Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Eexo.gr
JOURNAL OF CLINICAL ONCOLOGY
Phase III Randomized Trial of FOLFIRI VersusFOLFOX4 in the Treatment of Advanced ColorectalCancer: A Multicenter Study of the Gruppo OncologicoDell'Italia Meridionale
Giuseppe Colucci, Vittorio Gebbia, Giancarlo Paoletti, Francesco Giuliani, Michele Caruso,Nicola Gebbia, Giacomo Cartenı , Biagio Agostara, Giuseppe Pezzella, Luigi Manzione, Nicola Borsellino,Andrea Misino, Sante Romito, Ernesto Durini, Stefano Cordio, Marisa Di Seri, Massimo Lopez,and Evaristo Maiello
From the Oncology Institute, Bari;
University; La Maddalena Hospital; M.
Ascoli Hospital, Palermo; Regina Elena
Institute; La Sapienza University, Rome;
Centro Oncologico; S. Luigi Hospital,
We performed this phase III study to compare the irinotecan, leucovorin (LV), and fluorouracil
Catania; Cardarelli Hospital, Naples;
(FU) regimen (FOLFIRI) versus the oxaliplatin, LV, and FU regimen (FOLFOX4) in previously
Ospedale Nord, Taranto; S. Carlo Hospi-
untreated patients with advanced colorectal cancer.
tal, Potenza; Cardarelli Hospital, Campo-
basso; G. Panico Hospital, Tricase, Italy.
Patients and Methods
A total of 360 chemotherapy-naive patients were randomly assigned to receive, every 2
Submitted July 19, 2004; accepted
weeks, either arm A (FOLFIRI: irinotecan 180 mg/m2 on day 1 with LV 100 mg/m2
January 21, 2005.
administered as a 2-hour infusion before FU 400 mg/m2 administered as an intravenous
Presented in part at the 38th Annual
bolus injection, and FU 600 mg/m2 as a 22-hour infusion immediately after FU bolus
Meeting of the American Society of
Clinical Oncology, Orlando, FL,
injection on days 1 and 2 [LV5FU2]) or arm B (FOLFOX4: oxaliplatin 85 mg/m2 on day 1
May 18-21, 2002; and at the 39th
with LV5FU2 regimen).
Annual Meeting of the American
Society of Clinical Oncology,
One hundred sixty-four and 172 patients were assessable in arm A and B, respectively.
Chicago, IL, May 31-June 3, 2003.
Overall response rates (ORR) were 31% in arm A (95% CI, 24.6% to 38.3%) and 34% in arm
Authors' disclosures of potential con-
B (95% CI, 27.2% to 41.5%;
P
flicts of interest are found at the end of
⫽ .60). In both arms A and B, median time to progression
this article.
(TTP; 7
v 7 months, respectively), duration of response (9
v 10 months, respectively), andoverall survival (OS; 14
v 15 months, respectively) were similar, without any statistically
Address reprint requests to Giuseppe
significant difference. Toxicity was mild in both groups: alopecia and gastrointestinal
Colucci, MD, Medical and Experimental
Oncology Unit, Oncology Institute, Via
disturbances were the most common toxicities in arm A; thrombocytopenia and neurosen-
Amendola 209, 70126 Bari, Italy; e-mail:
sorial were the most common toxicities in arm B. Grade 3 to 4 toxicities were uncommon in
both arms, and no statistical significant difference was observed.
2005 by American Society of Clinical
There is no difference in ORR, TTP, and OS for patients treated with the FOLFIRI or
FOLFOX4 regimen. Both therapies seemed effective as first-line treatment in these patients.
The difference between these two combination therapies is mainly in the toxicity profile.
J Clin Oncol 23:4866-4875. 2005 by American Society of Clinical Oncology
tients. In the past, the standard treatment for
patients with advanced CRC was fluoroura-
The availability of new active drugs in the
cil (FU) biochemically modulated by leuco-
clinical practice of the treatment of ad-
vorin (LV), which globally demonstrated a
vanced colorectal cancer (CRC) patients has
response rate of 23%,1 with a median sur-
deeply changed the prognosis of these pa-
vival time that rarely exceeded 10 to 12
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
FOLFIRI Versus FOLFOX4 in Advanced CRC
months. Therefore, the results of systemic treatment were
ever, OHP in combination with FU and LV (mainly high-
disappointing, and patient prognosis remained poor.
dose LV and FU in infusional administration) resulted in
New approaches were clearly needed to improve clinical
objective response rates from 18% to 46%, with median
results. A bimonthly schedule of FU, administered as a bolus
survival time ranging from 10 to 17 months.15-18 In first-
and continuous infusion, combined with high-dose LV
line therapy, a European randomized phase III trial19
(LV5FU2) was randomly compared with the monthly North
demonstrated significant superiority of the combination
Central Cancer Treatment Group–Mayo Clinic regimen (FU
regimen of OHP, LV, and bolus plus infusional FU
bolus and low-dose LV). In the patients with measurable dis-
(FOLFOX4) over the Mayo Clinic regimen in terms of
ease, the bimonthly schedule obtained significantly better re-
response rate (50%
v 22%, respectively;
P ⫽ .0001) and
sults in terms of response rate and median progression-free
progression-free survival (8.2
v 6.0 months, respectively;
survival and resulted in fewer grade 3 and 4 toxicities (granu-
P ⫽ .0003), with no statistical difference in median OS time
locytopenia, diarrhea, and mucositis) than the monthly sched-
(16.2
v 14.7 months, respectively;
P ⫽ .12).
ule.2 Therefore, this regimen became the new standard option
After the results of the two large previously mentioned
for advanced CRC patients in some European countries.
randomized French studies,9,19 the FOLFOX4 and the
In recent years, a number of new treatment options
CTP-11 plus LV5FU2 combination regimens represented
have become available. In particular, two new cytotoxic
the reference first-line treatments for patients with ad-
agents, irinotecan (CPT-11) and oxaliplatin (OHP), have
vanced CRC in many European countries. Subsequently,
been proven to have efficacy in the treatment of CRC. After
the CTP-11 plus LV5FU2 regimen was slightly modified to
encouraging observations of substained activity in colon
become the FOLFIRI regimen.
cancer cell lines,3 CPT-11, a specific inhibitor of topoisom-
Taking into account these studies, in 1999, the Gruppo
erase I, demonstrated, in several clinical studies, significant
Oncologico dell'Italia Meridionale (GOIM) started a ran-
single-agent activity against CRC resistant to FU-based
domized trial (GOIM protocol No. 9901) to compare the
first-line therapy in phase II4-6 and phase III studies,7,8 with
FOLFIRI regimen used in the study by Douillard et al9 with
significant improvement in results when CPT-11 was com-
the FOLFOX4 combination therapy reported by de
pared with best supportive care alone or with FU by contin-
Gramont et al19 in patients with advanced CRC. The pri-
uous infusion. Therefore, because of these results, CPT-11
mary end point of our study was response rate, and the
was considered the reference treatment for patients with
secondary end points were TTP, OS, and toxicity profile.
FU-refractory advanced CRC. Furthermore, two first-linephase III trials9,10 showed a significant improvement inresults with the addition of CPT-11 to FU-LV combination
PATIENTS AND METHODS
therapy (FOLFIRI). In particular, in the European study,9the FOLFIRI regimen, compared with LV5FU2 (AIO regi-
men), obtained a significant difference in terms of overall
The elegibility criteria included the following: age ⱖ 18 and ⱕ 75
survival (OS; 17.4
v 14.1 months, respectively;
P ⫽ .031),
years, histologically confirmed locally advanced and/or metastatic
response rate (35%
v 22%, respectively;
P ⫽ .005), and
CRC with bidimensionally measurable disease, anticipated life ex-
time to progression (TTP; 6.7
v 4.4 months, respectively;
pectancy of at least 3 months, Eastern Cooperative Oncology Groupperformance status of 0 to 2, and adequate bone marrow (platelet
P ⬍ .001). The overall response rate (ORR) of the FOLFIRI
count ⱖ 100,000/L, WBC count ⱖ 4,000/L, granulocyte count of
arm in this study was similar to that observed in our previ-
ⱖ 1500/L, and a hemoglobin level of ⱖ 10.0 mg/dL), renal (serum
ous randomized phase II trial11 comparing FOLFIRI with
creatinine concentration ⱕ 2.0 mg/dL), and hepatic functions (serum
LV5FU2 (40%
v 18%, respectively). In addition, in an
bilirubin level ⱕ 2.0 mg/dL and AST ⬍ 3⫻ the institutional normal
American trial,10 the addition of CPT-11 to an FU bolus
level in the absence of liver involvement with cancer or up to 5⫻ the
administration (IFL) obtained significantly better results
institutional normal level when cancer was present in the liver).
than CPT-11 alone or FU-LV alone, and therefore, this
Patients had to be previously untreated for advanced disease,
except for those patients in whom this therapy had been per-
regimen represented the standard treatment for patients
formed in the adjuvant phase at least 6 months before enrollment.
with advanced CRC in the United States.
No concurrent uncontrolled medical illness was allowed.
OHP, a new cytotoxic agent from the diaminocyclo-
Patients were excluded if any of these criteria were not met
hexane platinum family, has a mechanism of action similar
and if they had any active or uncontrolled infections; known brain
to the other platinum derivates, with a different spectrum of
metastases or carcinomatous meningitis; interstitial pneumonia
antitumor activity against some tumor models; in particu-
or interstitial fibrosis; history of myocardial infarction within the
lar, activity against colon cell lines and synergistic activity of
previous 6 months or current clinical evidence of congestive heartfailure (patients taking medication for congestive heart failure and
OHP and FU in experimental models have been demon-
showing no clinical signs or symptoms were eligible); symptoms
strated.12,13 Activity of OHP as a single agent in previously
of coronary artery disease; history of thromboembolic disease;
treated patients with CRC was demonstrated in phase II
history in the past 5 years of a prior malignancy, except for ade-
trials, with a response rate of approximately 10%.14 How-
quately treated basal cell or squamous cell skin cancer or in situ
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
Colucci et al
cervical cancer; and any psychiatric or psychological disorders that
estimation of tumor size (⬎ or ⬍ 10 cm2) was determined accord-
interfere with consent and precluded treatment or adequate
ing to the sum of the products of the largest perpendicular diam-
follow-up. Pregnant or lactating women and patients with periph-
eters of all measurable lesions for site of the disease. It was found
eral neuropathy were also excluded. Radiotherapy was allowed
that this stratification method could be easily reproduced in the
only in sites other than those measurable for response evaluation.
various centers participating in the study and did not seem to be
Pretreatment evaluation included a complete medical and
subject to investigator bias. Thus, the stratification factors were as
clinical-physical examination, performance status evaluation,
follows: (1) size of disease (limited or extensive disease; ⬍ or ⬎ 10
baseline measurement of tumor size based on scans, x-ray exami-
cm2, respectively) and (2) liver involvement (with or without liver
nation or other radiographic means (comprising full assessment of
involvement; H⫹ and H⫺, respectively). The following four patient
all known metastatic disease), chest x-ray, ECG, CBC count with
categories were obtained: group 1, H⫹ and tumor more than 10 cm2;
leukocyte differential, platelet count, and serum chemistries and
group 2, H⫹ and tumor less than 10 cm2; group 3, H⫺ and tumor
electrolytes and tumor markers. Patients were required to agree to
more than 10 cm2; and group 4, H⫺ and tumor less than 10 cm2.
and sign a statement of informed consent before entry onto the
Patients were randomly assigned to receive either arm A or
study. Informed consent was previously approved by the Scientific
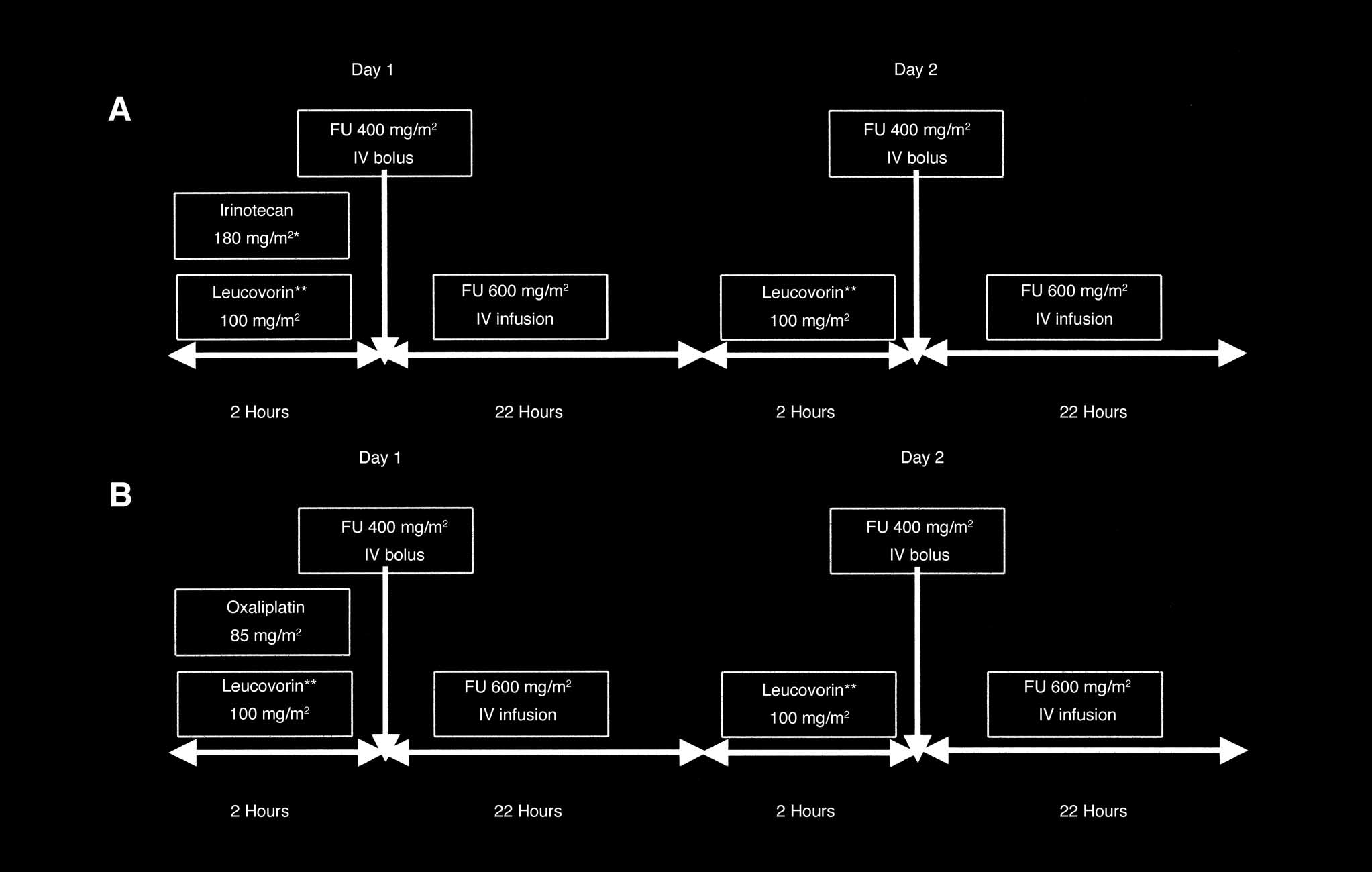
arm B (Fig 1). Arm A (FOLFIRI regimen) consisted of CPT-11 180
Committee of the GOIM and the ethics committees of each indi-
mg/m2 (150 mg/m2 for patients age ⱖ 70 and ⬍ 75 years) only on
vidual participating institution.
day 1, with LV 100 mg/m2 (L-isomer form) administered as a
2-hour infusion before FU 400 mg/m2 administered as an intrave-
Randomization to either the FOLFIRI regimen (arm A) or
nous bolus injection; FU 600 mg/m2 was administered as a 22-
FOLFOX4 regimen (arm B) was performed centrally at the GOIM
hour infusion immediately after FU bolus injection. LV and FU
headquarters in Bari, Italy. The random ratio between the two
were repeated on days 1 and 2 according to a previously reported
arms (A v B) was 1:1.
schedule.9 Arm B (FOLFOX4) consisted of OHP 85 mg/m2 only
According to our previous experiences,11,20 the size and site
on day 1, with LV 100 mg/m2 (L-isomer form) administered as a
of disease were considered prognostic variables for the stratifica-
2-hour infusion before FU 400 mg/m2 administered as an intrave-
tion of patients, and therefore, patients were stratified according to
nous bolus injection; FU 600 mg/m2 was administered as a
presence or absence of hepatic disease and by total tumor burden,
22-hour infusion immediately after FU bolus injection. LV and FU
which was defined as limited or extensive disease using 10 cm2 as
were repeated on days 1 and 2 according to a previously reported
the cutoff value. This cutoff value was arbitrarily chosen. The
schedule.19 Both regimens were administered at 2-week intervals.
Fig 1. Chemotherapy regimens. FU, fluorouracil; IV, intravenous; FOLFIRI, irinotecan, leucovorin, and fluorouracil regimen; FOLFOX4, oxaliplatin, leucovorin,
and fluorouracil regimen. (*) Patients between 70 and 75 years old: 150 mg/m2; (**) L-isomer form.
JOURNAL OF CLINICAL ONCOLOGY
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
FOLFIRI Versus FOLFOX4 in Advanced CRC
Evaluation of Response and Toxicity
to 150 mg/m2 (125 mg/m2 for patients with between the ages of
Survival, response duration, and TTP were determined from
70 and 75 years), FU bolus was reduced to 300 mg/m2, and FU
the date of first treatment until death or last follow-up and pro-
continuous infusion was reduced to 500 mg/m2. In the case of
gression. Objective response was first evaluated after four cycles of
persistent grade 3 toxicity or whenever grade 4 toxicity was re-
treatment and then every 2 months, according to a slight modifi-
corded, chemotherapy was definitively stopped. In the presence of
cation of WHO criteria.21 Briefly, a complete response (CR) was
grade 2 to 3 hematologic toxicity, treatment was delayed for 1 week
considered the complete disappearance of all evident tumor signs
or until hematologic recovery. If recovery was not reached, the
as estimated by two observations not less than 4 weeks apart.
dose level was reduced. For grade 0 to 2 gastrointestinal toxicity,
Partial response (PR) was defined as a 50% or greater decrease in
dose administration was 100%, and for grade 3 toxicity, after a
the sum of the products of the largest perpendicular diameters
1-week delay, the dose level was reduced. For neurosensory toxic-
of all measurable disease lasting at least 4 weeks, without occur-
ity, recommended dose modifications are listed in Table 1.
rence of new lesions. Stable disease (SD) was defined as a
change of less than 50% in the size of disease, and progressivedisease was defined as an increase of greater than 25% in the
Evaluation of objective response was the primary end point in
area of the measurable tumoral deposits or the appearance of
this trial. Objective responses were reported as relative rates with their
new lesions. The sum of CRs and PRs was reported as ORR.
95% CIs. Secondary end points were TTP, OS, and toxicity.
Response rates were provided for all patients (ie, intent-to-treat
As derived from published data9,19 at the time when the study
analysis [ITT]) and for assessable patients.
was started, the expected response rates were 35% and 50% for the
All toxicities, other than peripheral neuropathy, were graded
FOLFIRI and the FOLFOX4 regimens, respectively. Therefore, the
according to the National Cancer Institute Common Toxicity
study was designed to have the power to detect a 15% difference in
Criteria. Peripheral neuropathy was graded according to the spe-
objective response rate between the two arms, using a two-sided
cific grading system of Levı et al.22
log-rank test with an ␣ risk of .05 and a  risk of .20. The number
Prophylactic antiemetics were routinely administered before
of patients to be included in each arm was calculated to be 176.
each administration of the two regimens. Diarrhea or abdominal
All of the randomly assigned patients were included in the
cramping or important symptoms of a cholinergic syndrome that
survival analysis. To compare the difference between treatment
occurred during or within 1 hour after receiving CPT-11 were
groups for the proportion of patients with objective response, the
treated with atropine (0.25 mg subcutaneously). Routine use of a
z test of normal distribution and 95% CIs for proportions were
granulocyte colony-stimulating factor was not used in this trial.
used. The Wilcoxon rank sum test was used to evaluate the differ-
For symptoms of diarrhea and/or abdominal cramping that oc-
ence in the response duration between the treatment groups.23
curred more than 12 hours after receiving treatment, patients were
A univariate analysis of survival according to the product-
instructed to begin taking loperamide as soon as the first liquid stool
limit (Kaplan-Meier) estimate was performed.24 Comparisons be-
occurred (2 mg orally every 2 hours for at least 12 hours and up to 12
tween survival distributions were made by the log-rank test. In
hours after the last liquid stool, without exceeding a total treatment
multivariate analyses, the Cox proportional hazards model was
duration of 48 hours). Oral rehydration with large volumes of water
used to study the effects of different variables on survival.25
and electrolytes was prescribed during the whole diarrhea episode. If
Statistical significance was defined as P ⱕ .05 for univariate and
diarrhea persisted for more than 24 hours despite the recommended
multivariate analyses. All P values were based on two-sided testing.
loperamide treatment, a 7-day, prophylactic, oral, broad spectrumantibiotic therapy with fluoroquinolone was initiated.
If multiple toxicities were observed, the dose administered
was based on the most severe toxicity experienced. The dose ad-justment schedule was evaluated at the beginning of a new course
Patient and Clinical Characteristics
(based on laboratory analyses on the scheduled day of treatment
Between March 1999 and November 2002, 360 consec-
and on maximum toxicity encountered during the previous
utive patients were admitted onto the trial from the partic-
course). Dose reductions or treatment delays were calculated ac-
ipating centers. Of these patients, 178 were assigned to the
cording to the nonhematologic toxicity or myelosuppression re-corded at the time of the planned recycling (day 14). The drug dose
FOLFIRI regimen (arm A), and 182 were assigned to the
level was reduced in the case of severe or persistent toxicity; the LV
FOLFOX4 regimen (arm B). The arms were well balanced
dose remained fixed (100 mg/m2), whereas CPT-11 was reduced
with respect to stratification factors and other baseline
Table 1. Recommended Dose Modifications
Duration of Toxicity
⬎ 7 Days, ⱕ 14 Days
Persistent Between Courses
Cold-related dysesthesia
Paresthesia without pain
Stop until recovery; then restart at 75 mg/m2
Paresthesia associated with pain
Reduction: 75 mg/m2ⴱ
Paresthesia with functional impairment
Reduction: 75 mg/m2ⴱ
ⴱIf complete recovery and no neurologic symptoms at time of visit. In case of unbearable symptoms during the course, even if fully recovered at time of
visit, oxaliplatin administration was delayed until the next cycle and reintroduced at the dosage of 75 mg/m2.
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
Colucci et al
characteristics (Table 2). The median age in both groups
patients in arm B had previously received LV plus FU–
was 62 years. In arms A and B, 55 and 52 patients previously
based adjuvant chemotherapy.
received adjuvant therapy, respectively. The majority ofpatients had a primary colon cancer (66% and 68% in arms
A and B, respectively) and liver metastases (72% in arm A
A total of 336 patients were deemed assessable for re-
and 73% in arm B). Slightly more patients assigned to the
sponse (164 in arm A, 92%; and 172 in arm B, 95%). Overall,
FOLFIRI regimen had metachronous metastatic disease,
24 patients (14 in arm A and 10 in arm B) were considered
whereas, in the FOLFOX4 arm, more patients had lymph
nonassessable for the following reasons. In arm A, four pa-
node involvement. About half of the patients had multiple
tients were not assessable because of noneligibility or protocol
sites of disease in both arms (FOLFIRI, 44%; FOLFOX4,
violation, six patients refused to continue the treatment de-
46%). Moreover, 31% of patients in arm A and 32% of
spite low toxicity, three patients were not assessable because oftoxicity, and one patient was not assessable because of earlydeath unrelated to chemotherapy. In arm B, four patients werenot assessable because of noneligibility or protocol violation,
Table 2. Patient Characteristics
five patients refused to continue treatment (not for toxicity),
and one patient was not assessable because of toxicity.
Among the patients excluded for toxicity in arm A, one
patient had a cardiac ischemic episode after the second cycle
of treatment, and two patients died for hematologic reasons
(one severe treatment-related febrile neutropenia and one
disseminated intravascular coagulation); whereas in arm B,
one patient stopped the treatment because of grade 3 he-
patic toxicity (transaminase) after the first cycle of therapy.
A total of 1,264 cycles of the FOLFIRI regimen were
Performance status, ECOG
administered during the study, with a median of eight cycles
per patient (range, one to 22 cycles). A total of 1,321 cycles
of the FOLFOX4 combination therapy were administered,
with a median of eight cycles per patient (range, one to 15
Previous adjuvant therapy
cycles). The average number of cycles (ITT analysis) was
7.14 and 7.26 cycles in arms A and B, respectively. More
than 12 cycles were administered to four patients in arm A
and two patients in arm B.
The response rates for the two treatment arms are listed
Metastatic disease
in Table 3. Response rates between the FOLFIRI arm and
the FOLFOX4 arm did not statistically differ, whether eval-
Stratification groups
uated as ITT analysis (P ⫽ .60) or as assessable patients only
H⫹, tumor ⬎ 10 cm2
(P ⫽ .71). The ORR in the assessable patients was 34% in
H⫹, tumor ⬍ 10 cm2
arm A (95% CI, 26.9% to 41.4%) and 36% in arm B (95%
H⫺, tumor ⬎ 10 cm2
CI, 28.9% to 43.2%). When ITT analysis was performed,
⫺, tumor ⬍ 10 cm2
ORR was 31% in arm A (95% CI, 24.6% to 38.3%) and 34%
in arm B (95% CI, 27.2% to 41.5%). When also considering
SD, the overall tumor growth control rate (CR ⫹ PR ⫹ SD)
was 76% and 74% in arms A and B, respectively (70% in
both arms in ITT analysis).
The median duration of response was 9 months in arm
A and 10 months in arm B (P ⫽ .06), whereas the median
Liver ⫹ other sites
TTP according to ITT analysis was 7 months in both arms
(Fig 2). According to the ITT analysis, the median OS (Fig
3) was 14 and 15 months for patients in arms A and B,
Abbreviations: FOLFIRI, irinotecan, fluorouracil, and leucovorin; FOLFOX4,
respectively (P ⫽ .28). The median follow-up time of the
oxaliplatin, leucovorin, and bolus plus infusional fluorouracil; ECOG, Eastern
study was 31 months (range, 11 to 56 months), and the
Cooperative Oncology Group; H⫹, with liver involvement; H⫺, withoutliver involvement.
1-year survival rate was 55% and 62% (P ⫽ .16) in arms Aand B, respectively.
JOURNAL OF CLINICAL ONCOLOGY
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.

FOLFIRI Versus FOLFOX4 in Advanced CRC
Table 3. Response Rates for the Treatment Arms
No. of patients entered
No. of patients assessable
Fig 2. Time to progression. FOLFIRI, irinotecan, leucovorin, and fluorou-
racil regimen; FOLFOX4, oxaliplatin, leucovorin, and fluorouracil regimen.
CR ⫹ PR, No.
Assessable population
significant results with the FOLFOX4 regimen (25% and 40%
for arms A and B, respectively, with ITT analysis; P ⫽ .11).
When objective response rates were analyzed accord-
ing to the primary site of tumor, in arm A and B patients
with rectal and colon cancer, we observed (ITT analysis)
response rates of 30% (18 of 60 patients) versus 37% (22 of
59 patients) and 32% (38 of 118 patients) versus 33% (40 of
123 patients), respectively. In the 64 patients in arm A in
whom liver represented the only metastatic disease site, we
obtained 26 objective responses (41%), whereas in arm B,
24 (35%) of 68 patients obtained an objective response.
Furthermore, in patients with liver plus other disease sites,
we observed 15 objective responses in arm A (15 of 64
patients; 23%) and 21 objective responses in arm B (21 of
Abbreviations: FOLFIRI, irinotecan, fluorouracil, and leucovorin; FOLFOX4,
65 patients; 32%). Secondary surgery to remove liver me-
oxaliplatin, leucovorin, and bolus plus infusional fluorouracil; CR, completeresponse; PR, partial response; SD, stable disease; PD, progressive disease;
tastases was performed in nine patients in the FOLFIRI arm
TTP, time to progression.
(5.1%) versus eight patients in the FOLFOX4 arm (4.4%).
Considering the four stratification groups, the re-
sponse rates in arms A and B were as follows (ITT analysis):H⫹ and tumor more than 10 cm2, 30% v 37%, respectively
Second-line therapy (mainly consisting of OHP regi-
(P ⫽ .31); H⫹ and tumor less than 10 cm2, 37.5% v 24%,
mens after CPT-11 and CPT-regimens after OHP) was ad-ministered to 61% of patients previously treated withFOLFIRI and to 58% of patients previously treated withFOLFOX4. Overall, patients receiving second-line ther-apy had a median OS of 17 months, whereas patients whodid not receive a second-line therapy had a median OSof 10 months.
According to the obtained objective response, the me-
dian OS in arms A and B were as follows: patients withCR ⫹ PR, 18 v 20 months, respectively; patients with SD, 15v 15 months, respectively; and patients with progressivedisease, 8 v 9 months, respectively. In the group of patientswith hepatic metastatic disease, no difference was foundbetween the arms A and B in response rate (33% and 34%,respectively, with ITT analysis; P ⫽ .86), whereas patients
Fig 3. Overall survival. FOLFIRI, irinotecan, leucovorin, and fluorouracil
with lung metastases obtained better but not statistically
regimen; FOLFOX4, oxaliplatin, leucovorin, and fluorouracil regimen.
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
Colucci et al
respectively (P ⫽ .25); H⫺ and tumor more than 10 cm2,
The most frequent toxicity in FOLFIRI arm was gastro-
25% v 29%, respectively (P ⫽ .76); and H⫺ and tumor less
intestinal; as expected, in this arm, more alopecia and
than 10 cm2, 36% v 43%, respectively (P ⫽ .66). In the
gastrointestinal toxicities (mainly grade 1 to 2) were
patients with only a single site of disease, we observed a 38%
observed. Symptoms related to a cholinergic syndrome
ORR in arm A and a 34% ORR in arm B (P ⫽ .60), whereas
occurred in 18 patients treated with CPT-11 (10%). All
in patients with multiple sites of disease, the ORRs were
these events were manageable.
23% and 34% in arms A and B, respectively (P ⫽ .13).
In arm B, more grade 1 to 2 thrombocytopenia was
Multivariate analysis of prognostic factors related to
observed; furthermore, as expected, neurologic toxicity was
response rate did not show any statistical difference. In
more frequent. In the case of neurologic toxicity, we ob-
Table 4, multivariate analysis of prognostic factors related
served mainly cold-sensitive dysesthesias or paresthesias,
to OS is reported. The only factor predictive of improved
which occurred at low total cumulative doses and which
OS was the number of metastatic sites, and in the four
were reversible and did not require discontinuation of
stratification groups, only patients with the absence of
treatment. Only eight patients developed grade 3 neuropa-
metastatic liver disease less than 10 cm2 had statistically
thy (4%), and reversibility of this sensory neurotoxicity was
better survival than patients with liver metastases more
observed in all patients.
than 10 cm2.
Hypersensitivity reactions were observed only in the
FOLFOX4 arm and occurred mainly as grade 1 to 2 toxicity
after five to six cycles of treatment; premedication with
All patients were assessable for toxicity. In arm A, there
dexamethasone and antihistamine drugs in subsequent cy-
were two therapy-related deaths as a result of hematologic
cles enabled these patients to continue therapy with full-
toxicity (febrile neutropenia); another patient died of a
dose treatment. In the two patients with grade 3 to 4
disseminated intravascular coagulation (not related to the
hypersensitive toxicity, treatment continued without OHP.
treatment) because of concomitant progressive disease.
The death rates within the first 60 days of treatment were
There were no treatment-related deaths in arm B.
2.8% for patients receiving FOLFIRI and 1.1% for patients
The observed toxicities, according to the National Can-
receiving FOLFOX4 (P ⫽ .24). In the FOLFIRI arm, one pa-
cer Institute Common Toxicity Criteria, are listed in Table
tient died of disseminated intravascular coagulation, two pa-
5. Overall, toxicity was mild in both patient groups; grade 3
tients died of therapy-related febrile neutropenia, and two
to 4 toxicities were uncommon in both arms, with no sta-
patients died of progressive disease. In the FOLFOX4 arm,
tistical difference. When all grades of toxicities were ana-
two patients died of progressive disease. No chemotherapy-
lyzed, significant statistical differences between the two
related deaths were observed in the FOLFOX4 arm.
arms (A v B) were found for thrombocytopenia (15% v43%, respectively; P ⬍ .0001), nausea and vomiting (72%
v 59%, respectively; P ⫽ .009), diarrhea (63.5% v 46%,respectively; P ⫽ .0007), loss of hair (42% v 19%, respec-tively; P ⬍ .0001), and neurologic toxicity (5% v 45%,
Our study is the first randomized trial of a head-to-head
respectively; P ⬍ .0001).
comparison between the FOLFIRI and FOLFOX4 regimens
Table 4. Multivariate Analysis of Prognostic Factors
Single/multiple sites
PS ECOG, 0 v 1
PS ECOG, 0 v 2
H⫺, tumor ⬍ 10 cm2 v H⫺, tumor ⬎ 10 cm2
H⫺, tumor ⬍ 10 cm2 v H⫹, tumor ⬍ 10 cm2
H⫺, tumor ⬍ 10 cm2 v H⫹, tumor ⬎ 10 cm2
Abbreviations: HR, hazard ratio; PS, performance status; ECOG, Eastern Cooperative Oncology Group; H⫺, without liver involvement; H⫹, with
liver involvement.
JOURNAL OF CLINICAL ONCOLOGY
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
FOLFIRI Versus FOLFOX4 in Advanced CRC
Table 5. Observed Toxicities for Both Treatment Arms
Cholinergic syndrome
Abbreviations: FOLFIRI, irinotecan, fluorouracil, and leucovorin; FOLFOX4, oxaliplatin, leucovorin, and bolus plus infusional fluorouracil.
ⴱPeripheral neuropathy was graded according to the specific grading system of Levı et al.22
in the treatment of advanced CRC. For the last few decades,
nostic factors in both arms, such as multiple liver disease,
FU-based chemotherapy remained the mainstay of treat-
tumor burden, and previous adjuvant chemotherapy.
ment of CRC patients, and its biomodulation with LV
These considerations might explain the relatively lower re-
obtained better response rates than FU alone, but meta-
sponse rates observed in our study compared with the re-
analysis data failed to demonstrate a survival benefit com-
sponse rates of previous studies.9,19
pared with FU alone.1 Prolonged infusion of FU showed
In particular, when we considered the FOLFIRI arm,
better results in terms of response rate and OS than FU
the characteristics of patients entered onto our study
alone,26 and a hybrid regimen of FU (LV5FU2) with a bolus
seemed similar to the characteristics of patients in the study
and an infusional administration of FU obtained significant
by Douillard et al9; in addition, the objective response rate
improvements in response rate and TTP compared with
reported in our study according to an ITT analysis (31%)
the standard low-dose LV-FU bolus schedule of the
was similar that reported in the study by Douillard et al9
North Central Cancer Treatment Group regimen.2 Fur-
(32%). In the Douillard study, it was not possible to know
thermore, the addition to LV5FU2 of CPT-11 or OHP
the number of patients with a single site of metastatic dis-
showed better results than LV5FU2 alone in two large
ease, and a slightly smaller percentage of patients than in
studies,9,19 and therefore, these regimens were com-
our trial had previously received adjuvant chemotherapy
monly used in European countries as first-line therapy in
(26% v 31%, respectively). In the study by Tournigand et
advanced CRC patients.
al,27 the objective response rate obtained with the FOLFIRI
To verify and compare the activity of these two regi-
first-line treatment was 56%, but in this trial, there were
mens, the GOIM, in 1999, started protocol No. 9901. Three
fewer patients with multiple sites of disease (41%) and with
hundred sixty consecutive, nonseletected patients were en-
previously adjuvant therapy (17%) than in our study. These
tered onto this trial and randomly assigned to receive either
findings were reported as negative prognostic factors in the
the FOLFIRI regimen according to Douillard et al9 or the
analysis of previous experiences.10,19 The FOLFOX4 regi-
FOLFOX4 regimen according to de Gramont et al.19
men obtained a 34% response rate in our study. With the
Our results showed that no difference in terms of tu-
same regimen, de Gramont et al19 observed an ITT response
mor regression rate was observed in the two arms, with
rate of 50%. In this latter study, some characteristics of
objective responses observed in 31% of patients in the
enrolled patients appeared prognostically more favorable
FOLFIRI arm and 34% of patients in the FOLFOX4 arm
than in our study (20% had received adjuvant therapy and
(P ⫽ .60). The characteristics of patients were well balanced
33% had metachronous metastases v 29% and 37%, respec-
in the two arms, and the patients were representative of
tively, in our study). Tournigand et al,27 with a slightly
candidates for first-line chemotherapy in clinical practice,
different schedule and a higher OHP dose (FOLFOX6 reg-
with an elevated number of patients with unfavorable prog-
imen), observed tumor regression in 54% of patients; again,
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
Colucci et al
in this study, 21% of patients had received adjuvant ther-
toxicities were uncommon in both arms, with no statistical
apy, and 41% had multiple sites of disease.
difference. In the FOLFIRI arm, the most frequent toxicity
However, apart from considering the demographic
was gastrointestinal (mainly grade 1 to 2 diarrhea and nau-
characteristics of the entered patients, the patients entered
sea and vomiting), and the rates of toxic effects were similar
onto the present trial were representative of candidates for
overall to those observed in the study by Douillard et al.9
first-line chemotherapy in clinical practice. Most patients
More grade 1 to 2 thrombocytopenia and, as expected,
had unfavorable prognostic factors such as high liver in-
sensory neuropathy (mainly cold-sensitive dysesthesias or
volvement (⬎ 25%).
paresthesias) occurred in our study in the FOLFOX4 arm
Previous studies have shown that, in first-line therapy,
compared with the FOLFIRI arm. Only eight patients expe-
the addition of CPT-11 or OHP to the LV5FU2 regimen or
rienced grade 3 neuropathy, which was reversible in all
to bolus FU has an impact on survival of patients with
patients and did not require discontinuation of treatment.
metastatic CRC,9,10,19 and the median OS observed in these
The median number of FOLFOX4 cycles administered in
trials was between 15 and 17 months. No difference in OS
this study was eight, and this justifies the observed low rate
was observed between the FOLFIRI and FOLFOX4 arms in
of neurosensory toxicity. With the same regimen in the de
our study (14 v 15 months, respectively; P ⫽ .28). These
Gramont et al19 study, 18% of patients experienced grade 3
results are somewhat lower than those reported by other
neurologic toxicity, and the frequency of this adverse event
authors with the same or similar regimens.9,19,27,28 This
was clearly related to the exposure to OHP; 10% of
difference may be a result of the patient characteristics
patients had grade 2 to 3 neuropathy after three and nine
selected in our trial, as commented on earlier.
cycles of FOLFOX4, 25% had grade 2 to 3 neuropathy
As previously reported in other trials, in addition to the
after eight and 12 cycles, and 50% had grade 2 to 3
presence of negative prognostic factors, second-line thera-
neuropathy after 10 and 14 cycles. Furthermore, with an
pies have also shown an impact on survival. In our study,
enhanced dose of OHP and with a higher median number
second-line therapy was administered to 61% of the
of cycles,12 severe neurologic toxicity occurred in 34% of
FOLFIRI arm and to 58% of the FOLFOX4 arm. In the
patients treated with the FOLFOX6 regimen,28 and 19%
Tournigand et al27 study, 74% and 62% of patients re-
of patients had grade 3 neuropathy at the beginning of
ceived second-line chemotherapy in the FOLFIRI and the
FOLFIRI second-line therapy.
FOLFOX arms, respectively. In the three-arm study (IFL,
In conclusion, this study has demonstrated that there is
FOLFOX4, and irinotecan plus oxaliplatin [IROX]) re-
no difference in response rate, TTP, and OS for patients
ported by Goldberg et al,2 second-line therapy was admin-
treated with the FOLFIRI or FOLFOX4 regimens, and both
istered to 67%, 75%, and 70% of patients, respectively.
combination therapies seemed effective as first-line treat-
Second-line chemotherapy could have contributed to OS in
ment in advanced CRC patients. Our results confirm the
this trial; in fact, because OHP was not readily available in
efficacy of the addition of OHP or CTP-11 to the LV5FU2
North America, only 24% of patients received this drug
schedule and the mild toxicity of both regimens. Further-
after discontinuing the IFL arm, whereas CPT-11 was
more, FU still remains the basic component of the most
administered to 60% of patients after discontinuing the
efficacious regimens, and infusional FU is the best partner
FOLFOX4 regimen. This difference could have contrib-
in combination with OHP or CTP-11.29 The difference
uted to the statistically significant improvement in OS in
between these two combination therapies is mainly the
the FOLFOX4 arm compared with the IFL arm (19.5 v 15
toxicity profile; more gastrointestinal side effects and alope-
months, respectively; P ⫽ .0001), but the exact role of
cia were observed in the FOLFIRI arm, and more thrombo-
second-line chemotherapy in determining results re-
cytopenia, neurotoxicity, and hypersensitivity reactions
mains unclear.
were observed in the FOLFOX4 arm. Therefore, in clinical
When the three main effective drugs (FU, OHP, and
practice, the treatment choice must be individually tailored
CPT-11) were used in a higher percentage of patients, better
on these bases.
results were observed in terms of OS. In the Tournigand et
Improvement of the results obtained with these two
al27 study, 82% and 74% of patients received second-line
combination treatments should be possible with the addi-
treatment after the FOLFIRI and FOLFOX6 first-line regi-
tion of new drugs. In particular, targeted therapies, such as
mens, respectively, and OS was greater than 20 months in
two monoclonal antibodies directed against epidermal
both groups. In our study, the median OS time of patients
growth factor receptors or vascular endothelial growth
treated with the three main drugs was 18 months.
factor (cetuximab and bevacizumab, respectively), showed
As expected, the toxicity profile of both regimens
antitumor activity alone and in combination with chemo-
showed some differences. Also, for the safety evaluation,
therapy in advanced CRC.30,31 Phase II and III trials are
our study is the first study with a head-to-head comparison
ongoing in the United States and Europe.
between FOLFIRI and FOLFOX4. The adverse event profilewas favorable overall for both regimens, and grade 3 to 4
JOURNAL OF CLINICAL ONCOLOGY
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
FOLFIRI Versus FOLFOX4 in Advanced CRC
pital, Palermo: Vita Leonardi; Ospedale Nord, Taranto:
We thank Dr Vito Guerra for the statistical analysis and
Salvatore Pisconti; S. Carlo Hospital, Potenza: Gerardo Ro-
Dr Antonella Colucci for her assistance in the preparation
sati; Cardarelli Hospital, Campobasso: Francesco Carrozza;
of the manuscript.
Miulli Hospital, Acquaviva delle Fonti: Giuseppe Nettis;
Buccheri La Ferla Hospital, Palermo: Matteo Valdesi; Hos-
The following investigators are members of the Southern
pital, Paola: Gianfranco Filippelli; Analysis Center, Catania:
Italy Oncology Group (GOIM) and are coauthors of the
Santo Fortunato; Hospital, Campi Salentina: Sergio Man-
article: Oncology Institute, Bari: Severino Montemurro,
carella; and Hospital, Manduria: Cosimo Brunetti.
Antonio Cramarossa, Vito Lorusso, Maurizio Di Bisceglie;Centro
Authors' Disclosures of Potential
Chiarenza; University, Palermo: Maria Rosaria Valerio;
Conflicts of Interest
Cardarelli Hospital, Naples: Teresa Guida; M. Ascoli Hos-
The authors indicated no potential conflicts of interest.
static colorectal cancer: Irinotecan Study Group.
recombinant interferon-2b in advanced colorectal
N Engl J Med 343:905-914, 2000
cancer patients: A randomized multicenter study
11. Maiello E, Gebbia V, Giuliani F, et al:
with stratification for tumor burden and liver
1. Advanced
5-Fluorouracil and folinic acid with or without
involvement by the Southern Italy Oncology
Analysis Project: Modulation of fluorouracil by
CPT-11 in advanced colorectal cancer patients: A
Group. Cancer 85:535-545, 1999
leucovorin in patients with advanced colorectal
multicenter randomised phase II study of the
21. Miller AB, Hoogstraten B, Staquet M, et al:
cancer: Evidence in terms of response rate.
Southern Italy Oncology Group. Ann Oncol 11:
Reporting results of cancer treatment. Cancer
J Clin Oncol 10:896-903, 1992
2. de Gramont A, Bosset JF, Rougier P, et al:
12. Raymond E, Chaney SG, Taamma A, et al:
22. Levı F, Misset JL, Brienza S, et al: A
Randomized trial comparing monthly low-dose
Oxaliplatin: A review of preclinical and clinical
chronopharmacologic phase II clinical trial with
leucovorin and fluorouracil bolus with bimonthly
studies. Ann Oncol 9:1053-1071, 1998
5fluorouracil, folinic acid, and oxaliplatin using an
high-dose leucovorin and fluorouracil bolus plus
13. Raymond E, Buquet-Fagot C, Djelloul S, et
ambulatory multichannel programmable pump:
continuous infusion for advanced colorectal can-
al: Antitumor activity of oxaliplatin in combination
High antitumor effectiveness against metastatic
cer: A French Intergroup Study. J Clin Oncol
with 5-fluorouracil and the thymidylate synthase
colorectal cancer. Cancer 69:893-900, 1992
inhibitor AG337 in human colon, breast, and
23. Fisher LD, van Belle G: Biostatistics: A
3. Potmesil M: Camptothecins: From bench
ovarian cancers. Anticancer Drugs 8:876-885,
Methodology for the Health Sciences. New York,
research to hospital wards. Cancer Res 54:1431-
NY, John Wiley & Sons, 1993
14. Machover D, Diaz-Rubio E, de Gramont A,
24. Kaplan EL, Meier P: Nonparametric esti-
4. Rougier PH, Bugat R, Douillard JY, et al:
et al: Two consecutive phase II studies of oxali-
mation from incomplete observation. J Am Stat
Phase II study of irinotecan in the treatment of
platin (L-OHP) for treatment of patients with
Assoc 53:457-481, 1958
advanced colorectal cancer in chemotherapy-
advanced colorectal carcinoma who were resis-
25. Cox DR: Regression models and life ta-
naive patients and patients pretreated with 5-FU-
tant to previous treatment with fluoropyrimi-
bles. J R Stat Soc B 34:187-220, 1972
based chemotherapy. J Clin Oncol 15:251-260,
dines. Ann Oncol 7:95-98, 1996
26. Meta-Analysis Group in Cancer: Efficacy of
15. De Gramont A, Vignoud J, Tournigand C,
intravenous continuous infusion of fluorouracil
5. Rothenberg ML, Eckardt JR, Kuhn JG, et
et al: Oxaliplatin with high-dose leucovorin and5-fluorouracil 48-hour continuous infusion in pre-
compared with bolus administration in advanced
al: Phase II trial of irinotecan in patients with
treated metastatic colorectal cancer. Eur J Can-
colorectal cancer. J Clin Oncol 16:301-308, 1998
progressive or rapidly recurrent colorectal can-
cer 33:214-219, 1997
cer. J Clin Oncol 14:1128-1135, 1996
27. Tournigand C, André T, Achille E, et al:
16. André T, Bensmaı ne MA, Louvet C, et al:
6. Van Cutsem E, Cunningham D, Ten
FOLFIRI followed by FOLFOX6 or the reverse
Multicenter phase II study of bimonthly high-
Bokkel Huinink WW, et al: Clinical activity and
sequence in advanced colorectal cancer: A ran-
dose leucovorin, fluorouracil infusion, and oxali-
benefit of irinotecan (CPT-11) in patients with
domized GERCOR study. J Clin Oncol 22:229-
platin for metastatic colorectal cancer resistant
colorectal cancer truly resistant to 5-fluouracil
to the same leucovorin and fluorouracil regimen.
(5-FU). Eur J Cancer 35:54-59, 1999
28. Goldberg RM, Sargent DJ, Morton RF, et
J Clin Oncol 17:3560-3568, 1999
7. Rougier P, Van Cutsem E, Bajetta E, et al:
al: A randomized controlled trial of fluorouracil
17. Maindrault-Goebel F, Louvet C, André T,
Randomised trial of irinotecan versus fluorouracil
plus leucovorin, irinotecan, and oxaliplatin com-
et al: Oxaliplatin added to the simplified bi-
by continuous infusion after fluorouracil failure in
binations in patients with previously untreated
monthly leucovorin and fluorouracil regimen as
patients with metastatic colorectal cancer. Lan-
metastatic colorectal cancer. J Clin Oncol 22:23-
second-line therapy for metastatic colorectal
cet 352:1407-1412, 1998
cancer (FOLFOX6). Eur J Cancer 35:1338-1342,
8. Cunningham D, Pyrhonen S, James RD, et
29. Sobrero AF: Scheduling of fluorouracil: A
al: Randomised trial of irinotecan plus supportive
forget-me-not in the jungle of doublets. J Clin
18. Maindrault-Goebel F, de Gramont A, Louvet
care versus supportive care alone after fluorou-
Oncol 22:4-6, 2004
C, et al: High-dose intensity oxaliplatin added to the
racil failure for patients with metastatic colorec-
simplified bimonthly leucovorin and fluorouracil
30. Saltz LB, Meropol NJ, Loehrer PJ, et al:
tal cancer. Lancet 352:1413-1418, 1998
regimen as second-line therapy for metastatic
Phase II of cetuximab in patients with refractory
9. Douillard JY, Cunningham D, Roth AD,
colorectal cancer (FOLFOX7). Eur J Cancer 37:
colorectal cancer that expresses the epidermal
et al: Irinotecan combined with fluorouracil
growth factor receptor. J Clin Oncol 22:1201-
compared with fluorouracil alone as first-line
19. De Gramont A, Figer A, Seymour M, et al:
treatment for metastatic colorectal cancer: A
Leucovorin and fluorouracil with or without oxali-
31. Kabbinavar F, Hurwitz HI, Fehrenbacher L,
multicentre randomised trial. Lancet 355:1041-
platin as first-line treatment in advanced colorec-
et al: Phase II, randomized trial comparing bev-
tal cancer. J Clin Oncol 18:2938-2947, 2000
acizumab plus fluorouracil (FU)/leucovorin (LV)
10. Saltz LB, Cox JV, Blanke C, et al: Irinote-
20. Colucci G, Maiello E, Gebbia V, et al:
with FU/LV alone in patients with metastatic
can plus fluorouracil and leucovorin for meta-
5-Fluorouracil and levofolinic acid with or without
colorectal cancer. J Clin Oncol 21:60-65, 2003
Downloaded from www.jco.org by Ioannis Kaklamanos on November 3, 2005 .
Copyright 2005 by the American Society of Clinical Oncology. All rights reserved.
Source: http://www.eexo.gr/site/Education/Colorectal/Folfox.pdf
"Arts", "Food Nutrition", "Health & Society", "Humanities", "Social Sciences" Stanford University, Organizational Analysis The University of British Columbia, Climate Literacy : Navigating Climate Change Conversa- Stanford University, Democratic Development University of Virginia, The Kennedy Half Century Case Western Reserve University, Inspiring Leadership through Emotional Intelligence
The role of NMIs in providing reference measurements for the clinical sector - an LGC perspective Dr Ruth Hearn Biomedical Analysis & Metrology Laboratories Workshop Rabat, Morocco 19 – 20 November 2012 • Introduction to LGC • LGC, the National Measurement Institute • Scientific capabilities • Isotope dilution methods





