Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Ebot.gmu.edu
Individual Differences in Locomotion, Anxiety-like behavior, and Reward After Nicotine
and Baclofen Administration
A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at George Mason University
Adriana M. Falco
George Mason University, 2010
Bachelor of Arts
University of Maryland, 2003
Director: Robert F. Smith, Professor
Department of Psychology
Fall Semester 2012
George Mason University
Copyright 2012 Adriana M. Falco
All Rights Reserved
This is dedicated to the memory of my brother, Nicholas R. Falco, III.
ACKNOWLEDGEMENTS
I would like to thank the many friends, relatives, and supporters who have made this project possible. My parents, Nicholas Falco, Patricia Pruss, and Frank Pruss have been supportive throughout my graduate career. Drs. Smith, McDonald, and Fryxell, as members of my committee, all gave invaluable research guidance. Finally, thanks go to Charles Blanchard and Gina Fernandez who gave technical assistance during the project. This project was funded in part by the Virginia Foundation for Healthy Youth (VFHY).
TABLE OF CONTENTS
Page List of Tables……………………………………………………………………………v List of Figures………………………………………………………………………….vi List of Abbreviations………………………………………………………………….vii Abstract.…….viii 1. Introduction .…1 2. Material and Methods .5
Animals .5 Drugs .5 Pretesting.6 Open Field (OF) .6 Place Conditioning .7 Statistics .9
4. Discussion .26 5. References .33
Table Page 1. List of drug group denotations for open field (OF)………………………………….9 2. List of drug group denotations in conditioned place preference (CPP) .……11 3. Total Distance Traveled by 5 minute Intervals in HA Animals .……15 4. Total Distance Traveled by 5 minute Intervals in LA Animals .16 5. Comparisons Between Drug Groups and Saline on the Variable of Difference Score in HA Animals .26 6. Comparisons Between Drug Groups and Saline on the Variable of Difference Score in LA Animals .29
Figure Page
1. Means of all Drug Conditions for the Total Distance Traveled Variable
in HA Animals .……17
2. Means of the nicotine and baclofen + nicotine Groups for the Total Distance
Traveled Variable in HA Animals .18
3. Means of all Drug Conditions for the Total Distance Traveled Variable
in LA Animals.19
4
. Means of the nicotine and baclofen + nicotine Groups for the Total Distance
Traveled Variable in LA Animals .20
5. Means of the baclofen and saline Groups for the Total Distance
Traveled Variable in HA animals .21
6. Means of the baclofen and saline Groups for the Total Distance
Traveled Variable in LA animals .21
7. Means of all Drug Conditions for the Distance Traveled in the Center
Variable in HA Animals .22
8. Means of all Drug Conditions for the Distance Traveled in the Center
Variable in LA Animals .23
9. Means of all Drug Conditions on Center Time in HA Animals .24
10. Means of all Drug Conditions on Center Time in LA Animals .25
11. Means of all Drug conditions on the Variable of Difference Score
in HA Animals .27
12. Means of all Drug conditions on the Variable of Difference Score
in LA Animals.28
INDIVIDUAL DIFFFERENCES IN LOCOMOTION, ANXIETY-LIKE BEHAVIOR, AND REWARD AFTER NICOTINE AND BACLOFEN ADMINISTRATION Adriana M. Falco, Ph.D. George Mason University, 2012 Dissertation Director: Dr. Robert F. Smith
Tobacco use is a significant health problem that began in adolescence for many adult
smokers. Anxiety may also be a risk factor in who develops nicotine dependence
disorders. This study uses adolescent male Sprague-Dawley rats (n = 160) and splits
them into a high anxiety (HA) and low anxiety (LA) group based on the results of pretest
day of a conditioned place preference (CPP) protocol with a biased chamber. These rats
are further divided into drug groups that receive either saline or 0.6 mg/kg baclofen (i.p.)
30 minutes before testing and then either saline or 0.5 mg/kg nicotine (s.c.) immediately
Open field testing showed a significant difference between HA and LA rats in
locomotor activity, as well as significant differences between drug groups when
compared to saline. Notably, baclofen administration significantly decreased locomotor
behavior from saline levels in HA animals, but did not do so in LA animals. In both HA
and LA groups, baclofen and nicotine co-administration significantly decreased
locomotor behavior from locomotor activity levels in animals administered nicotine
alone. Additionally, the open field was used to examine potential differences in anxiety-
like behavior. Baclofen administration failed to produce differences in anxiety-like
behavior between HA and LA groups, but nicotine administration and baclofen + nicotine
co-administration had slightly more of an effect on anxiety-like behavior in LA than HA
animals. Single-trial nicotine CPP testing found that HA rats formed significant CPP to
nicotine and baclofen + nicotine, but LA rats did not. This study shows that innate
anxiety-like behavior plays a significant factor in formation of locomotor responses to
baclofen as well as later anxiety-like responses to nicotine and baclofen administration in
adolescent rats. This study also serves to highlight the role that innate anxiety-like
behavior plays in nicotine reward in adolescents.
Tobacco use represents a serious health epidemic, constituting the leading
preventable cause of premature death (US Health and Human Services, 2010). Of
particular interest are the prevention of and/or intervention in nicotine dependence
disorders prior to costly outcomes. Adolescence forms a unique period of vulnerability to
nicotine. The majority of smokers begin smoking prior to age 17 and demonstrate a
decreased ability to quit smoking as compared to smokers who begin smoking later in life
(Breslau & Peterson, 1996; Chen & Millar, 1998). Adolescent smokers also report higher
levels of tolerance and dependence than adult counterparts (Kandel & Chen, 2000).
Research with rodent models also supports the risk of adolescents to the development of
nicotine dependence and addiction. The rewarding effects of nicotine are heightened in
adolescent rats, marking a critical period for the development of nicotine dependence
(Adriani, et al., 2003; Belluzzi, Lee, Oliff, & Leslie, 2004; Brielmaier, McDonald, Smith,
2007; Torres, Tejada, Natividad, & O'Dell, 2008).
The coexistence of anxiety disorders and substance use disorders is present in
numerous populations, including adolescents. However, the direction of causation of
anxiety disorders and substance use disorders has yet to be clearly ascertained. Human
research has noted that significantly higher percentages of individuals with anxiety
disorders will develop substance dependence disorders than those in the general
population (Liang, Chikritzhs, & Lenton, 2011). Adolescents who report social fears and
social anxiety have a significantly higher risk of using cigarettes and developing nicotine
dependence (Henry, Jamner, & Whalen, 2012; McKenzie, Olsson, Jorm, Romaniuk, &
Patton, 2010; Sonntag, Wittchen, Höfler, Kessler, & Stein, 2000). Rates of social anxiety
or generalized anxiety disorder are also correlated with an earlier age of first tobacco use
(Mamorstein, White, Loeber, & Stouthamer-Loeber, 2010). Research with rodent models
has also investigated the impact of anxiety-like behavior on reward, mainly in adulthood,
with unclear results. When age is condensed into a homogenous group, one study found
that low anxiety-like behavior predicted higher levels of drug seeking in cocaine self-
administration (Schramm-Sapyta, et al., 2011). However, other work has found that high
anxiety-like behavior is associated with greater intake of cocaine in self-administration
and increases in place conditioning stimulated by cocaine (Dilleen, et al., 2012; Pelloux,
Costentin, & Duterte-Boucher, 2009).
Anxiety and substance use disorders may be comorbid, but the underlying
pathophysiology that links them has yet to be determined. Dysfunction of the γ-
aminobutyric acid (GABA) system has been implicated in both anxiety (Millan, 2003)
and substance use and abuse (Heilig, Goldman, Berrettini, & O'Brien, 2011; Shorter &
Kosten, 2011). The metabotropic GABAB receptor has been of particular research
interest in both anxiety disorders (Mombereau, et al., 2004; Ong & Kerr, 2005; Partyka,
et al., 2007) and drug addiction (Bowery, 2006; Cousins, Roberts, & de Wit, 2002;
Tyacke, Lingford-Hughes, Reed, & Nutt, 2010). To date, the specific roles that GABAB
receptors play in these disorders has not been elucidated. One drug under investigation
for both anxiety and substance abuse disorders is baclofen, a GABAB agonist currently
approved by the U.S. Food and Drug Administration (FDA) to treat muscle spasticity
(US Food and Drug Administration, 2011). There is scant research addressing baclofen's
involvement in anxiety and anxiety-like behavior. Research has shown that baclofen
administration has anxiolytic effects in the elevated plus maze (EPM) in male mice
(Amikishieva & Semendyaeva, 2007), but fails to modify nicotine-induced anxiety-like
behavior in mice (Varani & Balerio, 2012).
The effects of baclofen on drug addiction have been far better addressed, both in
clinical and preclinical populations. The use of baclofen in clinical populations has
highlighted a potential role for its use in the treatment of drug addiction and substance
use disorders. Baclofen has been found to alter the sensory aspects of smoking,
decreasing the enjoyment of cigarettes (Cousins, Stamat, & de Wit, 2001) as well as
reducing the number of cigarettes smoked (Franklin, et al., 2009). Baclofen
administration has also been found to decrease daily alcohol intake among alcoholics
(Addolorato, et al., 2011), in addition to reducing craving and withdrawal symptoms
(Addolorato & Leggio, 2010). There has also been some implication that baclofen may
be useful in decreasing craving in some cocaine dependent subjects (Haney, Hart, &
Work with preclinical samples is also showing promise for the use of baclofen as
a treatment for drug dependence and addiction. The acute administration of baclofen in
Sardinian alcohol-preferring rats was found to suppress extinction phase responding for
alcohol in a two bottle choice paradigm (Colombo, et al., 2003). Direct intracerebral
injections of baclofen into the ventral tegmental area (VTA) of rats were found to reduce
cocaine self-administration (Brebner, Childress, & Roberts, 2002). Baclofen
administration has also been found to prevent reinstatement of heroin (Spano, Fattore,
Fratta, & Fadda, 2007) and nicotine (Fattore, et al., 2009) self-administration as well as
reducing rates of nicotine self-administration (Fattore, Cossu, Martellotta, & Fratta, 2002;
Paterson, Forestl, & Markou, 2004). Baclofen pretreatment has also been shown to block
nicotine conditioned place preference (CPP) effects (Le Foll, Wertheim, & Goldberg,
2008) and enhance extinction of morphine CPP (Heinrichs, Leite-Morris, Carey, &
Kaplan, 2010). In addition, pretreatment with baclofen was found to attenuate
sensitization and locomotor effects of cocaine (Frankowska, Nowak, & Filip, 2009),
amphetamine (Bartoletti, Gubellini, Ricci, & Gaiardi, 2005), morphine (Bartoletti, Ricci,
& Gaiardi, 2007), and nicotine (Lobina, et al., 2011; Palmatier & Bevins, 2002).
While baclofen is generally considered a safe substance with limited abuse
potential (Evans & Bisaga, 2009), there have been cases of baclofen overuse and abuse
(Dore, Lo, Juckes, Bezyan, & Latt, 2011; May, 1983; Nasti & Brakoulias, 2011; Perry,
Wright, Shannon, & Woolf, 1998). Some of the previous citations show some evidence
that anxious users may be at a higher risk to abuse baclofen due its anxiolytic effects,
which have led to cases of overuse and abuse. Baclofen overdose is known to cause
numerous ill effects, including seizures, coma, and delirium (Chong & Wang, 2005;
Wall, Wasiak, & Hicklin, 2006).
The present study examined the effects of acute doses of baclofen and nicotine on
locomotion, anxiety-like behavior, and reward in adolescent male Sprague-Dawley rats
that were split into high anxiety (HA) and low anxiety (LA) groups based on pretesting
with a biased CPP apparatus. Locomotor and anxiety-like behavior were assessed in the
open field (OF) while reward was measured via single-trial nicotine CPP using a "biased"
methodology. It was hypothesized that administration of baclofen would cause
differences in anxiety-like behavior, with HA animals showing larger changes in anxiety-
like behavior due to higher initial anxiety levels. In addition, it was hypothesized that
baclofen administration would increase rates of single-trial nicotine CPP due to possible
anxiolytic reduction of baclofen seen in humans.
MATERIALS AND METHODS
Male adolescent Sprague-Dawley rats (n = 160) were obtained from Harlan
(Indianapolis, IN, USA) and housed in groups of four or five on a 12 h light/12 h dark
schedule (lights on at 0700). Food and water were available
ad libitum, with animals
being given additional food pulp (chow mixed with water) at arrival to supplement the
diet. Subjects were acclimatized to the colony for seven days prior to testing. Behavioral
testing began at postnatal day 28 (P28). All experiments were approved by the George
Mason University animal care committee and in accordance with the National Institutes
of Health Guide for Care and Use of Laboratory Animals (2011).
(-)-Nicotine hydrogen tartrate and R (+) baclofen hydrochloride were purchased
from Sigma-Aldrich (St. Louis, MO). All drugs were administered at an injection
volume of 1 mL/kg body weight. Baclofen and saline were administered
intraperitoneally (i.p.), and nicotine and saline were administered subcutaneously (s.c.)
between the shoulder blades for both open field and CPP experiments. Baclofen and
nicotine were dissolved in saline solution (0.9% NaCl). Dose levels of nicotine are
expressed as free base equivalent, and the pH was adjusted to 7.1-7.4.
Pretesting
Animals were divided into HA and LA groups on the basis of pretesting.
Pretesting utilized the CPP chamber to determine innate levels of anxiety-like behavior.
The chamber is akin to the light-dark box, a well-known apparatus for testing anxiety-like
behavior, in that it is composed of a black chamber and a white chamber. Similarly to the
light-dark box, animals spend varying amounts of time in the white chamber; those that
spend more time in the white chamber were considered to have low anxiety, those that
spend less time in the white chamber were considered to have high anxiety.
The first day of behavioral testing for all animals consisted of pretesting in order
to divide into high or low anxiety groups based on median split within each drug group.
Animals were given access to both sides of the apparatus for 15 minutes and after testing,
animals were divided into HA and LA animals and utilized in the next portions of testing.
Open Field (OF)
Locomotor and anxiety-like behavioral testing was performed in four OF
chambers, created from white Plexiglas, measuring 42 x 42 x 30 cm, and located in a
dimly lit (4-6 lx) testing room. A camera mounted above the apparatus recorded the 15-
minute trials and data were acquired in 3 x 5-minute intervals using Videotrack software
(Viewpoint, Montreal, QC, Canada). Between each set of animals, each chamber was
cleaned with 70% EtOH to eliminate odor cues.
Eighty animals from pretesting divided into the following groups: HA (n = 40) and LA (n
= 40). Each group was further split into one of the following four drug treatment groups:
saline+saline, saline + nicotine, baclofen + saline, and baclofen + nicotine (see Table 1).
Therefore, each drug treatment group consisted of a HA (n =10) and a LA (n =10)
On test days, animals were housed in individual hanging wire cages and permitted
to habituate to the testing room for 20 minutes. Thirty minutes prior to OF, rats were
injected (i.p.) with either baclofen or saline, depending on the treatment group.
Immediately before testing, another injection, of either nicotine or saline (s.c.) was given,
again depending on the treatment group. After drug injections, animals were tested in the
OF chamber for 15 minutes, with data being collected over 3 x 5 minute intervals.
Place Conditioning
Conditioned place preference (CPP) testing occurred in a two chambered
apparatus (Med Associates, VT) in the same testing room as OF. Each chamber of the
apparatus consisted of Plexiglas and had dimensions 21 x 42 x 30 cm. One chamber
consisted of white walls with a mesh floor over a white paper lining, while the opposite
chamber consisted of black walls with a stainless steel rod floor over a black paper lining.
A black removable door separated the two chambers.
Table 1. List of drug group denotations for open field (OF).
Label
baclofen + saline
saline + nicotine
baclofen + nicotine
Eighty animals from pretesting were split into HA (n =40) and LA (n = 40)
groups and were further designated to received (saline + saline)CPP, (baclofen +
saline)CPP, (saline + nicotine)CPP, or (baclofen + nicotine)CPP during CPP testing (see
Table 2). All animals underwent single-trial nicotine CPP testing in a "biased" place
conditioning method modified from previous Smith lab protocols (Brielmaier,
McDonald, & Smith, 2007; Brielmaier, McDonald, & Smith, 2008). In a "biased"
procedure, animals were tested for their natural preference to a chamber and then
conditioned with a drug in the non-preferred chamber. Testing consisted of three aspects:
pretest, conditioning sessions, and posttest. Each day, animals were placed into
individual wire hanging cages and permitted to habituate to the testing room for 20
minutes prior to testing. On the pretest day, each animal was placed in the apparatus and
given free access to both chambers for 15 minutes. Natural, or unconditioned, preference
for a chamber was determined by recording the amount of time spent in the white
chamber. The definition of time spent in the white chamber was described as when the
rat had all 4 paws completely in the white chamber. All rats were started in the white
chamber, facing toward the removable door.
Animals underwent two conditioning sessions, one to administer drug (or saline in
the case of controls), and one where all animals received saline. Animals were
counterbalanced so that half received drug on the first conditioning session (and saline in
the second), and half received drug in the second conditioning session (and saline in the
first). During conditioning sessions, animals were weighed before being placed in the
Table 2. List of drug group denotations in conditioned place preference (CPP).
Label
(saline+saline)CPP
(baclofen+saline)CPP
(saline+nicotine)CPP
(baclofen+nicotine)CPP Baclofen
hanging cages and habituated. On drug conditioning days, animals then received an
injection of either saline or 0.6 mg/kg R (+) baclofen (i.p.) and waited a period of 30
minutes. Immediately prior to CPP, animals received an injection of either saline or 0.5
mg/kg nicotine (s.c.) and were placed in their initially non-preferred chamber, facing
away from the door, for 15 minutes. On saline conditioning days, all animals received an
injection of saline (i.p.) 30 minutes before CPP, and then an injection of saline (s.c.)
immediately prior to CPP testing and were placed in their initially preferred chamber,
facing away from the door, for 15 minutes.
On the posttest day, animals were again given free access to the testing apparatus
to determine chamber preference during a 15-minute drug-free posttest. All animals were
again started in the white chamber facing the removed door. Preference was determined
by time spent in the white chamber. Between all trials, both chambers were cleaned with
70% EtOH and paper was changed after each animal to remove odors.
Statistics
Locomotor and anxiety-like behavioral variables were analyzed in quantified
records of OF activity using simple regressions with dummy coding in order to take into
account the categorical variables. For the CPP experiment, difference scores were
calculated for each animal by subtracting time in seconds spent in the initially non-
preferred chamber on the posttest day from time in seconds spent in the initially non-
preferred chamber on the pretest day. Again, simple regressions with dummy coding
were conducted to analyze data and take into account the categorical variables.
Regressions were used over ANOVA due to flexibility of the model should
circumstances become more complex and due to the presence of slightly unequal group
sizes, which is a violation of ANOVA assumptions. Where justified, additional t-tests
were conducted to supplement regression analyses. All analyses were conducted using
IBM SPSS 19.0 statistical software.
Locomotor and anxiety-like behavioral variables were analyzed using simple
regressions for both HA and LA groups of animals. The regression was calculated by
setting each drug group against saline to test for statistical significance. This allows a
predictive equation to be calculated so that y = b0 + b(drug group) + error. However,
where warranted, additional t-tests were used to determine differences between HA and
LA animals and between drug groups other drug groups of interest. Three variables were
considered, total distance traveled in the arena, distance traveled in the center of the
arena, and time spent in the center of the arena.
The main locomotor variable assessed was the total distance traveled in the OF
arena. This variable was measured in 3 x five minute intervals over the 15 minute test.
Regression output for the total distance traveled variable is summarized in Tables 3 and
4. Initially, each drug group, baclofen (baclofen +saline), nicotine (saline + nicotine),
and baclofen + nicotine, was compared to the saline group to determine statistical
differences. Each 5 minute interval was analyzed independently of the others. In the HA
group, after 5 minutes, there was a statistically significant difference in the total distance
traveled variable for each of the three drug groups when compared to saline animals,
p <
0.05 (see Figure 1).
Table 3. Total distance traveled by 5 minute intervals in HA animals. Means on the
graph are represented by y = b0 + b (drug group). In this condition, all drug groups were
compared against saline for statistical significance. At 5 minutes, R2 = .706, at 10
minutes, R2 = .377, and at 15 minutes, R2 = .238.
Drug Group/Time
Constant (Bo)/ @ 5
Nicotine/@ 5 minutes Baclofen/@ 5
minutes Nicotine/@ 5
minutes Constant (Bo)/ @
Nicotine/@ 10 minutes Baclofen/@ 10
minutes Nicotine/@ 10
minutes Constant (Bo)/ @
Nicotine/@ 15 minutes Baclofen/@ 15
Table 4. Total distance traveled by 5 minute intervals in LA animals. Means on the
graph are represented by y = b0 + b (drug group). In this condition, all animals were
compared against saline for statistical significance. At 5 minutes, R2 = .434, at 10
minutes, R2 = .191, and at 15 minutes, R2 = .207.
Drug Group/Time
Constant (Bo)/@ 5
Nicotine/@ 5 minutes Baclofen/@ 5
minutes Nicotine/@ 5
minutes Constant (Bo)/ @
Nicotine/@ 10 minutes Baclofen/@ 10
minutes Nicotine/@ 10
minutes Constant (Bo)/@ 15 756.267
Nicotine/@ 15 minutes Baclofen/@ 15
minutes Nicotine/@ 15
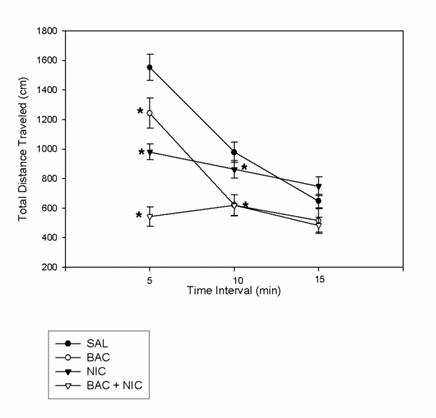
Figure 1. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), for the total distance
traveled variable at time intervals of 5 minutes, 10 minutes, and 15 minutes in HA
animals. * = a significant difference between drug group and saline, p < .05.
At the 10 minute interval, both the baclofen and baclofen + nicotine groups showed
statistically significant differences compared to saline (p < .05), but nicotine did not. By
the 15 minute interval, none of the drug groups showed significant differences with
respect to saline. However, at the 5 minute timepoint, there was a significant difference
between the baclofen and baclofen + nicotine groups, t(18) = 5.786, p < .001. And, at all
three time points, there were significant differences between nicotine and baclofen +
nicotine groups among HA animals, t(17) =5.097, 2.613, and 3.197 respectively, p < .05
Figure 2. Means of the saline + nicotine (NIC) and baclofen + nicotine (BAC + NIC)
groups for the total distance traveled variable at time intervals of 5, 10, and 15 minutes in
HA animals. *p < .05
Among LA animals, there were also statistical differences between drug groups
on the total distance traveled variable (see Figure 3). There were statistically significant
differences between saline animals and the animals that received either nicotine or
baclofen + nicotine in the first 5 minutes, p < .05. By 10 minutes and 15 minutes, these
differences were no longer significant. There were also statistically significant
differences on the total distance traveled variable among other drug groups in LA
animals. In the first 5 minutes there was a significant difference between baclofen and
baclofen + nicotine, t(18) = 3.317, p < .01. There were also significant differences
between nicotine and baclofen + nicotine groups at 5, 10, and 15 minutes, t(18) = 3.144,
3.488, 3.552 respectively, p < .01 (see Figure 4).
Figure 3. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), for the total distance
traveled variable at time intervals of 5 minutes, 10 minutes, and 15 minutes in LA
animals. * = a significant difference between drug group and saline, p < .05.
Figure 4. Means of the saline + nicotine (NIC) and baclofen + nicotine (BAC + NIC)
groups for the total distance traveled variable at time intervals of 5, 10, and 15 minutes in
LA animals. Statistically significant differences occurred at all three time points. +p <
.01
While not many substantial differences existed between HA and LA animals in
locomotor behavior, one notable difference was in the effect of baclofen on the total
distance traveled variable. Among HA animals, baclofen animals were statistically
significantly different from saline animals on the total distance traveled variable at the 5
minute, t(18) = -2.763, p < .01, and at the 10 minute intervals, t(18) = -3.809, p ≤ .001
(see Figure 5). In LA animals, there are no significant differences between baclofen and
saline animals (see Figure 6).
Figure 5. Means of the baclofen + saline (BAC) and saline + saline (SAL) groups for the
total distance traveled variable at time intervals of 5, 10, and 15 minutes in HA animals.
Statistically significant differences existed at the 5 and 10 minute intervals. *p < .01
Figure 6. Means of the baclofen + saline (BAC) and saline + saline (SAL) groups for the
total distance traveled variable at time intervals of 5, 10, and 15 minutes in LA animals.
No statistically significant differences existed.
The second variable measured was distance traveled in the center. Once again,
this variable was assessed at 5, 10, and 15 minute intervals. Distance traveled in the
center was linked to both locomotor and anxiety-like behavior. Among HA animals, only
one comparison showed statistical significance on this variable. When compared to
saline animals, nicotine animals were statistically significantly different at the 15 minute
interval of distance traveled in the center of the arena, t(19) = 2.170, p < .05 (see Figure
7). Among LA animals, there were no significant differences on this variable (see Figure
Figure 7. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), for the distance traveled
in the center variable at 5, 10, and 15 minute intervals in HA animals. Only the
comparison between SAL and NIC at 15 minutes was significantly different. *p < .05
Figure 8. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), for the distance traveled
in the center variable at 5, 10, and 15 minutes in LA animals. There were no significantly
different relationships.
The third variable evaluated was time spent in the center of the arena, a variable
used to gauge anxiety-like behavior. Again, this variable was measured at 5, 10, and 15
minutes. Almost all of the statistically significant comparisons involved the baclofen +
nicotine drug groups. Among HA animals, there was a significant difference between the
animals that received baclofen + nicotine and those that received saline on time spent in
the center at 5 minutes and 15 minutes, t(19) = 2.413 and 2.275 respectively, p < .05 (see
Figure 9). Additionally, there was a significant difference between the baclofen and
baclofen + nicotine groups on time spent in the center at the 5 minute mark, t(18) =
-2.235, p < .05. Among LA animals, there was a significant difference between baclofen
+ nicotine and saline animals on time spent in the center at 5 and 10 minutes, t(19) =
2.430 and 2.452 respectively, p < .05, and between nicotine and saline animals at 15
minutes, t(19) = 2.236, p < .05 (see Figure 10). In addition, there were significant
differences between the baclofen and baclofen + nicotine animals at 5, t (18) = -2.299, p
< .05, and 10 minutes, t(18) = -3.061, p < .01.
Figure 9. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), on center time at 5, 10,
and 15 minutes in HA animals. There was a statistically significant difference between
BAC + NIC and SAL at 5 and 15 minutes,*p < .05.
Figure 10. Means of all drug conditions, saline + saline (SAL), baclofen + saline (BAC),
saline + nicotine (NIC), and baclofen + nicotine (BAC + NIC), on center time at 5, 10,
and 15 minutes in LA animals. There were significant differences between BAC + NIC
and SAL animals at 5 and 10 minutes, and between NIC and SAL at 15 minutes. *p < .05
CPP
Linear regressions were analyzed comparing each drug condition to saline on the
variable of difference score. When a preliminary t-test was run comparing nicotine to
saline using the sample as a homogenous group, there was no statistically significant
distinction between nicotine and saline, t(35) = -1.320, p = .196, suggesting that CPP
training had not been successful. However, when the sample was divided into HA and
LA groups, this concept was no longer the case.
Among HA animals there were statistically significant variations between animals
administered nicotine and baclofen + nicotine and animals administered saline.
Regression output is summarized in Table 5.
Table 5. Comparisons between drug groups and saline on the variable of difference
score in HA animals. Means on the graph are represented by y = b0 + b (drug group). R2
= .294
Drug Condition
Nicotine Baclofen
Both the nicotine and baclofen + nicotine groups had a significantly higher difference
score than the saline group (see Figure 11).
Figure 11. Means of all drug conditions, (saline + saline)CPP [(SAL)CPP], (baclofen +
saline)CPP [(BAC)CPP], (saline + nicotine)CPP [(NIC)CPP], and (baclofen +
nicotine)CPP [(BAC + NIC)CPP], on the variable of difference score in HA animals.
Significant differences existed between (SAL)CPP animals and both (NIC)CPP and
(BAC + NIC)CPP animals. *p < .01
Among LA animals, there were no significant distinctions between the saline group and
any of the drug groups (see Figure 12). Regression output is summarized in Table 6.
Figure 12. Means of all drug conditions, (saline + saline)CPP [(SAL)CPP], (baclofen +
saline)CPP [(BAC)CPP], (saline + nicotine)CPP [(NIC)CPP], and (baclofen +
nicotine)CPP [(BAC + NIC)CPP], on the variable of difference score in LA animals.
There were no significant differences between saline and any drug group.
Table 6. Comparisons between drug groups and saline on the variable of difference
score. Means on the graph are represented by y = b0 + b (drug group). R2 = .158
Drug Condition
Baclofen + Nicotine 54.867
The present study examined the impact individual differences in anxiety-like
behavior had on locomotion, anxiety-like behavior, and reward after administration of
nicotine and baclofen in adolescent rats. Adolescent Sprague-Dawley rats were separated
into HA and LA groups using a median split analysis based on time spent in the white
chamber of a biased CPP chamber. Subsequent testing using OF found some notable
differences in innate anxiety-like behavior and later locomotor and anxiety-like behavior
in rats dependent on HA/LA status and drug administered. Notably, baclofen
administration significantly decreased locomotor behavior from saline levels in HA
animals, but did not do so in LA animals. In both HA and LA groups, baclofen and
nicotine co-administration significantly decreased locomotor behavior from locomotor
activity levels in animals administered nicotine alone. The open field was also used to
examine potential differences in anxiety-like behavior. Baclofen administration failed to
produce differences in anxiety-like behavior between HA and LA groups, but nicotine
administration and baclofen + nicotine co-administration had a slightly more profound
effect on anxiety-like behavior in LA than HA animals. Additionally, in single-trial
nicotine CPP testing, only HA rats formed CPP to nicotine and baclofen + nicotine
administration. LA rats failed to form CPP to any drugs tested.
Rats were assigned to HA or LA groups using performance in the CPP chamber, either
prior to OF testing, or data from the pretest day of CPP testing. This method was utilized
because pilot testing found that pretesting with elevated plus maze (EPM) prevented
adolescents from forming single-trial nicotine CPP (unpublished pilot data). However,
the biased CPP chamber employed is highly similar to the light-dark box, so that it is
likely that it acts as a viable measure of anxiety-like behavior. This method also has the
benefit of reducing number of testing days in rats, as the window of time to obtain single-
trial nicotine CPP is very narrow, approximately P28-P32 in Sprague Dawleys (Belluzzi,
et al., 2004; Brielmaier, et al., 2007; Brielmaier, et al., 2008). This methodology also
alleviates the issues of EPM blocking the ability to achieve single-trial nicotine CPP and
the inability to use OF as a pretest due to its inclusion later in the protocol.
One particularly notable finding of this study is that HA rats show a statistically
significant difference between those administered baclofen + saline and controls (saline +
saline) [see Figure 5]. This relationship is no longer statistically significant in the LA
group (see Figure 6). Administration of baclofen is known to sedate locomotion in rats,
though often at higher doses (Le Foll, et al., 2008; Frankowska, et al., 2009; Palmatier &
Bevins, 2002). This study is of note in that adolescents may be slightly more susceptible
to the sedating effects of baclofen, even though the R (+) baclofen enantiomer is used
here and is relatively more active; 0.6 mg/kg is a lower dose than used in other literature
and pretest data in adults showed that this dose had no sedating effects (unpublished
pretest data). In addition, it is of interest, that at least in adolescents, innate anxiety-like
behavior is a variable that determines reaction to the locomotor effects of baclofen. It is
entirely possible that this is due to the systemic action of the GABAB agonist activating
receptors which play a role in both anxiety-like behavior and locomotion (Amikishieva &
Semendyaeva, 2007; Bowery, 2006; Mombereau, et al., 2004). It is also possible that
these findings are only applicable to adolescents as adolescents are known to exhibit
higher levels of anxiety-like behavior than adults in numerous paradigms (Lynn &
Brown, 2010); clearly these results would have to be replicated in adults.
Another noticeable finding is that in both HA and LA groups, dosing with
baclofen + nicotine significantly reduced the locomotor behavior in comparison to rats
dosed only with nicotine (see Figures 2 and 4). This is supported by literature which
shows that baclofen reduces the activity levels of adult rodents dosed with nicotine
(Lobina, et al., 2011; Palmatier & Bevins, 2002) and cocaine (Frankowska, et al., 2009).
The means of rats dosed with nicotine and rats dosed with baclofen + nicotine are
significantly lower than saline-dosed rats at several time points. It appears that the
GABAB activation in the rats dosed with baclofen + nicotine is playing a role in the
further suppression of locomotor activity due to the fact that the addition of baclofen
suppresses locomotor activity further than in nicotine dosed rats.
It was expected that dosing with baclofen and baclofen + nicotine would have a
more significant impact on anxiety-like behavior. This hypothesis was driven by several
studies suggesting a link for the GABAB receptor in anxiety-like behavior. Genetic work
has shown that GABAB(1) -/- mice, which lack functional GABAB(1) receptors, were more
anxious than wildtype littermates in the light-dark box and staircase test (Mombereau, et
al., 2004). Studies with baclofen have shown that baclofen administration has anxiolytic
effects on the EPM in male mice (Amikishieva & Semendyaeva, 2007), however, that
baclofen administration was unable to alter the dose-dependent anxiety-like behavior
produced by nicotine in male mice (Varani & Balerio, 2012). It is possible that baclofen
has little effect on anxiety-like behavior in this study because the subjects are
adolescents, whereas most work has been done with adults. Though GABA is the main
inhibitory neurotransmitter in adults, it actually serves as an excitatory neurotransmitter
in early postnatal development (Ben-Ari, Khazipov, Leinekugel, Caillard, & Gaiarsa,
1997) and studies have shown that during early adolescence, GABA neurons respond
more weakly to GABA agonists due to immaturity of the neurons (Cohen, Lin, &
Coulter, 2000). It is, therefore, entirely possible that these findings would not be
replicated in adults. This is also supported by the body of work delineating the increased
vulnerability of the adolescent to nicotine (see O'Dell & Khroyan, 2009). However, this
hypothesis is in opposition to this study's findings that adolescents could show profound
locomotor effects to baclofen. There is a possibility that the GABA neurons in the
movement areas of the brain are maturing more quickly than areas associated with
anxiety-like behavior, but at this moment, this question does not seem to have been
The present study also found that HA rats that were dosed with either nicotine or
baclofen + nicotine were able to form single-trial CPP, while no group among LA rats
were able to achieve CPP. This suggests that high anxiety-like behavior plays a role in
nicotine CPP and that using the pretest day is a valid measure of naïve anxiety-like
behavior. Previous work with cocaine has suggested that high anxiety rats achieve higher
rates of CPP (Pelloux, et al., 2009), in addition to higher rates of cocaine self-
administration (Dilleen, et al., 2012; Schramm-Sapyta, et al., 2011), though this
relationship has not been seen with alcohol (Langen & Fink, 2004). Among the HA rats,
it seems likely that nicotine is driving the CPP effect among rats dosed with baclofen +
nicotine, as there was no significant alterations in the difference scores between the
nicotine and baclofen + nicotine groups. However, it is noteworthy that baclofen co-
administration did nothing to alter nicotine CPP. Previous studies using baclofen have
shown that administration of 3 mg/kg of R (+) baclofen, though neither 0.3 or 1 mg/kg of
baclofen blocked nicotine CPP (Le Foll, et al., 2008). In addition, administration of
baclofen was capable of preventing reinstatement of nicotine CPP in mice (Fattore, et al.,
2009). It may be that baclofen has an ability to block nicotine CPP, but only at high
doses. However, it would seem that, at least in adolescents, the sedative effects at such a
high dose may be problematic. The administration of baclofen did not have a long-
lasting locomotor impact that impaired CPP, as is demonstrated by the fact the baclofen +
nicotine group acquired single-trial nicotine CPP at roughly the same rate as nicotine rats.
It is also noteworthy, that during OF testing, nicotine dosing did not alter anxiety-like
behavior in either HA or LA groups, supporting the concept that the anxiety-like
behavioral difference here is innate and not drug-induced.
This study has areas that are worth expanding on. Since previous work has found
higher doses of baclofen effective in attenuating nicotine CPP, it may be worthwhile to
examine if adolescents can be treated at the higher dose without severe locomotor
sedation. However, at this point, it does appear that baclofen, while it may be useful as a
treatment in adults, is not an option as a preventative or blocking agent of nicotine reward
in adolescents. In addition, it is may also be beneficial to apply the anxiety aspect of this
work to adults to see if anxiety status can select out adults that will form nicotine CPP
over multiple conditioning sessions.
In summary, testing using OF found some notable differences in innate anxiety-
like behavior and later locomotor and anxiety-like behavior in rats dependent on HA/LA
status and drug administered. Notably, baclofen administration significantly decreased
locomotor behavior from saline levels in HA animals, but did not do so in LA animals.
In both HA and LA groups, baclofen and nicotine co-administration significantly
decreased locomotor behavior from locomotor activity levels in animals administered
nicotine alone. The open field was also used to examine potential differences in anxiety-
like behavior. Baclofen administration failed to produce differences in anxiety-like
behavior between HA and LA groups, but nicotine administration and baclofen + nicotine
co-administration had a slightly more profound effect on anxiety-like behavior in LA
than HA animals. In addition, the dose of baclofen used had no effect on single trial
nicotine CPP in adolescents, but anxiety status emerged as a predictor of which rats
would form CPP. Therefore, this study does not lend support to the use of baclofen as a
treatment for nicotine addiction, but elucidates the coexistence of adolescence and high
anxiety as dual roles in forming nicotine reward.
Addolorato, G., & Leggio, L. (2010). Safety and efficacy of baclofen in the treatment of
alcohol-dependent patients. Current Pharmaceutical Design, 16, 2113-2117.
Addolorato, G., Leggio, L., Ferrulli, A., Cardone, S., Bedogni, G., Caputo, F., et al.
(2011). Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol and Alcoholism, 46, 312-317.
Adriani, W., Spijker, S., Deroche-Gamonet, V., Laviola, G., Le Moal, M., Smit, A.B., et
al. (2003). Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. The Journal of Neuroscience, 23, 4712-4716.
Amikishieva, A.V., & Semendyaeva, S.N. (2007). Effects of baclofen on anxiety, sexual
motivation, and olfactory perception in male mice in different psychoemotional states. Neuroscience and Behavioral Physiology, 37, 929-937.
Balla, A., Nattini, M.E., Sershen, H., Lajtha, A., Dunlop, D.S., & Javitt, D.C. (2009).
GABAB/NMDA receptor interaction in the regulation of extracellular dopamine
levels in rodent prefrontal cortex and striatum. Neuropharmacology, 56, 915-921.
Bartoletti, M., Gubellini, C., Ricci, F., & Gaiardi, M. (2005). Baclofen blocks the
development of sensitization to the locomotor stimulant effect of amphetamine. Behavioural Pharmacology, 16, 553-558.
Bartoletti, M., Ricci, F., & Gaiardi, M. (2007). A GABAB agonist reverses the
behavioral sensitization to morphine in rats. Psychopharmacology, 192, 79-85.
Belluzzi, J.D., Lee, A.G., Oliff, H.S., & Leslie, F.M. (2004). Age-dependent effects of
nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology, 174, 389-395.
Ben-Ari, Y., Khazipov, R., Leinekugel, X., Caillard, O., & Gaiarsa, J.L. (1997).
GABA , NMDA and AMPA receptors: a developmentally regulated ‘ménage à
trois'. Trends in Neurosciences, 20, 523-529.
Bowery, N.G. (2006). GABAB receptor: a site of therapeutic benefit. Current Opinion
in Pharmacology, 6, 37-43.
Brebner, K., Childress, A.R., & Roberts, D.C.S. (2002). A potential role for GABAB
agonists in the treatment of psychostimulant addiction. Alcohol & Alcoholism, 37, 478-484.
Breslau, N., & Peterson, E.L. (1996). Smoking cessation in young adults: age at
initiation of cigarette smoking and other suspected influences. American Journal of Public Health, 86, 214-220.
Brielmaier, J.M., McDonald, C.G., & Smith, R.F. (2007). Immediate and long-term
behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicology and Teratology, 29, 74-80.
Brielmaier, J.M., McDonald, C.G., & Smith, R.F. (2008). Nicotine place preference in a
biased conditioned place preference design. Pharmacology, Biochemistry and Behavior, 89, 94-100.
Chen, J., & Millar, W.J. (1998). Age of smoking initiation: Implications for quitting.
Health Reports, 9, 39-46.
Chen, Y., Phillips, K., Minton, G., & Sher, E. (2005). GABAB receptor modulators
potentiate baclofen-induced depression of dopamine neuron activity in the rat ventral tegmental area. British Journal of Pharmacology, 144, 926-932.
Chong, C.F., & Wang, T.L. (2005). An unusual presentation of baclofen overdose.
Emergency Medicine, 22, 673-674.
Cohen, A.S., Lin, D.D., & Coulter, D.A. (2000). Protracted postnatal development of
inhibitory synaptic transmission in rat hippocampal area CA1 neurons. Journal of Neurophysiology, 84, 2464-2476.
Colombo, G., Vacca, G., Serra, S., Brunetti, G., Carai, M.A.M., & Gessa, G.L. (2003).
Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology, 167, 221-224.
Cousins, M.S., Stamat, H.M., & de Wit, H. (2001). Effects of a single dose of baclofen
on self-reported subjective effects and tobacco smoking. Nicotine & Tobacco Research, 3, 123-129.
Cousins, M.S., Roberts, D.C.S., & de Wit, H. (2002). GABAB receptor agonists for the
treatment of drug addiction: a review of recent findings. Drug and Alcohol Dependence, 65, 209-220.
Dore, G.M., Lo, K., Juckes, L., Bezyan, S., & Latt, N. (2011). Clinical experience with
Baclofen in the management of alcohol-dependent patients with psychiatric comorbidity: a selected case series. Alcohol and Alcoholism, 46, 714-720.
Dilleen, R., Pelloux, Y., Mar, A.C., Molander, A., Robbins, T.W., Everitt, B.J., et al.
(2012). High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology. E-pub ahead of print. DOI 10.1007/s00213-011-2626-4
Evans, S.M., & Bisaga, A. (2009). Acute interaction of baclofen in combination with
alcohol in heavy social drinkers. Alcoholism Clinical and Experimental Research, 33, 19-30.
Fadda, P., Scherma, M., Fresu, A., Collu, M., & Fratta, W. (2003). Baclofen antagonizes
nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse, 50, 1-6.
Fattore, L., Cossu, G., Martellotta, M.C., & Fratta, W. (2002). Baclofen antagonizes
Intravenous self-administration of nicotine in mice and rats. Alcohol & Alcoholism, 37, 495-498.
Fattore, L., Spano, M.S., Cossu, G., Scherma, M., Fratta, W., & Fadda, P. (2009).
Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. European Neuropsychopharmacology, 19, 487-498.
Franklin, T.R., Harper, D., Kampman, K., Kildea-McCrea, S., Jens, W., Lynch, K.G., et
al. (2009). The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug and Alcohol Dependence, 103, 30-36.
Frankowska, M., Nowak, E., & Filip, M. (2009). Effects of GABAB receptor agonists on
cocaine hyperlocomotor and sensitizing effects in rats. Pharmacological Reports, 61, 1042-1049.
Haney, M., Hart, C.L., & Foltin, R.W. (2006). Effects of baclofen on cocaine self-
administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology, 31, 1814-1821.
Heinrichs, S.C., Leite-Morris, K.A., Carey, R.J., & Kaplan, G.B. (2010). Baclofen
Enhances extinction of opiate conditioned place preference. Behavioural Brain Research, 207, 353-359.
Heilig, M., Goldman, D., Berrettini, W., & O'Brien, C.P. (2011). Pharmacogenetic
approaches to the treatment of alcohol addiction. Nature Reviews Neuroscience, 12, 670-684.
Henry, S.L., Jamner, L.D., & Whalen, C.K. (2012). I (should) need a cigarette:
adolescent social anxiety and cigarette smoking. Annals of Behavioral Medicine. E-pub ahead of print. DOI 10.1007/s12160-011-9340-7
Jayaram, P., & Steketee, J.D. (2004). Effects of repeated cocaine on medial prefrontal
cortical GABAB receptor modulation of neurotransmission in the mesocorticolimbic dopamine system. Journal of Neurochemistry, 90, 839-847.
Kandel, D.B., & Chen, K. (2000). Extent of smoking and nicotine dependence in the
United States: 1991-1993. Nicotine & Tobacco Research, 2, 263-274.
Karler, R., Calder, L.D., Thai, D.K., & Bedingfield, J.B. (1998). The role of dopamine
and GABA in the frontal cortex of mice in modulating a motor-stimulant effect of
amphetamine and cocaine. Pharmacology, Biochemistry and Behavior, 60, 237- 244.
Langen, B., & Fink, H. (2004). Anxiety as a predictor of alcohol preference in rats?
Progress in Neuro-Psychopharmacology & Biological Psychiatry, 28, 961-968.
Le Foll, B., Wertheim, C.E., & Goldberg, S.R. (2008). Effects of baclofen on
conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neuroscience Letters, 443, 236-240.
Liang, W., Chikritzhs, T., & Lenton, S. (2011). Affective disorders and anxiety
disorders predict the risk of drug harmful use and dependence. Addiction, 106, 1126-1134.
Lobina, C., Carai, M.A.M., Froestl, W., Mugnaini, C., Pasquini, S., Corelli, F., et al.
(2011). Activation of the GABAB receptor prevents nicotine-induced locomotor stimulation in mice. Frontiers in Psychiatry, 2, 1-5.
Lynn, D.A., & Brown, G.R. (2010). The ontogeny of anxiety-like behavior in rats from
adolescence to adulthood. Developmental Psychobiology, 52, 731-739.
Marmorstein, N.R., White, H.R., Loeber, R., & Stouthamer-Loeber, M. (2010). Anxiety
as a predictor of age at first use of substances and progression to substance use problems among boys. Journal of Abnormal Child Psychology, 38, 211-224.
May, CR. (1983). Baclofen overdose. Annals of Emergency Medicine, 12, 171-173. McKenzie, M., Olsson, C.A., Jorm, A.F., Romaniuk, H., & Patton, G.C. (2010).
Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: Findings from a 10-year longitudinal study. Addiction, 105, 1652-1659.
Millan, M.J. (2003). The neurobiology and control of anxious states. Progress in
Neurobiology, 70, 83-244.
Mombereau, C., Kaupmann, K., Froestl, W., Sansig, G., van der Putten, H., & Cryan, J.F.
(2004). Genetic and pharmacological evidence of a role for GABAB receptors in
the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology, 29, 1050-1062.
Nasti, J.J., Brakoulias, V. (2011). Chronic baclofen abuse and withdrawal. Australia
and New Zealand Journal of Psychiatry, 45, 86-87.
O'Dell, L.E., & Khroyan, T.V. (2009). Rodent models of nicotine reward: What do they
tell us about tobacco abuse in humans? Pharmacology, Biochemistry and Behavior, 91, 481-488.
Ong, J., & Kerr, D.I.B. (2005). Clinical potential of GABAB receptor modulators. CNS
Drug Reviews, 11, 317-334.
Palmatier, M.I., & Bevins, R.A. (2002). Examination of GABAergic and dopaminergic
compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology, 45, 87-94.
Partyka, A., Klodzińska, A., Szewczyk, B., Wierońska, J.M., Chojnacka-Wójcik, E.,
Librowski, T., et al. (2007). Effects of GABAB receptor ligands in rodent tests of anxiety-like behavior. Pharmacological Reports, 59, 757-762.
Paterson, N.E., Froestl, W., Markou, A. (2004). The GABAB receptor agonists baclofen
and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology, 172, 179-186.
Pelloux, Y., Costentin, J., Duterte-Boucher, D. (2009). Anxiety increases the place
conditioning induced by cocaine in rats. Behavioural Brain Research, 197, 311-316.
Perry, H.E., Wright, R.O., Shannon, M.W., & Woolf, A.D. (1998). Baclofen overdose:
drug experimentation to a group of adolescents. Pediatrics, 101, 1045-1048.
Santiago, M., Machado, A., & Cano, J. (1993). Regulation of the prefrontal cortical
dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Research, 630, 28-31.
Schramm-Saptya, N.L., Cauley, M.C., Stangl, D.K., Glowacz, S., Stepp, K.A., Levin,
E.D., et al. (2011). Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology, 215, 493-504.
Shorter, D., & Kosten, T.R. (2011). Novel pharmacotherapeutic treatments for cocaine
addiction. BMC Medicine, 9, 119-128.
Sonntag, H., Wittchen, H.U., Höfler, M., Kessler, R.C., & Stein, M.B. (2000). Are
social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? European Psychiatry, 15, 67-74.
Spano, M.S., Fattore, L., Fratta, W., & Fadda, P. (2007). The GABAB receptor agonist
baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology, 52, 1555-1562.
Torres, O.V., Tejeda, H.A., Natividad, L.A., & O'Dell, L.E. (2008). Enhanced
vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology, Biochemistry and Behavior, 90, 658-663.
Tyacke, R.J., Lingford-Hughes, A., Reed, L.J., & Nutt, D.J. (2005). GABAB receptors
in addiction and its treatment. Advances in Pharmacology, 58, 373-396.
U.S. Department of Health and Human Services, Centers for Disease Control and
Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. (2010). How tobacco smoke causes disease: the biology and behavioral basis for smoking attributable disease. Access
U.S. Food and Drug Administration (FDA). (20 Access: http://www.fda.gov
Varani, A.P., & Balerio, G.N. (2012). GABAB receptors involvement in the effects
induced by nicotine on anxiety-related behaviour in mice. Phamacological Research, 65, 507-513.
Wall, G.C., Wasiak, A., & Hicklin, G.A. (2006). An initially unsuspected case of
baclofen overdose. American Journal of Critical Care, 15, 611-613.
CURRICULUM VITAE
Adriana M. Falco attended the University of Maryland, where she received her B.S. in Psychology in 2003. She then enrolled at George Mason University where she completed her M.A. in Psychology in 2010 and Ph.D. in Psychology in 2012.
Source: http://ebot.gmu.edu/bitstream/handle/1920/7980/Falco_dissertation_2012.pdf?sequence=1&isAllowed=y
Menopause: The Journal of The North American Menopause SocietyVol. 19, No. 7, pp. 724/734DOI: 10.1097/gme.0b013e31825a28f2 * 2012 by The North American Menopause Society 2011 NAMS/PFIZER V WULF H. UTIAN ENDOWED LECTURE History and experience: the direction of Alzheimer's disease William E. Reichman, MD1 and Nathan S. Rose, PhD2 As the global population is projected to age substantially in coming decades, the number of individuals who
Cardiovascular Formulary for the Hypertensive Cat Lusitrope, Vasodilator, Negative chronotrope 180, 240 mg caps. 180, 240 mg caps. Enacard (Vasotec) 1, 2.5, & 5 mg tablets ACE-I (CHF, Hypertension) Lotensin (Foretkor) 5 & 10 mg tablets .25-.5 mg/kg PO qd-bid Negative chronotrope, 6.25-12.5 mg PO qd Antiarrhythmic, Lusi-trope, Antihypertensive 1/8–¼ inch topically tid




