Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
031041u487
Expression of Macrophage Inflammatory
Protein-3
/CCL19 in Pulmonary Sarcoidosis
Agata Gibejova, Frantisek Mrazek, Daniela Subrtova, Veronika Sekerova, Jaroslava Szotkowska, Vitezslav Kolek,
Roland M. du Bois, and Martin Petrek
Departments of Immunology and Respiratory Medicine, Palacky University, Olomouc, Czech Republic; and Interstitial Lung Disease Unit,Royal Brompton Hospital, London, United Kingdom
In this study, messenger RNA (mRNA) expression for novel T
described using bioinformatics (5), including the leukotac-
lymphocyte chemoattractants, leukotactin-1, macrophage inflam-
tin-1 (Lkn-1)/CC chemokine ligand (CCL) 15/hemofiltrate CC
matory protein (MIP)-3␣
and MIP-3
was investigated in bronchoal-
chemokine-2 (6), macrophage inflammatory protein (MIP)-3␣/
veolar lavage fluid (BALF) cells from patients with sarcoidosis, a T
CCL20/liver and activation-regulated chemokine (7), and
cell–mediated disease with typical CD4⫹
lymphocyte alveolitis. Of
MIP-3/CCL19/Epstein-Barr virus–induced molecule 1 ligand
these three chemokines, only MIP-3
mRNA was upregulated in
chemokine/Exodus 3/CK-11 (7, 8).
sarcoidosis, and therefore, protein levels of this chemokine, its phar-
The chemokine MIP-3 is expressed especially in lymphoid
macologic regulation, and association with disease clinical course
tissues, whereas production of MIP-3␣ is found also in periph-
were explored. MIP-3
protein concentrations were elevated in
eral blood leukocytes and several fetal tissues (7, 8). Expres-
BALF from sarcoid patients compared with control subjects (p ⫽
sion of Lkn-1 is observed in the liver, intestine, and lung
0.001) and in patients with chest X-ray stage II chemokine protein
leukocytes (9). The gene encoding chemokine MIP-3 maps
levels were increased compared with stage I (p ⫽
0.003). MIP-3
on human chromosome 9, whereas most other CC chemokine
protein was associated predominantly with alveolar macrophages
genes (including MIP-3␣ and Lkn-1) are clustered on chro-
and correlated with BALF lymphocytes and T cell subsets. mRNA
expression for the MIP-3
mosome 17 (8). Although there are significant differences in

receptor, CC chemokine receptor 7, was
increased in sarcoidosis and correlated with MIP-3
protein levels.
tissue distribution of Lkn-1, MIP-3␣, and MIP-3, mRNA
MIP-3
mRNA and protein expression in BALF cells was suppressed
transcripts for all three chemokines have been previously
by dexamethasone and cyclosporine A in vitro. In conclusion, MIP-
found in samples of human lung tissue (7, 9, 10).
3
is implicated in T lymphocyte recruitment in sarcoidosis, is associ-
The chemokine MIP-3 is chemoattractant for T and B
ated with disease progression, and is downregulated by drugs used
lymphocytes (11, 12), dendritic cells (13), macrophage pro-
for sarcoidosis treatment. This novel chemokine, therefore, repre-
genitor cells (14), and natural killer cells (15). It might, there-
sents a candidate for studies of sarcoidosis pathobiologic mecha-
fore, play an important role in the trafficking of T cells in
the thymus and migration of T and B cells to secondarylymphoid organs (12, 16). Furthermore, MIP-3 has been
Keywords: chemokine; leukotactin-1; CC chemokine receptor 7; dexa-
recently shown to mediate rapid adhesion of naive CD4⫹ T
methasone; cyclosporine A
lymphocytes to activated endothelial cells supporting the role
Sarcoidosis is a multiorgan granulomatous disorder most fre-
of this chemokine in regulation of lymphocyte homing (17).
quently affecting the lung that results from the accumulation
MIP-3 acts through CC chemokine receptor 7 (CCR7) (8).
of CD4⫹ T lymphocytes and macrophages (1). The mecha-
The chemokines Lkn-1 and MIP-3␣ are also lymphocyte
nism of accumulation of inflammatory cells in the lung af-
attractants, although compared with MIP-3, their promigra-
fected by sarcoidosis is not clear. However, proinflammatory
tory effect on lymphocytes is less intensive and their chemo-
cytokines and chemokines have been previously implicated
tactic activity is more promiscuous. Lkn-1 attracts monocytes,
in this process (2).
lymphocytes, and eosinophils via the chemokine receptors
Chemotactic cytokines (chemokines) are low-molecular-
CCR1 and CCR3 (6, 18). MIP-3␣ exhibits promigratory ef-
weight cytokines traditionally divided into four subgroups
fects on lymphocytes, neutrophils, and immature dendritic
(CC, CXC, CX3C, and C) based on the structure of N-termi-
cells via the CCR6 receptor (13, 19).
nal cysteine motifs; the current nomenclature in line with
Regarding their promigratory effect on lymphocytes, we
this division assigns serial numbers to individual chemokines
hypothesized that novel lymphocyte attractant chemokines
(3). Chemokines regulate distribution of leukocytes and play
MIP-3 and also Lkn-1, and MIP-3␣ may contribute to the
an essential role in inflammation (4). Recently, a number of
development of the CD4⫹ lymphocyte alveolitis, which ac-
novel members of the chemokine superfamily have been
companies pulmonary sarcoidosis. We have, therefore, inves-tigated the mRNA expression of these three chemokines inbronchoalveolar lavage fluid (BALF) cells from patients withsarcoidosis in comparison with cells from healthy subjects
(
Received in original form May 30, 2002; accepted in final form March 4, 2003)
and have also examined the relationship between chemokine
Supported by the Czech Ministry of Health (IGA grant no. 3768–3 to M.P.); partial
mRNA expression and BALF cellular profile. These expres-
funding from the Czech government fund (MSMT J14/98.151100002) and also
sion studies revealed upregulation of MIP-3 transcripts,
from a Travel Fellowship granted to M.P. by the British Society for Immunology.
which was associated with lymphocyte alveolitis. We have,
Correspondence and requests for reprints should be addressed to Dr. Martin
therefore, focused on MIP-3. Protein levels of MIP-3 were
Petrek, M.D., Department of Immunology, Palacky University, I. P. Pavlova str. 6,
measured in BALF of patients in comparison to control sub-
Olomouc CZ-775 20, Czech Republic. E-mail:
[email protected]
jects and their relationship to clinical course of sarcoidosis,
This article has an online supplement, which is accessible from this issue's table
as assessed by chest X-ray stage and need for treatment, was
of contents online at www.atsjournals.org
evaluated. Furthermore, cell-associated MIP-3 protein in
Am J Respir Crit Care Med
Vol 167. pp 1695–1703, 2003
BALF was identified by immunocytochemistry, and mRNA
Originally Published in Press as DOI: 10.1164/rccm.200205-487OC on March 5, 2003
Internet address: www.atsjournals.org
expression of MIP-3 receptor, CC chemokine receptor
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
(CCR) 7, was determined in BALF cells. Finally, to explore
TABLE 1. CLINICAL AND LABORATORY DATA
OF INVESTIGATED SUBJECTS
how current therapeutic approaches to sarcoidosis interferewith MIP-3 chemokine expression, we have studied the
effects of dexamethasone and cyclosporine A on MIP-3
mRNA and protein expression
in vitro. Some of the results
(
n ⫽
78)
(
n ⫽
11)
of these studies have been previously reported in the form
of an abstract (20).
Bronchoalveolar lavage was performed according to a standard proce-
dure in 78 patients with sarcoidosis and 11 control subjects. The control
group consisted of subjects who at the time of presentation and subse-
BALF cell concentration, 105/ml
quently showed no clinical signs of lung inflammation; they had no
lung disease in their medical history. All had normal BALF cytology,
BALF differential count
immunology, and microbiology.
The diagnosis of pulmonary sarcoidosis was based on typical clinical
features together with granulomas on lung biopsies and supported by
the BALF cellular profile and was compatible with the criteria contained
in the International Statement on Sarcoidosis (21). Regarding chest
radiography, patients were divided into stages (stage 0: n ⫽ 2, stage I:
n ⫽ 48, stage II: n ⫽ 25, and stage III: n ⫽ 3). An additional subdivision
of patients was established to provide an index of disease course: pa-
tients requiring corticosteroid treatment (n
⫽ 40) and patients in whom
treatment was not necessary (i.e., the disease resolved spontaneously)
BALF CD4⫹/CD8⫹ ratio
(n ⫽ 38). No patient received corticosteroid treatment before bron-
choalveolar lavage. The treatment scheme did not differ from that
Corticosteroid treatment, yes/ no*
recommended in the International Statement on Sarcoidosis (21).
Chest X-ray stage, I/II/III/IV
Treatment with steroids was indicated according to an accepted proto-col: (
1 ) all patients with chest X-ray stage III disease at presentation,
Definition of abbreviations: BALF ⫽ bronchoalveolar lavage fluid; NE ⫽ not
(
2 ) patients with progressing and/or symptomatic stage II disease, and
(
3 ) patients with persistent stage I or II. Patients with persistent disease
Data are means ⫾ SD (min/max). Particular data were not available in a small
were treated after at least 6 months of disease observation. Detailed
number of individuals.
clinical characterization of the study groups is shown in Table 1. The
* Treatment initiated only after bronchoalveolar lavage.
study was performed with the approval of the Ethics Committee of theMedical Faculty of Palacky University Olomouc.
Semiquantification of Lkn-1, MIP-3␣
, MIP-3
, and CCR7
calf serum (Flow Labs, Irvine, UK) and 10 ng/ml of tumor necrosis
mRNA Expression in Bronchoalveolar Lavage Cells by
factor-␣ (NIBSC, Potters Bar, UK) in 5% CO2 atmosphere at 37⬚C.
Reverse Transcription-Polymerase Chain Reaction
Three types of cultures were set up: cells cultured alone and cells cul-tured in the presence of dexamethasone or cyclosporine A (dexametha-
The methods used for isolation of mRNA from unseparated BALF
sone acetate, 10⫺6 M [Le´cˇiva, Prague, Czech Republic]; and cyclosporine
cells and for semiquantification of chemokine mRNA expression are
A, 10 ng/ml [Sandimmune, Sandoz, Switzerland]). After a 21-hour cul-
described elsewhere (22, 23). Chemokine and receptor-specific poly-
ture, the supernatants were used for determination of protein by ELISA,
merase chain reaction were performed using modified amplification
and the cells were used for mRNA extraction for subsequent reverse
protocols (24, 25); primer sequences are shown in Table 2. Authenticity
transcription-polymerase chain reaction experiments.
of MIP-3 amplification was confirmed by direct sequencing (data notshown). -Actin–specific polymerase chain reaction has been describedelsewhere (23).
Optical densities of amplicons were determined using the software
Quantiscan (Biosoft, Ferguson, MO). mRNA expression was normal-
TABLE 2. CHARACTERIZATION OF OLIGONUCLEOTIDE
ized to the expression of the -actin gene as the optical density ratio
SEQUENCES OF THE PRIMERS USED FOR SPECIFIC
(ODR). This approach (description in detail in Petrek [23]) has been pre-
POLYMERASE CHAIN REACTION
viously validated in mRNA semiquantification of other chemokines (26).
MIP-3 protein levels were measured in BALF and cultured superna-tants by specific DuoSet ELISA Development kit (R&D Systems, Abing-
don, UK) according to the manufacturer's instructions. The detection
5⬘TTC CAC TGG AAA ATC CAG TAG
limit of this assay was 4 pg/ml. The method is described in detail in the
5⬘CTG GGT TTG GCA CAG AC
online supplement (section A).
5⬘TTT GAC TGC TGT CTT GGA TAC
5⬘GGC TAT GTC CAA TTC CAT TC
MIP-3 protein was detected on cytocentrifuge preparations of BALF
5⬘GCC CTG CTA CTG GCC CTC
cells using the streptavidin-biotin/horseradish peroxidase method with
5⬘GTC CTG GCT GGT CAG GTC
anti–MIP-3 monoclonal antibody (R&D Systems, Abingdon, UK). The
method is described in detail in the online supplement (section B).
5⬘CGC GTC CTT CTC ATC AGC AA
5⬘GTG CCG ACA GGA AGA CCA CT
Effect of Immunomodulators: In Vitro Regulation Experiments
Bronchoalveolar cells were cultured (106/ml cells) in RPMI-1640 me-
Definition of abbreviations: CCR7 ⫽ CC chemokine receptor 7; Lkn-1 ⫽
´ SOL, Prague, Czech Republic) supplemented with 8% fetal
leukotactin-1; MIP ⫽ macrophage inflammatory protein.
Gibejova, Mrazek, Subrtova,
et al.: Expression of MIP-3 in Sarcoidosis
Relationship between Lkn-1, MIP-3␣
, and MIP-3
mRNA
Expression and BALF Cells
The data are reported as median with first to third quartile (interquartilerange [IQR]). Comparisons of mRNA and protein expression between
Because Lkn-1, MIP-3␣, and MIP-3 are novel lymphocyte at-
study groups were performed using the nonparametric Mann-Whitney
tractant chemokines, we investigated a possible relationship of
U-test. Spearman's rank correlation was used to assess the relationships
their mRNA expression with BALF lymphocyte numbers.
between chemokine expression and cellular profile of BALF and the
mRNA expression of Lkn-1 and MIP-3␣ did not correlate with
clinical course of sarcoidosis. Paired
t test analysis was used to investi-
the number of BALF lymphocytes nor with the CD4⫹/CD8⫹
gate differences in expression
in vitro culture experiments. Differences
T cell ratio and BALF lymphocyte subsets. MIP-3 mRNA
with a p value of less than 0.05 were considered statistically significant.
expression correlated with absolute (
r ⫽
0.389, p ⫽ 0.017) and
relative (
r ⫽
0.373, p ⫽ 0.019) BALF lymphocyte count and
moreover with CD4⫹ lymphocytes (
r ⫽
0.488, p ⫽ 0.003) but
Lkn-1, MIP-3␣
, and MIP-3
mRNA Expression in BALF Cells
not with the CD8⫹ subset. Furthermore, there was a strikingassociation between MIP-3 mRNA expression and BALF
To investigate chemokine mRNA expression in patients with
CD4⫹/CD8⫹ T cell ratio (
r ⫽
0.541, p ⬍ 0.001; Figure 2B).
sarcoidosis and control subjects, mRNA extracted from unsepa-
The relationship between Lkn-1, MIP-3␣, and MIP-3 mRNA
rated BALF cells by biomagnet separation was reverse tran-
expression and number of BALF macrophages, neutrophils, and
scribed to cDNA and specific polymerase chain reaction for the
eosinophils was also investigated, but no association was ob-
chemokine Lkn-1, MIP-3␣, and MIP-3 genes were performed.
served (p ⬎ 0.05).
The levels of mRNA expression (ODR) were determined foreach individual by normalizing to the expression of the -actin
MIP-3
Protein Levels in BALF and Their Relationship
to BALF Cell Profile
Lkn-1 mRNA transcripts were found in similar frequency in
25 of 30 (83%) of patients with sarcoidosis and in 11 of 11
Because of the finding of upregulated mRNA for MIP-3 and
(100%) of control subjects. Lkn-1 mRNA expression was similar
its association with numbers of total BALF lymphocytes, we
in both groups tested (ODR, median [IQR]; control subjects,
explored this chemokine also at the protein level. BALF protein
0.66 [0.38–0.93]; sarcoidosis, 0.62 [0.25–0.85]; p ⫽ 0.814; Figure
concentrations were significantly increased in patients with sar-
coidosis in comparison to control subjects (median [IQR], pg/
MIP-3␣ mRNA transcripts were observed in similar fre-
ml; control subjects, 5.9 [5.2–7.0]; sarcoidosis, 11.2 [6.9–47.6]; p ⫽
quency in 28 of 30 (93%) of patients with sarcoidosis and in 10
0.001; Figure 3A). MIP-3 BALF protein levels correlated with
of 11 (91%) of control subjects. MIP-3␣ mRNA expression in
mRNA expression (
r ⫽
0.320, p ⫽ 0.036). The MIP-3 BALF
BALF cells from patients with sarcoidosis and from control
protein levels strongly correlated with absolute (
r ⫽
subjects was similar (ODR, median [IQR]; control subjects, 0.57
0.001; Figure 3B) and relative (
r ⫽
0.53, p ⬍ 0.001) numbers
[0.26–0.95]; sarcoidosis, 0.62 [0.42–0.91]; p ⫽ 0.659; Figure 1B).
of total BALF lymphocytes. There was a strong correlation be-
MIP-3 mRNA was detected in 70% (21 of 30) of patients
tween MIP-3 BALF protein concentrations and absolute num-
with sarcoidosis but only in 55% (6 of 11) of control subjects.
ber of both CD4⫹ and the CD8⫹ BALF lymphocyte subsets
Importantly, MIP-3 mRNA expression was increased in patients
0.370, p ⫽ 0.002 and rs
0.340, p ⫽ 0.005, respectively). The
with sarcoidosis in comparison with control subjects (ODR, me-
relationship between MIP-3 protein expression and number
dian [IQR]; control subjects, 0.00 [0.00–0.17]; sarcoidosis, 0.33
of BALF macrophages, neutrophils, and eosinophils was also
[0.00–0.47]; p ⫽ 0.035; Figure 2A).
investigated, but no association was observed (p ⬎ 0.05).
Figure 1. Messenger RNA (mRNA) expression of the chemokine leukotactin (Lkn)-1 (
A ) and macrophage inflammatory protein (MIP)-3␣ (
B ) in
bronchoalveolar lavage fluid (BALF) cells obtained from control subjects (n ⫽ 11) and patients with sarcoidosis (n ⫽ 30). mRNA expression is
semiquantified using an ODR (group medians are indicated by
horizontal bars).
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Figure 2. (
A ) mRNA expression of the chemokine MIP-3 in BALF cells obtained from control subjects (n ⫽ 11) and patients with sarcoidosis (n ⫽
30). mRNA expression is semiquantified using an optical density ratio (ODR) (group medians are indicated by
horizontal bars). (
B ) Relationship of
MIP-3 mRNA expression (ODR MIP-3/-actin) with BALF CD4⫹/CD8⫹ T cell ratio. MIP-3 mRNA expression versus BALF CD4⫹/CD8⫹ T cell
ratio (
r ⫽
0.541, p ⬍ 0.001).
Open triangles ⫽ control subjects;
closed circles ⫽ subjects with sarcoidosis.
MIP-3
Protein Levels in BALF and Clinical Course
Determination of Cell-associated MIP-3
Protein
To evaluate whether MIP-3 is associated with the course of
To identify the cellular source of MIP-3, cytocentrifuge prepa-
sarcoidosis, its protein expression was analyzed in subgroups of
rations of BALF cells obtained from 17 patients with sarcoidosis
patients with different chest X-ray stage and distinct requirement
and from 6 control subjects were immunostained for MIP-3
for treatment. MIP-3 concentrations were significantly elevated
protein. The protein was found to be expressed in all investigated
in patients with chest x-ray stage II (n ⫽ 25) in comparison to
patients and control samples. Strong, uniform expression of MIP-3
patients with stage I (n ⫽ 48) (median [IQR], pg/ml; stage I, 9.6
was observed in alveolar macrophages (Figure 5). MIP-3 protein
[6.4–24.2]; stage II, 48.6 [9.3–213.0]; p ⫽ 0.003; Figure 4A). The
was associated also with lymphocytes; however, its expression
BALF protein levels of MIP-3 were higher in patients requiring
was limited to approximately one-third of BALF lymphocytes,
treatment (n ⫽ 40, median [IQR], pg/ml; 16.9 [8.5–106.8]) than
and it was less intensive than in the case of macrophage-associ-
in patients with spontaneous remission (n ⫽ 38, median [IQR],
ated MIP-3. Semiquantitative analysis of MIP-3–immuno-
pg/ml; 10.1 [6.5–38.9]; Figure 4B); the difference did not, how-
stained preparations revealed a trend to higher proportion of
ever, attain significance (p ⫽ 0.07).
MIP-3 strongly positive macrophages in patients with sar-
Figure 3. (
A ) Concentrations of MIP-3 protein (pg/ml, expressed by log) in BALF obtained from control subjects (n ⫽ 11) and patients with
sarcoidosis (n ⫽ 78) (group medians are indicated by
horizontal bars). (
B ) Relationship of MIP-3 protein concentrations (pg/ml, expressed by
log) with BALF lymphocytes. MIP-3 protein versus BALF absolute lymphocyte number (
r ⫽
0.500, p ⬍ 0.001).
Open triangles ⫽ control subjects;
closed diamonds ⫽ subjects with sarcoidosis.
Gibejova, Mrazek, Subrtova,
et al.: Expression of MIP-3 in Sarcoidosis
Figure 4. Concentrations of MIP-3 protein (pg/ml, expressed by log) in BALF obtained from patients with different chest X-ray stage (
A ) and
distinct disease course (
B ) (group medians are indicated by
horizontal bars).
coidosis in comparison to control subjects (
see Figure E1 in the
from 8 (75%) cultures affected by cyclosporine A (dexametha-
online supplement).
sone, p ⫽ 0.020; cyclosporine A, p ⫽ 0.029).
CCR7 mRNA Expression in BALF Cells and Its Relationship
to BALF Cell Profile
The accumulation of immune cells (macrophages and CD4⫹ T
Because MIP-3 is a ligand for CCR7, we have attempted to
lymphocytes) in the pulmonary interstitium and alveoli is a typi-
analyze mRNA expression of this receptor molecule. CCR7
cal feature of patients with lung inflammation in sarcoidosis (1).
mRNA was detected in 67% (20 of 30) of patients affected by
Several chemokines, such as regulated upon activation, normal
sarcoidosis and only in 36% (4 of 11) of control subjects. Patients
T cell expressed and secreted (CCL5), MIP-1␣ (CCL3), MIP-1
with sarcoidosis had higher CCR7 mRNA expression (ODR,
(CCL4), monocyte chemoattractant protein-1 (CCL2), and inter-
median [IQR]; sarcoidosis, 0.25 [0.00–0.51]) than control subjects
feron-␥ inducible protein-10 (CXCL10), have already been im-
(ODR, median [IQR]; control subjects, 0.00 [0.00–0.21]; Figure
plicated in the recruitment of leukocytes to the lungs of these
6A); the difference did not, however, attain significance (p ⫽
patients (27–30). In this study, we have focused on novel candi-
date mediators of this migration process: lymphocyte attractant
CCR7 mRNA expression correlated with absolute (
r ⫽
chemokines Lkn-1, MIP-3␣, and MIP-3, which were recently
p ⫽ 0.031) and relative (
r ⫽
0.338, p ⫽ 0.035) numbers of
identified by bioinformatics (5). Of these three novel chemo-
total BALF lymphocytes. It also strongly associated with CD4⫹
kines, MIP-3 expression was upregulated in BALF cells and
lymphocytes (absolute number, r ⫽
0.469, p ⫽ 0.005; relative
also in BALF fluid from patients with sarcoidosis by comparison
0.380, p ⫽ 0.019) but not with CD8⫹ T cell numbers.
with control subjects. Importantly, MIP-3 BALF protein levels
Furthermore, there was a relationship between CCR7 mRNA
paralleled the disease course and were associated with the extent
expression and BALF CD4⫹/CD8⫹ T cell ratio (
r ⫽
of CD4⫹ T lymphocyte alveolitis. Finally, studies directed at
0.006; Figure 6B). The association between CCR7 mRNA ex-
MIP-3 receptor, CCR7, suggested an increase of CCR7 mRNA
pression and number of other BALF cells was also investigated:
expression in BALF cells from sarcoid patients.
There was a relationship between CCR7 mRNA and absolute
The findings of this study are in compliance with our initial
0.422, p ⫽ 0.009) and relative (
rs
0.369, p ⫽ 0.021) number
hypothesis that the novel chemokines, including MIP-3, may
of BALF neutrophils. Interestingly, CCR7 mRNA expression
contribute to the recruitment of lymphocytes to the sarcoid lung.
correlated with MIP-3 BALF protein levels (
r ⫽
First, both MIP-3 mRNA and protein were upregulated in
BALF from patients with sarcoidosis. Second, there was a strong
Effect of Immunomodulators on MIP-3
mRNA and Protein
correlation between MIP-3 expression and total BALF lym-
Expression In Vitro
phocytes as well as with the absolute BALF CD4⫹ T cell count.
Furthermore, our immunocytochemistry experiments revealed
In vitro experiments investigating the effects of dexamethasone
that although virtually all alveolar macrophages were immuno-
and cyclosporine A on MIP-3 mRNA and protein expressions
stained for MIP-3 protein, only minor MIP-3 expression was
were performed to explore the modulatory effect of these drugson chemokine expression. The results are summarized in Figures
associated with some BALF lymphocytes. Semiquantitative
7A and 7B, respectively. Tumor necrosis factor-
analysis showed more intensive immunostaining of sarcoid mac-
␣–induced MIP-
3 expression was significantly suppressed in cells cultured in
rophages compared with macrophages from our control subjects,
the presence of dexamethasone and cyclosporine A. The levels
indicating that in disease there is more MIP-3 per cell. In this
of mRNA were reduced in 10 of 12 (83%) cultures treated by
context, the possibility that increased chemokine detected in
dexamethasone and in 7 of 8 (88%) cultures in the presence of
sarcoid macrophages arose from phagocytosis of external che-
cyclosporine A (dexamethasone, p ⫽ 0.004; cyclosporine A, p ⫽
mokine is unlikely. Other than macrophages, there are only
0.009). MIP-3 protein concentrations were downregulated in
minor producers of MIP-3 present in BALF. Dendritic cells,
9 from 12 (75%) cultures treated by dexamethasone and in 6
which also express this chemokine, account for no more than
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
cruitment. There are functional data on preferential attraction ofCD4⫹ T cells by MIP-3 (11). However, a definite role for thischemokine in the cell-migration process in sarcoidosis cannot beassigned before chemotaxis experiments with BALF cells areperformed.
In contrast to MIP-3, mRNA expression of the other two
chemokines, Lkn-1 and MIP-3␣, did not differ between patientsand control subjects, and their expression was not related to theextent of lymphocyte alveolitis. Rather than acting as diseasemediators, these chemokines may contribute to immune surveil-lance by participation in regulation of physiological T cell traf-ficking (16, 35). Although mRNA for these two chemokineswas expressed in the great majority of study subjects, MIP-3expression was much more heterogeneous. Apart from manynegative control subjects, no MIP-3 transcripts were detectedin approximately one-third of patients. We are not aware of anymethod confounder: The BALF procedure and its processingwere standardized as in our previous investigations (27, 29), andour mRNA isolation procedure and semiquantitative reversetranscription-polymerase chain reaction method were carefullyvalidated with regard to the specific conditions of this study (22,23). In protein experiments, free immunoreactive MIP-3 wasdetected in all study subjects, but the distribution of individualvalues of BALF chemokine concentrations resembled the pat-tern of the mRNA data; importantly, mRNA level and proteinconcentrations correlated. It is indeed possible that the commer-cial ELISA assay was more sensitive and detectable, albeit lowprotein levels were determined also in the samples in which noMIP-3 transcripts could be identified. The observed heterogene-ity of MIP-3 expression may be due to interindividual differencesin the hierarchy of chemokine expression. Microarray technologycould be used for future assessment of parallel expression of aset of chemokines and also for the analysis of the contribution ofgene polymorphisms to differences in interindividual expression.
As already mentioned, upregulated expression of several CC
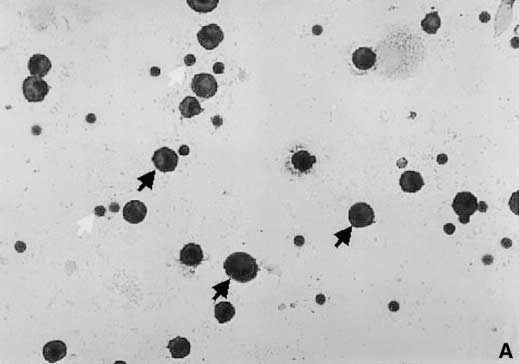
Figure 5. Detection of MIP-3 protein in BALF cells from patients with
and CXC chemokines has been reported before in sarcoidosis
sarcoidosis; representative result of immunocytochemistry experiments.
(36), and a relationship between monocyte chemoattractant
Positively stained cells were observed in BALF cytospin preparations
protein-1 (CCL2), MIP-1␣ (CCL3), and MIP-1 (CCL4) protein
incubated with anti–MIP-3 antibody (A ), but not with the irrelevant
levels and clinical course of disease has been described (28, 29,
control antibody (B ). MIP-3 was localized predominantly to the cyto-
37). In this context, it is interesting that the upregulation of MIP-
plasm of macrophages (black arrows); less intensive staining was associ-
3 protein was observed in the BALF of our patients affected
ated also with a minor proportion of BALF lymphocytes (white arrows)
by chest X-ray stage II in comparison to patients with stage I.
(streptavidin-biotin/horseradish peroxidase method with 3,9 amino-
There was also a difference in MIP-3 expression in patients
ethyl-carbazole as substrate and counterstained with hematoxylin;
subdivided according to the need for corticosteroid treatment (as
original magnification ⫻125).
a measure of disease evolution): MIP-3 protein levels tended tobe higher in patients requiring treatment than in patients withspontaneous remission. The lack of a significant difference be-tween active and inactive disease may be due to the fact that the
1% of the cells in the bronchoalveolar compartment (31), and
relatively small number of patients may have been insufficient to
interestingly, their typical phenotypic markers can be acquired
detect a difference in MIP-3 in patients with active disease.
by sarcoid alveolar macrophages (32). All of these facts imply
The treatment scheme did not differ from that recommended in
that alveolar macrophages are indeed the major source of BALF
the International Statement on Sarcoidosis (21), and therefore, it
MIP-3 protein, which is also concordant with reports of MIP-3
is less likely that lack of significance is caused by usage of differ-
cellular expression in other tissues (7, 33). However, a definitive
ent criteria for starting steroids by physicians involved in this
answer about the source of the chemokine would require evalua-
study. Further studies based on additional functional and clinical
tion of transcripts at a single-cell level. Finally, we report here
characteristics including long-term patient follow-up are, how-
that there is a relationship between the expression of MIP-
ever, necessary for a more definitive interpretation of the clinical
3 and its receptor molecule, CCR7: The number of CCR7
significance of chemokine upregulation.
transcripts correlated with MIP-3 expression, and in patients
From a practical point of view, it is important that drugs used
with sarcoidosis, there was a marked trend to an increase of
for sarcoidosis treatment, dexamethasone and cyclosporine A,
mRNA expression for the CCR7 molecule, which is, however,
suppressed in vitro MIP-3 expression in BALF cells from pa-
not fully specific for MIP-3, as it may bind another ligand,
tients with sarcoidosis. By analogy, our data have an in vivo
chemokine secondary lymphoid tissue chemokine (CCL21) (34).
correlate in the clinical observation of Hashimoto and coworkers
Taken together, our findings are in favor of the explanation
(37): After successful corticosteroid treatment of patients with
that macrophage-derived MIP-3 contributes to the development
sarcoidosis, the elevated serum levels of other chemokines
of alveolitis in sarcoid lung by promoting CD4⫹ lymphocyte re-
(monocyte chemoattractant protein-1 and MIP-1␣) returned to
Gibejova, Mrazek, Subrtova, et al.: Expression of MIP-3 in Sarcoidosis
Figure 6. (A ) mRNA expression of the chemokine receptor CCR7 in BALF cells obtained from control subjects (n ⫽ 11) and patients with sarcoidosis
(n ⫽ 30). mRNA expression is semiquantified using an ODR (group medians are indicated by horizontal bars). (B ) Relationship of CCR7 mRNA
expression (ODR CCR7/-actin) with BALF CD4⫹/CD8⫹ T cell ratio. CCR7 mRNA expression versus BALF CD4⫹/CD8⫹ T cell ratio (r ⫽
p ⫽ 0.006). Open triangles ⫽ control subjects; closed circles ⫽ subjects with sarcoidosis.
normal values. The potential practical importance of the re-
frequent (40–42), but there has been no report of its expression
ported elevation of MIP-3 in sarcoidosis, including data on its
in lung diseases. The aforementioned reports also imply the
pharmacologic regulation, would be strengthened by other in
novelty of our data, which means that their significance has to
vivo data. However, we did not find any report of work in animal
be verified in future independent studies.
model with this chemokine that could be used to support or
In conclusion, we report here for the first time that the chemo-
dispute its potential as a therapeutic target. Also, published
kine MIP-3 and its CCR7 (but not chemokines Lkn-1 and
information on in vivo expression of MIP-3 and other investi-
MIP-3␣) are upregulated in the bronchoalveolar fluid cells from
gated chemokines is limited. To our knowledge, MIP-3 expres-
patients with sarcoidosis. Furthermore, we demonstrate that
sion in human disease has so far been restricted to Sjo¨gren's
MIP-3 mRNA and protein levels and CCR7 mRNA expression
syndrome (38) and atherosclerosis (33), in which also Lkn-1 was
correlate with the number of lymphocytes in the bronchoalveolar
detected (39). Reports of disease expression of MIP-3␣ are more
compartment and that MIP-3 protein was localized mainly in
Figure 7. (A ) Effect of dexamethasone (Dx) on MIP-3 mRNA and protein expression in vitro. The effect is expressed as an index of modulation
that normalizes the data to the tumor necrosis factor (TNF)-stimulated values for mRNA and protein expression in 12 cultures treated with Dx
(mean ⫾ SEM). Paired t test was used to investigate differences in MIP-3 expression in modulated cultures in vitro. TNF-␣ induced MIP-3 protein
expression in cultures treated by Dx versus cultures without effect of Dx (p ⫽ 0.020). TNF-␣ induced MIP-3 mRNA expression in cultures treated
by Dx versus cultures without effect of Dx (p ⫽ 0.004). (B ) Effect of cyclosporine A (CyA) on MIP-3 mRNA and protein expression in vitro. The
effect is expressed as an index of modulation that normalizes the data to the TNF-stimulated values for mRNA and protein expression in 8 cultures
treated with CyA (mean ⫾ SEM). Paired t test was used to investigate differences in MIP-3 expression in modulated cultures in vitro. TNF-␣
induced MIP-3 protein expression in cultures treated by CyA versus cultures without effect of CyA (p ⫽ 0.029). TNF-␣ induced MIP-3 mRNA
expression in cultures treated by CyA versus cultures without effect of CyA (p ⫽ 0.009).
AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
EC. Chemokines and the arrest of lymphocytes rolling under flow con-
alveolar macrophages. These findings indicate that MIP-3 is
ditions. Science 1998;279:381–384.
implicated in the complex network between lymphocytes and
18. Pardigol A, Forssmann U, Zucht HD, Loetscher P, Schulz-Knappe P,
cytokines, which sets the stage for the subsequent development
Baggiolini M, Forssmann WG, Magert HJ. HCC-2, a human chemo-
of sarcoid granuloma. Finally, we have shown that MIP-3 ex-
kine: gene structure, expression pattern, and biological activity. Proc
pression can be suppressed by dexamethasone and cyclosporine
Natl Acad Sci USA 1998;95:6308–6313.
A at both the mRNA and protein level. These data and our
19. Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K,
observation of a relationship between MIP-3 protein and the
Nomiyama H, Yoshie O. Identification of CCR6, the specific receptorfor a novel lymphocyte-directed CC chemokine LARC. J Biol Chem
clinical course of disease suggest that this chemokine may be
an important mediator in the pathobiologic mechanisms of
20. Gibejova A, Mrazek F, Subrtova D, Szotkowska J, Kolek V, du Bois RM,
Petrek M. Relationship between macrophage inflammatory protein-3(MIP-3) levels and clinical course of sarcoidosis [abstract]. Eur Respir
Acknowledgment : Determination of lymphocyte subsets in BALF was performed
in part by Dr. M. Ordeltova. Dr. J. Drabek designed primer pairs for Lkn-1 andMIP-3␣. The authors gratefully acknowledge technical assistance from Ms. A.
21. American Thoracic Society/European Respiratory Society/World Associ-
Vevodova and the staff of Bronchology Unit, Department of Respiratory Medicine,
ation of Sarcoidosis and Other Granulomatous Disorders. Statement
Faculty Hospital Olomouc.
on sarcoidosis. Am J Respir Crit Care Med 1999;160:736–755.
22. Mrazek F, Petrek M. Processing of mRNA from human leukocytes by
biomagnetical separation: comparison with current methods of RNA
isolation. Acta Univ Palacki Olomuc Fac Med 1999;142:23–28.
1. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;
23. Petrek M. Analysis of chemokine gene expression in lung cells by poly-
merase chain reaction. Acta Univ Palacki Olomuc Fac Med 1999;
2. Keane MP, Standiford TJ, Strieter RM. Chemokines are important cyto-
kines in the pathogenesis of interstitial lung disease. Eur Respir J 1997;
24. Byrnes HD, Kaminski H, Mirza A, Deno G, Lundell D, Fine JS. Macro-
phage inflammatory protein-3 enhances IL-10 production by acti-
3. Zlotnik A, Yoshie O. Chemokines: a new classification system and their
vated human peripheral blood monocytes and T cells. J Immunol 1999;
role in immunity. Immunity 2000;12:254–257.
4. Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2001;2:108–
25. Bunce M, O'Neill CM, Barnardo MC, Krausa P, Browning MJ, Morris
PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A,
5. Wells TN, Peitsch MC. The chemokine information source: identification
B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer
and characterization of novel chemokines using the World Wide Web
mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens
and expressed sequence tag databases. J Leukoc Biol 1997;61:545–550.
6. Youn BS, Zhang SM, Lee EK, Park DH, Broxmeyer HE, Murphy PM,
26. Southcott AM, Jones KP, Li D, Majumdar S, Cambrey AD, Pantelidis
Locati M, Pease JE, Kim KK, Antol K, et al. Molecular cloning of
P, Black CM, Laurent GJ, Davies BH, Jeffery PK. Interleukin-8:
leukotactin-1: a novel human beta-chemokine, a chemoattractant for
differential expression in lone fibrosing alveolitis and systemic sclero-
neutrophils, monocytes, and lymphocytes, and a potent agonist at CC
sis. Am J Respir Crit Care Med 1995;151:1604–1612.
chemokine receptors 1 and 3. J Immunol 1997;159:5201–5205.
27. Petrek M, Pantelidis P, Southcott AM, Lympany P, Safranek P, Black
7. Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Iden-
CM, Kolek V, Weigl E, du Bois RM. The source and role of RANTES
tification through bioinformatics of two new macrophage proinflam-
in interstitial lung disease. Eur Respir J 1997;10:1207–1216.
matory human chemokines: MIP-3alpha and MIP-3beta. J Immunol
28. Capelli A, di Stefano A, Lusuardi M, Gnemmi I, Donner CF. Increased
macrophage inflammatory protein-1␣ and macrophage inflammatory
8. Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura
protein-1 levels in bronchoalveolar lavage fluid of patients affected
M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a
by different stages of pulmonary sarcoidosis. Am J Respir Crit Care
novel human CC chemokine EBI1-ligand chemokine that is a specific
functional ligand for EBI1, CCR7. J Biol Chem 1997;272:13803–13809.
29. Petrek M, Kolek V, Szotkowska J, du Bois RM. CC and C chemokine
9. Coulin F, Power CA, Alouani S, Peitsch MC, Schroeder JM, Moshizuki
expression in pulmonary sarcoidosis. Eur Respir J 2002;20:1206–1212.
M, Clark-Lewis I, Wells TN. Characterisation of macrophage inflam-
30. Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A,
matory protein-5/human CC cytokine-2, a member of the macrophage-
Piazza F, Siviero M, Facco M, Dziejman M, et al. Involvement of the
inflammatory-protein family of chemokines. Eur J Biochem 1997;
IP-10 chemokine in sarcoid granulomatous reactions. J Immunol 1998;
10. Hromas R, Gray PW, Chantry D, Godiska R, Krathwohl M, Fife K, Bell
31. Nicod LP, Cochand L, Dreher D. Antigen presentation in the lung:
GI, Takeda J, Aronica S, Gordon M, et al. Cloning and characterization
dendritic cells and macrophages. Sarcoidosis Vasc Diffuse Lung Dis
of exodus, a novel beta-chemokine. Blood 1997;89:3315–3322.
11. Kim CH, Pelus LM, White JR, Applebaum E, Johanson K, Broxmeyer
32. Nicod LP, Habre F. Adhesion molecules on human lung dendritic cells
HE. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-li-
and their role for T cell activation. Am J Respir Cell Mol Biol 1992;7:
gand chemokine is an efficacious chemoattractant for T and B cells.
J Immunol 1998;160:2418–2424.
33. Reape TJ, Rayner K, Manning CD, Gee AN, Barnette MS, Burnand KG,
12. Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1
Groot PH. Expression and cellular localization of the CC chemokines
ligand chemokine is expressed by dendritic cells in lymphoid tissues
PARC and ELC in human atherosclerotic plaques. Am J Pathol 1999;
and strongly attracts naive T cells and activated B cells. J Exp Med
34. Yoshida R, Nagira M, Kitaura M, Imagawa A, Imai T, Yoshie O. Second-
13. Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia
S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of
ary lymphoid-tissue chemokine is a functional ligand for the CC che-
immature and mature dendritic cells by distinct chemokines expressed
mokine receptor CCR7. J Biol Chem 1998;273:7118–7122.
in different anatomic sites. J Exp Med 1998;188:373–386.
35. Baggiolini M. Chemokines and leukocyte traffic. Nature 1998;392:565–
14. Kim CH, Pelus LM, White JR, Broxmeyer HE. Macrophage-inflamma-
tory protein-3 /CK -11, a CC chemokine, is a chemoattractant with
36. D'Ambrosio D. Mariani M, Panina-Bordignon P, and Sinigaglia F. Che-
a specificity for macrophage progenitors among myeloid progenitor
mokines and their receptors guiding T lymphocyte recruitment in lung
cells. J Immunol 1998;161:2580–2585.
inflammation. Am J Respir Crit Care Med 2001;164:1266–1275.
15. Kim CH, Pelus LM, Appelbaum E, Johanson K, Anzai N, Broxmeyer
37. Hashimoto S, Nakayama T, Gon Y, Hata A, Koura T, Maruoka S,
HE. CCR7 ligands, SLC/6Ckine/Exodus 2/TCA4 and CK-11/MIP-
Matsumoto K, Hayashi S, Abe Y, Horie T. Correlation of plasma
3/ELC, are chemoattractants for CD56⫹CD16- NK cells and late
monocyte chemoattractant protein-1 (MCP-1) and monocyte inflam-
stage lymphoid progenitors. Cell Immunol 1999;193:226–235.
matory protein 1-␣ (MIP-1␣) levels with disease activity and clinical
16. Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T
course of sarcoidosis. Clin Exp Immunol 1998;111:604–610.
and B cells for development and effector function. J Leukoc Biol 1999;
38. Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutso-
poulos HM. Lymphoid chemokine messenger RNA expression by
17. Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher
epithelial cells in the chronic inflammatory lesion of the salivary glands
Gibejova, Mrazek, Subrtova, et al.: Expression of MIP-3 in Sarcoidosis
of Sjo¨gren's syndrome patients: possible participation in lymphoid
41. Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M,
structure formation. Arthritis Rheum 2001;44:408–418.
Nishimura A, Endo H, Kitasato H, Kawai S, et al. Selective recruitment
39. Lee W-H, Kim S-H, Jeong E-M, Choi Y-H, Kim D-I, Lee BB, Cho
of CCR6-expressing cells by increasing production of MIP-3␣ in rheu-
YS, Kwon BS, Park J-E. A novel chemokine, leukotactin-1, induces
matoid arthritis. Clin Exp Immunol 2001;125:155–161.
chemotaxis, pro-atherogenic cytokines, and tissue factor expression in
42. Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased
serum levels of macrophage inflammatory protein-3␣ in chronic viral
40. Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial
hepatitis: prognostic importance of macrophage inflammatory protein-
cell are a major site of macrophage inflammatory protein 3␣ (MIP-3
␣) production in normal colon and inflammatory bowel disease. Gut
␣ during interferon therapy in chronic hepatitis C. J Viral Hepat 2002;
Source: http://dr-petrek.eu/pubs_pdf/17.%20Expression%20of%20macrophage%20inflammatory%20protein-3%20beta(CCL19)%20in%20pulmonary%20sarcoidosis.pdf
Editorial Guide on Hong Kong Clinical Terminology Table – Drugs (Medication Terminology Table) [Document Reference No. G52] Version 1.1 January 2015 The Government of the Hong Kong Special Administrative Region Editorial Guide on Hong Kong Clinical Terminology Table – Drugs (Medication Terminology Table)
MANUAL DE ACTUACIÓN MANUAL DE ACTUACIÓN Dr. Francisco Toquero de la TorreVicesecretario OMC. Dr. Miguel Muñoz-NavasDirector del Servicio de Digestivo. Clínica Universitaria de Navarra. Pamplona. Dr. Fernando Gomollón GarcíaMédico Adjunto. Servicio de Aparato Digestivo. Hospital Universitario Lozano Blesa. Zaragoza. Profesor Asociado. Facultad de Medicina. Zaragoza.





