.G, Chantal, Pharand. Resistance to Clopidogrel:
polymorphism is a major determinant ofclopidogrel responsiveness
A Review of the Evidence, Cardiol 2005; 45:1157-1164
in healthy subjects. Blood 2006; 108: 2244-2247
Jaremo, P., Lindahl, TL.,Fransson, SG., Richter, A.(2002). Individual
Kazui,M. ,Nishiya,Y., Ishizuka,T. (2010). Identification of the human
variationsof platelet inhibition after loading doses of clopidogrel.
cytochrome P450 enzymes involved in the two oxidativesteps in the
J Intern Med.2002; 252:233– 8
bioactivation of clopidogrel to its pharmacologically active
metabolite. Drug MetabDispos2010; 38: 92-¬99
Boris,T.Ivandica., Philipp Schlick., Peter Staritz., Kerstin Kurz.,
Hugo, AK., Evangelos, Giannitsis. (2006). Determination of clopidogrel
Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR.
resistance by whole blood platelet aggregometry and inhibitors of
Aspirin "resistance" and risk of cardiovascular morbidity: systematic
the P2Y12 receptor .Clinchem.2006;52:383-388-3
review and meta-analysis. BMJ 2008;336:195–198.
Kumar, S., Saran, RK., Puri, A., Gupta, N., Sethi, R., Surin, WR., et al.
Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW,
Profile and prevalence of clopidogrel resistance in patients of acute
Huisman, MV. Clopidogrelnonresponsiveness in patients
coronary syndrome. Indian Heart J.2007;59(2):152-6.
undergoing percutaneous coronary intervention with stenting: a
systematic review and meta-analysis. Am Heart J 2007;154:221–231.
Smith, SC., Feldman, TE.,Hirshfeld, JW. (2006). ACC/AHA/SCAI 2005
Guideline Update for Percutaneous Coronary Intervention--summary
Florian, K., Hae-Young, S., Volker, K. (2008) Antiplatelet drugs in
article: a report of the American College of Cardiology/American
cardiological practice: Established strategies and new developments
Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI
.ISVH 2008;4(3):637 – 45
Writing Committee to Update the 2001 Guidelines for Percutaneous
Coronary Intervention). Circulation 2006; 113:156–75
Harrison, P. The role of PFA-100 testing in the investigation and
management of haemostatic defects in children and adults. Br J
Ajzenberg, N., Aubry, P., Huisse, MG.,Cachier, A., El Amara, W., Feld-
Haematol 2005;130:3-10
man, LJ., et al. Enhanced shear-induced platelet aggregation in
patients who experience subacute stent thrombosis: a case-control
Spectre, G., Brill, A., Gural, A., Shenkman, B., Touretsky, N., Mosseri,
study. J Am Coll Cardiol.2005; 45:1753-1756.
E., et al. A new point-of care method for monitoring antiplatelet
therapy: Application of the cone and plate(let) analyzer. Platelets
Jean-Philippe,C., Wouter van Werkum. (2010). Individualised anti-
2005 ;16:293–9.
platelet therapy: Is there a role for platelet function testing in routine
clinical practice? European Cardiology.2010;6(1):41-49
Antiplatelet Trialist Collaboration Collaborative overview of
randomized trial of antiplatelet therapy. Prevention of death,
myocardial infarction and stroke in prolonged antiplatelet therapy in
various categories of patients.BMJ 1994; 308: 81-106.
EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL II ISSUE III
Source: http://www.healthcare-bulletin.com/uploads/media/Frequency_of_Clopidogrel__Resistance.pdf
The Medifast Program and What you and your doctor should know about the Medifast Program and Director of Nutrition 2 Medifast Meals _ 4 Vegetables for the Lean & Green Meal _ 5 From Medifast's Director of Nutrition Achieving optimal health and weight control is not always a "one-size-fits-all" equation. Lifestyle choices and medical conditions can affect both your food preferences and requirements, which in turn have an impact on how you can best lose weight.
International Geology Review, Vol. 49, 2007, p. 374–388. Copyright © 2007 by V. H. Winston & Son, Inc. All rights reserved. Review of the Lithium Isotope System as a Geochemical Tracer YAN-JIE TANG,1 HONG-FU ZHANG, AND JI-FENG YING State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences,






