Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Microsoft word - 11-이덕철
Biomedical Science Letters 2014, 20(3): 173 179
Brief Communication
eISSN : 2288-7415
Effect of Korean Red Ginseng on Artificial Sand Dust (ASD)
Induced Allergic Lung Inflammation
Jung-Ha Kim1, Tae-Jin Lee2, Im Jee-Aee3 and Duk-Chul Lee4,†
1Department of Family Medicine, Chung-Ang University Healthcare System, Seoul 156-756, Korea
2Department of Pathology, Chung-Ang University College of Medicine, Seoul 156-755, Korea
3Sports and Medicine Research Center, INTOTO Inc., Seoul 120-160, Korea
4Department of Family Medicine, Severance Hospital, Yonsei University, College of Medicine,
Seoul 120-752, Korea
Asian sand dust is known to promote various respiratory symptoms or disorders. For the prevention of harmful health
effects by Asian sand dust, the best strategy is known to avoid or reduce exposure to the Asian sand dust. Several studies
have shown that Korean red ginseng (RG) has anti-inflammatory and anti-allergic effects. The study aimed to clarify the
effect of Korean red ginseng intake on lung inflammation responses to artificial sand dust (ASD) similar to Asian sand
dust. BALB/c mice were divided into five groups (n=12) of control (saline), ovalbumin (OVA), OVA with ASD, OVA
plus RG with ASD, and OVA plus dexamethasone (DEXA) with ASD. Histopathologic evaluation of lung was conducted.
Interleukin (IL)-5, IL-12, interferon (IFN)-γ, IL-13, monocyte chemotactic protein (MCP)-1, and eotaxin within
bronchoalveolar lavage (BAL) fluid were measured by ELISA. OVA+ASD group significantly increased concentrations
of IL-5, IL-13, MCP-1, and eotaxin (
P<0.01) compared to the control. OVA+ASD+RG group showed significant
decreased levels of IL-2, IL-13, MCP-1 and eotaxin (
P<0.01) compared with OVA+ASD. Between RG and DEXA
treatment groups, there was no significant difference in all cytokines and chemokines. The inflammatory cells were
significantly decreased in treatment groups with RG or DEXA compared to OVA+ASD group. This study suggests a
beneficial effect of Korean RG administration in preventing inflammation of lung resulting from Asian sand dust.
Key Words: Asian sand dust, Korean red ginseng, Anti-inflammation, Allergic mouse model
Asian sand dust is a meteorological phenomenon that
respiratory diseases (Pope et al., 1995; Hong et al., 1999;
affects much of Northeast Asia. The dust originats in the
Kim, 2004). Moreover, water soluble ions (Park et al.,
deserts of China and Mongolia and is carried to the Korean
2004b), inorganic components (mineral elements) (Ichinose
Peninsula by prevailing westerly winds, resulting in social
et al., 2008b), or organic substances such as bacteria in the
and economic damage (Kwon and Cho, 2004). It is known
dust (Echigo et al., 2005; Ichinose et al., 2008a) have been
that particulate matter (PM) less than 10 μm in diameter
suggested to be associated with respiratory inflammation
(PM10) in sand dust affect human health. Increased PM10
responses. The pulmonary function of asthma patients
invading the lower trachea particularly promotes different
deteriorates and nocturnal asthma symptoms increase during
the dust storm season (Min et al., 2001; Song, 2001; Park
Received: July 26, 2014 / Revised: September 21, 2014 Accepted: September 24, 2014
et al., 2003b). Also, daily mortality rate and hospital
†Corresponding author: Duk-Chul Lee. Department of Family Medicine,
admission rate were reported to increase due to respiratory
Severance Hospital, Yonsei University, College of Medicine, 250 Seongsanno, Seodaemun-gu, Seoul 120-752, Korea.
illnesses during the Asian sand dust season (Kwon et al.,
Tel: +82-2-2228-2331, Fax: +82-2-362-2473
2002; Chen et al., 2004; Hwang et al., 2005). Furthermore,
e-mail:
[email protected]
C The Korean Society for Biomedical Laboratory Sciences. All rights reserved.
recent studies have reported that the dust may enhance
allergic rhinitis and rise in Th2 cytokines in particular seem
RG was greater than the effect of dexamethasone (DEXA)
to be associated in this process (Kim et al., 2009).
administration (Babayigit et al., 2008).
The main components of red ginseng (RG, the steamed
Therefore, this study aimed to clarify the effect of Korean
root of Panax ginseng C.A. Meyer, family Araliaceae) are
RG intake on lung inflammation responses in an allergic
ginsenosides and polysaccharides that have various medicinal
mice model using an artificial sand dust (ASD) similar to
effects including anti-inflammatory (Lee and Lau, 2011;
Asian sand dust.
Paul et al., 2012), anti-allergic (Kim and Yang, 2011), and
The study used a total of 60 five-week-old healthy
anti-tumor effects (Ho et al., 2012). Strong anti-allergic and
female BALB/c mice (Central Lab. Animal Inc., Korea)
anti-inflammatory effects have been identified particularly
weighing between 20 25 g. All mice were raised in the
in ginsenoside Rh1 (Park et al., 2004a), Rh2 (Park et al.,
animal facility at 20 23℃ and the humidity of 45 70%
2003a), and Rb1, and its metabolic substance compound K
from one week before the experiment. All experiments
(Park et al., 2005; Yang et al., 2007). In a recent study of
were performed in accordance with the National Institutes
mice model of allergic asthma, histological changes such
of Health guidelines for laboratory animals. The study was
as increased mast cell numbers and thickened basement
approved by the Institutional Animal Care and Use Com-
membrane, epithelium, and subepithelial smooth muscle,
mittees of the National Veterinary Research & Quarantine
in lungs have been improved in the RG group compared
with the placebo group. The histologic improvement of
The study used the 5000 mesh yellow soil powder
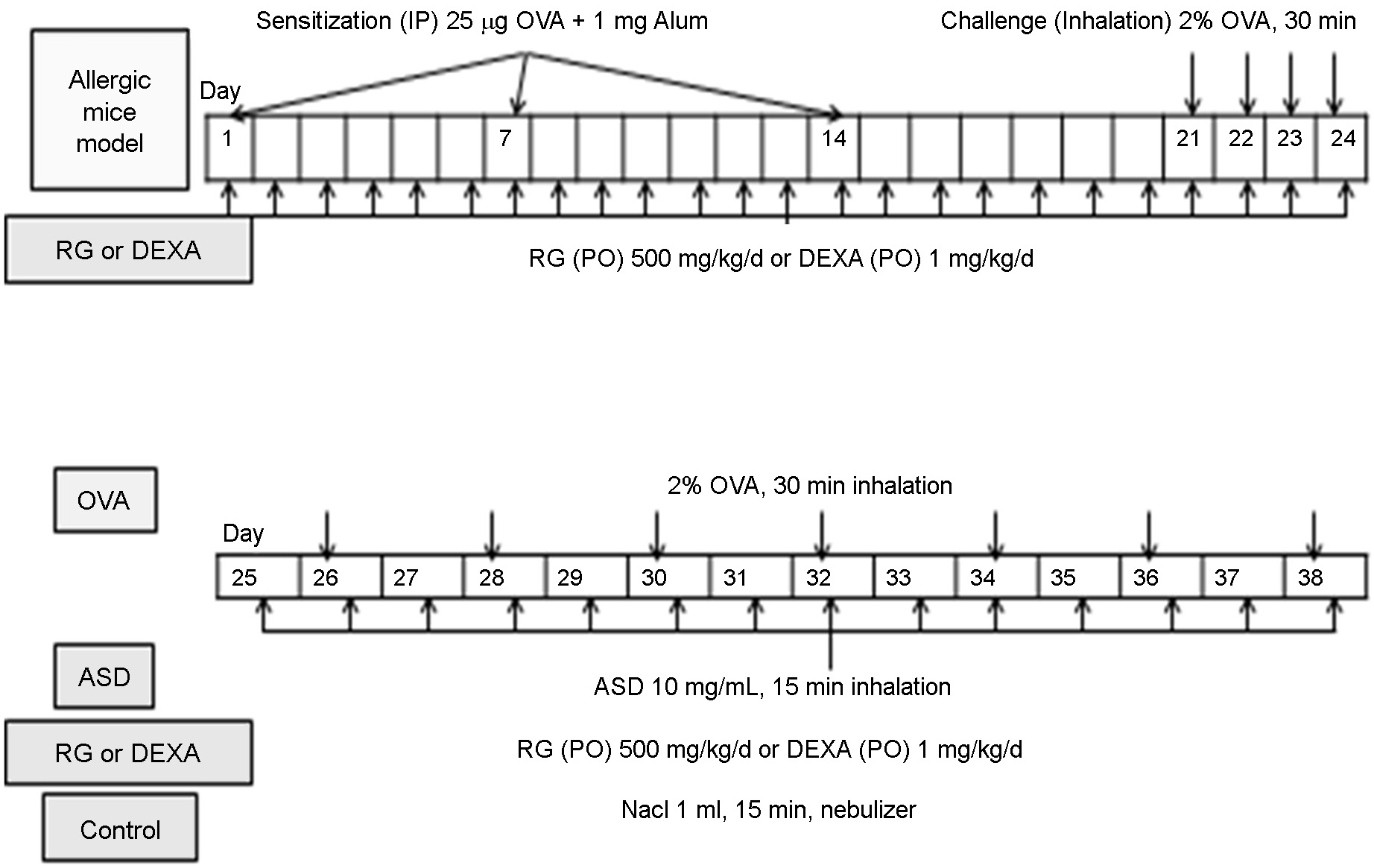
Fig. 1. Study protocol. For allergic mouse model, a mixture of 25 μg OVA and 1 mg Al(OH)3 gel was injected into the peritoneum
(systemic immunization) and intranasal sensitization was induced with 2% OVA for 4 days. OVA group inhaled 2% OVA every other day
for two weeks. OVA+ASD group inhaled 2% OVA every other day and 1 mL ASD (10 mg/mL) every day for two. OVA+ASD+RG or
OVA+ASD+DEXA group were orally administered with a 500 mg/kg/d of RG powder or a 1 mg/kg/d of DEXA, respectively, since the
first day of peritoneal injection. OVA and ASD inhalation protocol was the same as the OVA+ASD group. Control group was sprayed
with 1 mL of NaCl for two weeks. OVA: ovalbumin, ASD: artificial sand dust, RG: red ginseng, DEXA: dexamethasone
(Yellow soil, Hongikbio, Korea). It was near identical to
group was sprayed with 1 mL of NaCl for 15 minutes in
sand dust collected during the dust storm season of Korea
each session for two weeks using a nebulizer (Fig. 1).
in terms of chemical components, the contents of heavy
Among 12 mice in each group, 6 mice were used in
metals and particle size among commercially manufactured
histopathological examination of the lung. Lung samples
yellow soils (Seo, 2009). ASD is comprised of ions and
were fixed with 10% paraformaldehyde, and stained with
heavy metals such as Ca2+ 6.1 cmol/kg, K+ 2.5 cmol/kg,
haematoxylin and eosin (H&E) to evaluate the degree of
Mg2+ 6.2 cmol/kg, Na+ 0.3 cmol/kg, Cd 7.9 mg/kg, Pb 20.4
inflammatory cell infiltration. The degrees of observed
mg/kg, Zn 90.7 mg/kg, Cu 14.4 mg/kg, Cr 37.2 mg/kg, and
inflammation were distinguished into three stages in lung
Ni 17.6 mg/kg. ASD has pH 6.2 and electrical conductivity
tissue specimen. Grade 0 was established as a normal stage
of 35.5 dS/m (Seo, 2009).
with almost no inflammatory cell infiltration while grade 1
BALB/c mice were divided into five groups (n=12) each
was judged to be a mild inflammation stage with slight
of control (saline), ovalbumin (OVA), OVA+ASD, OVA+
inflammatory cell infiltration around bronchioles. Moreover,
ASD+RG, and OVA+ASD+DEXA. The generation of the
grade 2 was defined to be a stage of inflammatory cell
OVA-induced allergic animal model was described pre-
cluster detection with more severe inflammatory cell
viously (Kim et al., 2009). For systemic sensitization, 25 μg
infiltration. The interpretation of lung tissue samples was
OVA (Sigma, St. Louis, MO) and 1 mg Al(OH)3 gel (Pierce
carried out by two pathologists who were not informed
Chemical Co., Rockford, IL) were mixed with 3 mL of
with the categorized group of specimens.
saline and injected with 300 μL into the peritoneal cavity of
Among 12 mice in each group, the rest 6 mice were used
each mouse on day 1, 7 and 14. Intranasal sensitization was
in obtaining bronchoalveolar lavage (BAL) fluid. The lungs
implemented with 2% OVA for 30 minutes using a jet
were lavaged with 1 mL of sterile saline at 37℃ by syringe.
nebulizer (Omron, Japan) from day 21 to day 24 (Fig. 1).
The supernatants of BAL fluid were stored at -80℃ until
Subsequently, 2% OVA 5 mL inhalation was maintained in
analysis of cytokines and chemokines. Interleukin (IL)-5,
allergic mice in the OVA group for 30 minutes per session
IL-12, interferon (IFN)-γ, IL-13, monocyte chemotactic
every other day for two weeks. Along with OVA 5 mL
protein (MCP)-1, and eotaxin (R&D systems, Minneapolis,
inhalation, the OVA+ASD group inhaled 10 mg/mL ASD
USA) within BAL fluid were assessed using an Enzyme-
for 15 minutes per session. Moreover, RG and DEXA
Linked Immunosorbent Assay (ELISA) kit.
group was orally administered with 500 mg/kg/d of RG
Data are presented as mean ± standard deviation (SD).
powder (KT&G, Korea) and a 1 mg/kg/d of dexamethasone
Statistical analyses on the pathologic inflammation degrees,
(Dexa-S®, Ilsung, Korea), respectively, since the first day of
the levels of cytokines and chemokines in BAL fluid were
peritoneal injection for allergic animal model. The control
conducted using ANOVA followed by Tukey's test. Dif-
Table 1. Expression of inflammatory cytokines in bronchoalveolar larvage fluid
Control 2.50±0.59 2.24±0.08
OVA 3.65±0.59 2.13±0.64
OVA+ASD 4.57*±1.25 2.76±0.79 27.13*±2.33
1.93±0.61 2064.15*†±56.77
OVA+ASD+RG 2.61#±0.42 2.70±0.58
OVA+ASD+DEXA 3.36±1.07 2.84±0.51 1.29#†±0.42
2.84#±0.47 1833.61*#†±199.41 386.35*#±53.43
Each experiment consisted of six observations. All values were expressed as mean ± SE.
*P<0.01 compared to control by ANOVA.
#P<0.01 compared to OVA+ASD by ANOVA.
†P<0.01 compared to OVA by ANOVA.
OVA: ovalbumin, ASD: artificial sand dust, RG: Red ginseng, DEXA: dexamethasone
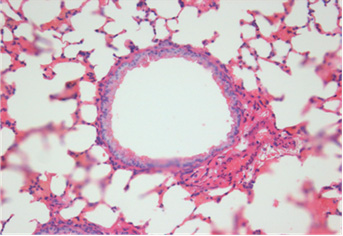
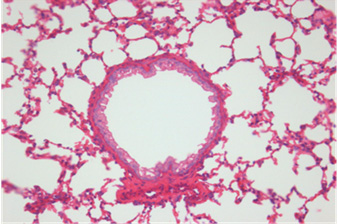
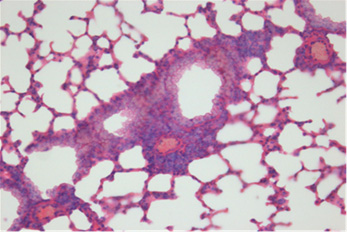
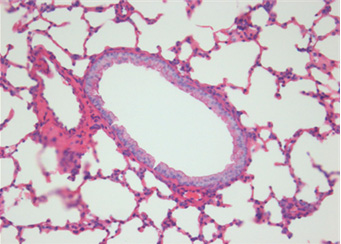
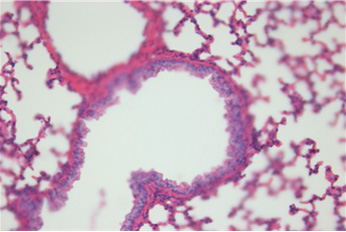
Fig. 2. Histopathologic changes of the lung. A: No pathological changes in the airway treated with saline (control). B: Moderate infiltration
of inflammatory cells in the airway treated with OVA alone. C: Marked infiltration of inflammatory cells in the airway treated with OVA+
ASD. D: Significantly decreased infiltration of inflammatory cells in the airway treated with OVA+ASD+RG compared to C. E: Significantly
decreased infiltration of inflammatory cells in the airway treated with OVA+ASD+DEXA compared to C. H&E stain (×400). OVA:
ovalbumin, ASD: artificial sand dust, RG: red ginseng, DEXA: dexamethasone
ferences among groups were determined as statistically significant at a level of P<0.05. All calculations were performed using the SAS 9.1 statistics package (SAS Institute, Inc., Cary, NC, US).
Table 1 shows the levels of cytokines and chemokines in
BAL fluid. OVA+ASD group significantly increased con- centrations of IL-5, IL-13, MCP-1, and eotaxin (P<0.01) compared to the control group. OVA+ASD+RG group showed significant decreased levels of IL-5, IL-13, MCP-1 and eotaxin (P<0.01) compared with the OVA+ASD group. IL-5 and IL-13 levels of OVA+ASD+RG group were not significantly different from the control group. OVA+ASD+
Fig. 3. Inflammation in lung. Each experiment consisted of six
observations. Inflammation of OVA+ASD+RG group was signifi-
DEXA group also showed decreased concentrations of
cantly lower than in that of OVA+ASD group.
*P<0.01 compared to control by ANOVA.
IL-13, MCP-1, and eotaxin (P<0.01), and increased IFN-γ
#P<0.01 compared to OVA+ASD by ANOVA.
level (P<0.01) compared to the OVA+ASD group. Between
OVA: ovalbumin, ASD: artificial sand dust, RG: red ginseng,
DEXA: dexamethasone
RG and DEXA treatment groups, there were no significant differences in all cytokines and chemokines. Fig. 2 and 3 shows the pathologic change and inflammation degree in lung according to the study groups. The inflammatory cells
The purpose of this study was to verify the preventive
were significantly decreased in treatment groups with RG or
effect of Korean RG intake on pulmonary diseases generated
DEXA group compared to the OVA+ASD group (P<0.01).
by sand dust using allergic mice model. Asian sand dust
induced inflammatory cell infiltration of lung and increased
Some studies have suggested that ions (Park et al., 2004b),
inflammatory cytokines and chemokines in BAL fluid. The
such as Na+, Mg2+, Ca2+, NH4+, SO 2-
4 , NO3 , toxins including
administration of RG reduced these lung inflammatory
LPS (Ichinose et al., 2008a), and heavy metals (Ichinose et
responses, which was effective comparable to steroid, well
al., 2008b) in the dust act as a new allergic antigen in
known as anti-inflammatory drug.
healthy individuals or an activating factor in allergic patients.
Several active components were reported to be involved
Among the measured cytokines, the levels of MCP-1
in the mechanism of anti-allergic and anti-inflammatory
and eotaxin of all groups increased in our study compared
efficacy of RG. Ginsenoside Rh2 was reported to have cell
to those in a previous study (Ichinose et al., 2008a). IL-12
membrane stabilizing effect by inhibiting the secretion of
and IL-γ concentrations were generally low in our study
β-hexosaminidase and anti-inflammatory efficacy by in-
(Ichinose et al., 2008a; Ichinose et al., 2008b). These
hibiting the formation of nitrogen oxide (NO) and pro-
findings may be caused by different sand dusts. Despite the
staglandin E2 (PGE2) (Park et al., 2003a). In particular, it
use of ASD, the study obtained similar results consistent
was found to be more powerful in cell membrane stabilizing
with previous studies. The BAL fluids of ASD exposed
effect compare with disodium cromolycate (Park et al.,
allergic mice model showed significant increases in not
2003a). Furthermore, ginsenoside Rh1 inhibited the release
only IL-5, a known key mediator of eosinophilic inflam-
of histamine in peritoneal mast cells of mice and inhibited
mation, but also MCP-1, eotaxin, and the Th2 cytokine
passive cutaneous anaphylaxis (PCA) by IgE in mice,
IL-13 compared to those of control mice (Ichinose et al.,
exhibiting a stronger efficacy than disodium cromolycate
2008a; Ichinose et al., 2008b). Both cytokines of BAL
(Park et al., 2004a). Moreover, ginsenoside Rb1 and its
fluids and inflammatory cell infiltration of lung tissues were
metabolic substance compound K were also reported to be
significantly decreased in Korean RG-treated (OVA+ASD+
inhibit the expression of iNOS, COX-2, and NF-κB (Park
RG) group compared to the ASD-exposed allergic mice
et al., 2004a; Park et al., 2005). In addition, compound K
(OVA+ASD) group. It is clinically significant outcome that
has been suggested to influence the role of TLR4 and
the anti-inflammatory effect of RG is not any different with
TLR9 in inflammatory responses (Yang et al., 2007).
Previous studies reported that respiratory diseases are
However, RG or DEXA can also influence OVA-induced
generated by eosinophil infiltration, increased eosinophil
allergy as well as dust-exposed allergy. In our study, because
relevant cytokine and chemokines, and allergic inflam-
OVA+RG and OVA+DEXA group were not included, it is
mations resulting from Th2 predominant response (Chen et
difficult to clarify whether the RG or DEXA has a
al., 2004; Hiyoshi et al., 2005; Ichinose et al., 2008b).
beneficial effect on inflammation by dust-exposed allergy.
However, the mechanism by which sand dust develops
In conclusion, the current study shows that sand dust
allergic responses is unclear. Although the dust may be one
boosts the allergic responses in the allergic mice model.
antigen directly involved in inducing allergies, further
This study suggests the beneficial effects of Korean RG
studies are essential to distinguish antigen substances among
administration in preventing inflammations of lung resulted
various components in sand dust. The sizes of Asian sand
from Asian sand dust. Further studies in human subjects
dust particles range between 0.1∼20 μm including fine
are needed to verify the effect of RG intake on preventing
particles under 2.5 μm and ultra-fine particles under 0.1 μm
respiratory diseases caused by sand dust.
(Choi et al., 2001; Mori et al., 2003). Titanium dioxide, a
known fine particle found in sand dust, is assumed to be a
direct cause as well as a deteriorating factor in pulmonary
This work was supported by the 2010 grant from the
diseases. It was suggested that phagocytosis of titanium
Korean Society of Ginseng funded by Korea Ginseng
dioxide by lung macrophages may affect the change and
Corporation. The authors would like to thank Seong-Jin
adjustment of respiratory cells (Donaldson et al., 2001).
Park, Ph.D. at Climate Change & Agroecology Division,
National Academy of Agricultural Science for providing the
Effects of asian sand dust, Arizona sand dust, amorphous
artificial sand dust.
silica and aluminum oxide on allergic inflammation in the
murine lung. Inhal Toxicol. 2008b. 20: 685-694.
REFERENCES
Kim DY, Yang WM. Panax ginseng ameliorates airway in-
flammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 2011. 136: 230-235.
Babayigit A, Olmez D, Karaman O, Bagriyanik HA, Yilmaz O,
Kim MY. Physical and chemical characteristics of Asian dust. J
Kivcak B, Erbil G, Uzuner N. Ginseng ameliorates chronic
Korean Med Assoc. 2004. 47: 453-464.
histopathologic changes in a murine model of asthma. Allergy
Kim ST, Lee EJ, Jung HJ, Gang IG, Cha HE, Kim DY, Do SH.
Asthma Proc. 2008. 29: 493-498.
The effect of Asian sand dust in allergic inflammation of
Chen YS, Sheen PC, Chen ER, Liu YK, Wu TN, Yang CY. Effects
allergic mouse. Korean J Otorhinolaryngol-Head Neck Surg.
of Asian dust storm events on daily mortality in Taipei, Taiwan.
2009. 52: 498-505.
Environ Res. 2004. 95: 151-155.
Kwon HJ, Cho SH. The health effects of Asian dust event. J Korean
Choi JC, Lee M, Chun Y, Kim J, Oh S. Chemical composition and
Med Assoc. 2004. 47: 465-470.
source signature of spring aerosol in Seoul. Korea J Geophys
Kwon HJ, Cho SH, Chun YS, Lagarde F, Pershagen G. Effects of
Res. 2001. 106: 18067-18074.
the Asian dust events on daily mortality in Seoul, Korea.
Donaldson K, Stone V, Clouter A, Ren wick L, MacNee W.
Environ Res. 2002. 90: 1-15.
Ultrafine particles. Occup Environ Med. 2001. 58: 211-216.
Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis
Echigo A, Hino M, Fukushima T, Mizuki T, Kamekura M, Usami
factor-α-mediated inflammation: a mini-review. Molecules.
R. Endospores of halophilic bacteria of the family Bacillaceae
2011. 16: 2802-2816.
isolated from non-saline Japanese soil may be transported by
Min PK, Kim CW, Yun YJ, Chang JH, Chu JK, Lee KE, Han JY,
Kosa event (Asian dust storm). Saline Systems. 2005. 1: 1-13.
Park JW, Hong CS. Effect of yellow sand on respiratory
Hiyoshi K, Ichinose T, Sadakane K, Takano H, Nishikawa M,
symptoms and diurnal variation of peak expiratory flow in
Mori I, Yanagisawa R, Yoshida S, Kumagai Y, Tomura S,
patients with bronchial asthma. J Asthma Allergy Clin Immunol.
Shibamoto T. Asian sand dust enhances ovalbumin-induced
2001. 21: 1179-1186.
eosinophil recruitment in the alveoli and airway of mice.
Mori I, Nishikawa M, Tanimura T, Quan H. Change in size
Environ Res. 2005. 99: 361-368.
distribution and chemical composition of Korea (Asian dust)
Ho YL, Li KC, Chao W, Chang YS, Huang GJ. Korean red
aerosol during long-range transport. Atmos Environ. 2003.
ginseng suppresses metastasis of human hepatoma SK-Hep1
cells by inhibiting matrix metalloproteinase-2/-9 and uro-
Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 pos-
kinase plasminogen activator. Evid Based Complement
sesses antiallergic and anti-inflammatory activities. Int Arch
Alternat Med. 2012. 2012: 965846.
Allergy Immunol. 2004a. 133: 113-120.
Hong YC, Leem JH, Ha EH, Christiani DC. PM10 exposure,
Park EK, Choo MK, Kim EJ, Han MJ, Kim DH. Antiallergic
gaseous pollutants, and daily mortality in Inchon, Korea.
activity of ginsenoside Rh2. Biol Pharm Bull. 2003a. 26:
Environ Health Perspect. 1999. 107: 873-878.
Hwang SS, Cho SH, Kwon HJ. Effects of the severe Asian dust
Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH.
events of daily mortality during the spring of 2002, in Seoul,
Inhibitory effect of ginsenoside Rb1 and compound K on
Korea. J Prev Med Public Health. 2005. 38: 197-202.
NO and prostaglandin E2 biosyntheses of RAW264.7 cells
Ichinose T, Yoshida S, Hiyoshi K, Sadakane K, Takano H,
induced by lipopolysaccharide. Biol Pharm Bull. 2005. 28:
Nishikawa M, Mori I, Yanagisawa R, Kawazato H, Yasuda A,
Shibamoto T. The effects of microbial materials adhered to
Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, Ju YS.
Asian sand dust on allergic lung inflammation. Arch Environ
Effects of ambient particulate matter (PM10) on peak
Contam Toxicol. 2008a. 55: 348-357.
expiratory flow and respiratory symptoms in subjects with
Ichinose T, Yoshida S, Sadakane K, Takano H, Yanagisawa R, Inoue K,
bronchial asthma during yellow sand period. Tuberc Respir
Nishikawa M, Mori I, Kawazato H, Yasuda A, Shibamoto T.
Dis. 2003b. 55: 570-578.
Park SH, Song CB, Kim MC, Kwon SB, Lee KW. Study on size
Seo KH. Chemical and microbiological characteristics and risk
distribution of total aerosol and water-soluble ions during an
assessment of Asian dust. National Academy of Agricultural
Asian dust storm event at Jeju Island, Korea. Environ Monit
Assess. 2004b. 93: 157-183.
Song HI. Effect of air pollution on childhood asthma living in
Paul S, Shin HS, Kang SC. Inhibition of inflammations and macro-
Seoul. J Asthma Allergy Clin Immunol. 2001. 21: 28-39.
phage activation by ginsenoside-Re isolated from Korean
Yang CS, Ko SR, Cho BG, Lee JY, Kim KH, Shin DM, Yuk JM,
ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol.
Sohn HJ, Kim SY, Wee JJ, Do JH, Jo EK. Compound K
2012. 50: 1354-1361.
(CK) rich fractions from Korean red ginseng Inhibit Toll-like
Pope CA 3rd, Bates DV, Raizenne ME. Health effects of particulate
receptor (TLR) 4- or TLR 9-mediated mitogen-activated
air pollution: Time for reassessment? Environ Health Perspect.
protein kinases activation and proinflammatory responses in
1995. 103: 472-480.
murine macrophages. J Ginseng Res. 2007. 31: 181-190.
Source: http://www.bslonline.org/journal/download_pdf.php?doi=10.15616/BSL.2014.20.3.173
Individual Differences in Locomotion, Anxiety-like behavior, and Reward After Nicotine and Baclofen Administration A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at George Mason University Adriana M. Falco George Mason University, 2010 Bachelor of Arts University of Maryland, 2003 Director: Robert F. Smith, Professor
Condiciones generales para el suministro de maquinaria y repuestos 1. Generalidades 1.1 El contrato se considerará concluido al recibir la confirmación por escrito del proveedor estipulando su aceptación del pedido (confirmación del pedido). Ofertas que no contienen un plazo de aceptación se entienden sin compromiso.









