Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Ribavirin and interferon therapy in patients infected with the middle east respiratory syndrome coronavirus: an observational study



Contents lists available at
International Journal of Infectious Diseases
Ribavirin and interferon therapy in patients infected with the Middle
East respiratory syndrome coronavirus: an observational study
Jaffar A. Al-Tawfiq , Hisham Momattin Jean Dib , Ziad A. Memish ,
a Internal Medicine, Saudi Aramco Medical Services Organization, PO Box 76, Room A-428-2, Building 61, Dhahran Health Center, Dhahran 31311,
b Indiana University School of Medicine, Indiana, USA
c Pharmacy Services Division, Saudi Aramco Medical Services Organization, Dhahran, Saudi Arabia
d WHO Collaborating Center for Mass Gathering Medicine, Ministry of Health, Riyadh, Saudi Arabia
e Al-Faisal University, Riyadh, Saudi Arabia
Background: The Middle East respiratory syndrome coronavirus (MERS-CoV) has been reported to have a
Received 9 December 2013
high case-fatality rate. Currently, there is no specific therapy or vaccine with proven effectiveness for
Accepted 9 December 2013
Corresponding Editor: Eskild Petersen,
Methods: A combination of ribavirin and interferon therapy was used for the treatment of five MERS-
CoV-positive patients. We reviewed the therapeutic schedule and the outcome of these patients.
Results: All patients were critically ill with acute respiratory distress syndrome treated with adjunctive
corticosteroids and were on mechanical ventilation at the time of initiation of therapy. The median time
from admission to therapy with ribavirin and interferon was 19 (range 10–22) days. None of the patients
responded to the supportive or therapeutic interventions and all died of their illness.
Conclusions: While ribavirin and interferon may be effective in some patients, our practical experience
Pegylated interferon
suggests that critically ill patients with multiple comorbidities who are diagnosed late in the course of
their illness may not benefit from combination antiviral therapy as preclinical data suggest. There is
clearly an urgent need for a novel effective antiviral therapy for this emerging global threat.
ß 2014 The Authors. Published by Elsevier Ltd on behalf of International Society for Infectious
Diseases. Open access under
outcome of the use of a combination of interferon-a2b and
ribavirin in the management of five patients with MERS-CoV
Since the discovery of Middle East respiratory syndrome
coronavirus (MERS-CoV), the virus has caused 163 cases of disease,
with a fatality rate of The disease was initially described
2. Materials and methods
in a patient from Saudi Arabia in June 2012.MERS-CoV has caused
sporadic cases and clusters in families and healthcare settings.
This was a retrospective observational study of five MERS-CoV-
The best treatment option for the virus is not known. In view of the
positive patients who received a combination therapy of interfer-
high case-fatality rate and the potential global spread of the virus,
on-a2b and ribavirin. We describe the therapeutic schedule, the
there is an urgent need to develop effective therapies for this
clinical course, clinical and laboratory parameters, and outcomes.
infection. In a recent review, based on therapies used for the
related severe acute respiratory syndrome (SARS) coronavirus, the
2.1. MERS-CoV testing
possible use of interferon and ribavirin was considered as a
therapeutic option.The purpose of this study was to describe the
Either Dacron flocked nasopharyngeal swabs or tracheal
aspirates were obtained from the patients, and these were
submitted to the Ministry of Health MERS-CoV laboratory for
MERS-CoV diagnosis. The clinical samples were screened at the
Ministry of Health laboratory using a real-time reverse transcrip-
tase PCR.Amplification targeted both the upstream E protein
(upE gene) and open reading frame 1a (ORF1a) for MERS-CoV
* Corresponding author. Tel.: +966 13 877 3524; fax: +966 13 877 3790.
testing, and a case was considered positive if both assays were
E-mail addresses: ,
(J.A. Al-Tawfiq).
positive, as described previously (
1201-9712 ß 2014 The Authors. Published by Elsevier Ltd on behalf of International Society for Infectious Diseases. Open access under
J.A. Al-Tawfiq et al. / International Journal of Infectious Diseases 20 (2014) 42–46
intravenous (IV) every 2 days for 15 days. Imipenem was added on
day 5 for 3 days. On day 21 after admission, she was started on
ribavirin for 5 days with a loading dose of 2000 mg via nasogastric
tube, followed by 400 mg per os (PO) every 8 h, one dose of
interferon-a2b 130 mg subcutaneously, and methylprednisolone
40 mg IV every 8 h for 1 day, then twice daily for 1 day, then daily
for 2 days. She had a mild rise in amylase and lipase (from 61 to 126
U/l and from 274 to 348 U/l, respectively). The patient remained
critically ill on mechanical ventilation requiring inotropic support.
She died 34 days after admission from respiratory and multi-organ
A 58-year-old man with diabetes, hypertension, and ESRD on
hemodialysis was admitted with cough, fever, and hypoxemic
respiratory failure of 2-day duration. He tested positive for MERS-
CoV by PCR. Baseline laboratory data are shown in
On admission, the patient was started on oseltamivir 75 mg once
daily for 5 days, levofloxacin 500 mg IV every 2 days for 6 days, and
imipenem 250 mg IV twice daily for 7 days. On day 6, he was
prescribed methylprednisolone 60 mg IV every 6 h for 6 days, then
every 8 h for 1 day, then 40 mg every 12 h for 1 day, followed by a
prednisolone tapered dose for another 10 days. On day 13 after
admission, he was started on ribavirin for 5 days with a loading
dose of 2000 mg PO, followed by 400 mg every 8 h and two doses of
interferon-a2b 100 mg subcutaneously once per week. Two weeks
after the initiation of the therapy, platelets dropped from 408 to
43 � 109/l ). The patient remained on the ventilator for
more than 30 days. He died 35 days after admission from multi-
organ failure.
A 63-year-old woman with a history of severe bronchial asthma
and obstructive sleep apnea (on continuous positive airway
pressure (CPAP)), with coronary artery disease, diabetes, hyper-
tension, and chronic kidney disease (estimated glomerular
filtration rate 15.7 ml/min), was admitted with fever, cough, and
dyspnea of 3-day duration and a diagnosis of pneumonia. She
tested positive for MERS-CoV by PCR. Baseline laboratory data are
shown in On admission, the patient was also started
on oseltamivir 75 mg once daily for 5 days, levofloxacin 750 mg IV
every 2 days for 7 days, and imipenem 500 mg IV every 6 h for 8
days. On day 11, she was started on ribavirin for 5 days, with a
loading dose of 2000 mg via nasogastric tube followed by 600 mg
PO every 8 h, one dose of interferon-a2b 144 mg subcutaneously,
and prednisone 40 mg PO daily, tapered to 10 mg PO daily for 10
days. One week after initiation of the antiviral and steroid therapy,
serum creatinine, aspartate aminotransferase (AST) ,
and amylase increased (from 0.6 to 2.9 mg/dl, from 6.3 to 20 � 109/
l, from 25 to 126 IU/l, and from 35 to 255 U/l, respectively). The
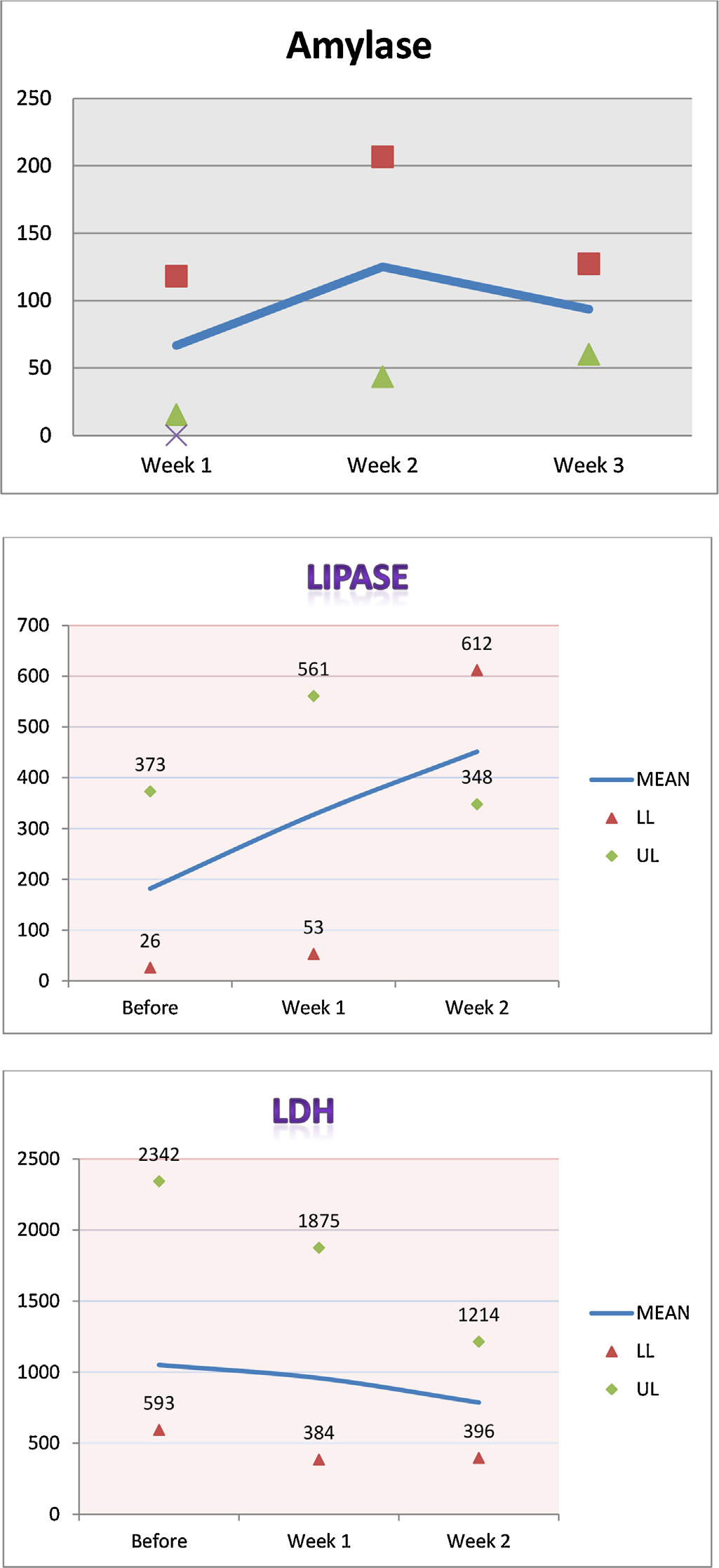
Figure 1. Median, minimum, and maximum values for amylase, lipase, and lactate
patient was diagnosed with pancreatitis and thus further
interferon and ribavirin was not given. The patient remained
critically ill on mechanical ventilation. She died 45 days after
admission from multi-organ failure.
An 81-year-old man with a history of atrial fibrillation, diabetes
A 62-year-old woman with diabetes, hypertension, and end-
mellitus, and hypertension was admitted with progressive
stage renal disease (ESRD) on hemodialysis was admitted with
respiratory distress and hypoxemia with fever for 2 days. He
fever, cough, and respiratory failure of 3-day duration (). She
tested positive for MERS-CoV by PCR. Baseline laboratory data are
tested positive for MERS-CoV by PCR. Baseline laboratory data are
shown in On the first day of hospitalization, the
shown in On admission, the patient was started on
patient was started on oseltamivir 75 mg once daily for 6 days,
oseltamivir 75 mg once daily for 5 days and levofloxacin 500 mg
levofloxacin 750 mg every 48 h for 5 days, and imipenem 250 mg
J.A. Al-Tawfiq et al. / International Journal of Infectious Diseases 20 (2014) 42–46
Patient demographics and underlying conditions
Other chronic conditions
Asthma, obstructive
sleep apnea, coronary
Atrial fibrillation
End-stage renal disease
ClCr, creatinine clearance; F, female; M, male; HD, hemodialysis.
Laboratory results of patients at baseline and at 1 and 2 weeks after the initiation of interferon and ribavirin therapy
Serum creatinine, mg/dl
Platelet count, � 109/l
WBC count, � 109/l
IV every 6 h for 6 days. On day 20 after admission, he was started
on ribavirin for 9 days, with a loading dose of 2000 mg via
nasogastric tube followed by 800 mg via nasogastric tube every
We reviewed the cases of five patients with MERS-CoV
8 h, two doses of interferon-a2b 100 mg subcutaneously once per
(). Their median age was 62 (range 24–81) years. The
week on hospital days 20 and 27, and methylprednisolone 40 mg
patients had chronic kidney disease and four were on maintenance
IV every 8 h for 4 days, then 40 mg IV twice daily for 2 days, then
hemodialysis. There were three men and two women. All patients
40 mg daily for 2 days (started on hospital day 22). Three weeks
tested negative for influenza. The median number of days from
after the initiation of therapy, his hemoglobin dropped (from 11.7
admission to therapy with ribavirin and interferon was 19 (range
to 8.7 g/dl) ), while bilirubin increased (from 0.7 to 7.4 mg/
10–22) All patients received adjunctive corticosteroid
dl), suggestive of hemolysis. The patient died 52 days after
therapy for acute respiratory distress syndrome. Two patients
admission from multi-organ failure.
developed increased lipase after the initiation of therapy, but both
of them were given corticosteroids at the same time as antiviral
therapy. One patient (case 2) developed thrombocytopenia, with a
platelet count that dropped from 408 to 49 � 109/l ()
A 24-year-old man with a history of ESRD on hemodialysis was
and one patient had possible hemolysis (case 3). All patients had
admitted with a febrile illness, a cough for 10 days, and respiratory
severe disease with progressive respiratory failure, developed
failure requiring mechanical ventilation. He tested positive for
multi-organ failure, and died a mean 39.6 (standard deviation 8.5)
MERS-CoV by PCR. Baseline laboratory data are shown in
days after admission. None of the patients had bacteremia or
On the first day of hospitalization, the patient was started on
fungemia during their hospital stay. shows the median,
oseltamivir 75 mg once daily for 2 days and imipenem 250 mg IV
minimum, and maximum values for amylase, lipase, and lactate
twice daily for 11 days. On day 19 of admission, he was started on
dehydrogenase (LDH) for all the patients before initiation of
ribavirin for 11 days, with a loading dose of 2000 mg via
therapy and at week1 and 2 after therapy initiation.
nasogastric tube, followed by 400 mg via nasogastric tube every
8 h, and two doses of interferon-a2b 100 mg subcutaneously once
per week on hospital days 19 and 26. On the same day he started
ribavirin, he was also started on methylprednisolone 40 mg IV
Since the emergence of MERS-CoV in 2012, the virus has caused
every 8 h for 4 days, then 40 mg IV twice daily for 2 days, and then
a total of 163 cases of disease, with a high case-fatality rate of
40 mg daily for 2 days. Two weeks after the initiation of therapy,
There is an urgent need for effective therapeutic agents. To date,
lipase increased (from 561 to 612 U/l). The patient died 32 days
no data are available on the use of any agents for the therapy of
after admission from multi-organ failure.
MERS-CoV-positive patients. In this report we have described the
J.A. Al-Tawfiq et al. / International Journal of Infectious Diseases 20 (2014) 42–46
Hepatic and pancreatic enzymes of patients at baseline and at 1 and 2 weeks after the initiation of interferon and ribavirin therapy
Liver function test
Pancreatic enzymes
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
a ALP, U/L; ALT, IU/L; AST, IU/L; LDH, IU/L; bilirubin, mg/dl.
b Amylase, U/L; lipase, U/L
in very mild radiographic evidence of pneumoniThe treatment
Days from admission to initiation of therapy or death
was given within 8 h after inoculation of the rhesus macaques.
Days to antiviral therapy
Days to steroid therapy
Treated animals had lower levels of systemic and local proin-
flammatory markers and fewer viral genome copieAlthough
these preclinical data are promising, our report illustrates some of
the real world issues of dealing with a novel emerging viral infection.
Our patients were not diagnosed until a week into their illnesses, by
which time all five were on mechanical ventilation. They had
multiple comorbidities, which most likely adversely affected their
therapy of five MERS-CoV patients who received interferon and
clinical outcomes. In addition, we had no serial virologic determi-
nation of MERS-CoV levels in airway secretions to shed light on the
The patients were treated during the initial phase of the Al-Hasa
possibility of virological clearance, virological failure, or clinical
outbreak in April and May 2013, a time at which the disease
failure of therapy.
epidemiology and clinical characteristics were not known. Hence,
In our small series, adverse effects from combination ribavirin
there was an inevitable time lapse from admission to the initiation
and interferon therapy were observed in three cases. These side
of therapy. All patients were already on mechanical ventilation by
effects have been noted Two patients had pancreatic
the time interferon and ribavirin therapy was instituted and all had
enzyme elevation and one had significant hemolysis. These
a fatal outcome. The patients received the therapy late in the
findings were complicated by the presence of abnormal laboratory
course of the disease, at a median of 19 (range 10–22) days after
findings even before the initiation of combination therapy, so it is
admission. Late therapy may have contributed to the poor
difficult to determine which side effects were due to disease
outcome, in addition to the severity of the disease and the
progression and which were due to the therapeutic drugs.
multiple comorbidities of the patients.
There is an urgent need for large-scale clinical trials to
The timing of initiation of antiviral therapy is critical in the
determine the safest and most effective regime for the treatment
treatment of most viral infections. In the treatment of SARS-CoV, no
of this novel highly fatal emerging infection. While ribavirin and
effect of oseltamivir or ribavirin was observed when these agents
interferon may show promise, their use needs to be prompt and
were started 6–14 days after symptom onset.Early therapy
adverse effects monitored closely. This therapeutic approach
with ribavirin, within 48 h of hospitalization or after the diagnosis of
should be tested in careful clinical studies.
SARS, has been shown to be associated with better outcomes,
although the numbers of patients enrolled in these studies has been
smalIn the treatment of influenza, oseltamivir therapy early
in the disease resulted in reduced mortality when this was started
The authors (JAT, HM, and JD) wish to acknowledge the use of
not later than 8 days after the onset of symptomsEarly in vitro
the Saudi Aramco Medical Services Organization (SAMSO) facilities
studies showed that ribavirin and interferon have anti-MERS-CoV
for the data and study, which resulted in this paper. Opinions
activity.The in vitro activity of interferon was augmented by
expressed in this article are those of the authors and not
the concomitant use of ribavirin.In a rhesus macaque model of
necessarily of SAMSO. The authors thank Dr Paul Anantharajah
MERS-CoV infection, the combination of interferon-a2b and
Tambyah of the National University of Singapore Department of
ribavirin therapy was effective in limiting the disease and resulted
Medicine for his critical review of the manuscript.
J.A. Al-Tawfiq et al. / International Journal of Infectious Diseases 20 (2014) 42–46
Financial support: All authors have no funding.
Conflict of interest: All authors have no conflict of interest to
2. World Health Orzination. Middle East respiratory syndrome coronavirus
(MERS-CoV)—update. Geneva: WHO; 2013, Available at:
last (accessed December 3).
18. Adisasmito W, Chan PK, Lee N, Oner AF, Gasimov V, Aghayev F, et al. Effective-
ness of antiviral treatment in human influenza A(H5N1) infections: analysis of a
global patient registry. J Infect Dis 2010;202
19. Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, et al. Broad-spectrum antivirals
for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013
Oct 3. pii: S0163-4453(13)00298-3.
Source: http://beid.ddc.moph.go.th/research2/file_upload/fulltext/fulltext_20150318_143045_bbcb8c6cf_1.pdf
Csir-Forestry research institute of Ghana R SCIENTIFIC AND INDUSTRIAL R Annual Report 2011 R SCIENTIFIC AND INDUSTRIAL RE CSIR-FORESTRY RESEARCH INSTITUTE OF GHANA Annual Report 2011 CSIR-FORESTRY RESEARCH INSTITUTE OF GHANA © Copyright CSIR-FORIG 2012 For more information please contact:The DirectorCSIR-Forestry Research Institute of GhanaU.P.O Box 63Kumasi, Ghana.
Handerwärmungstraining bei Morbus Raynaud-Syndrom Das Raynaud-Syndrom (Morbus Raynaud) ist eindie durch anfallsweises Erblassen der Hände oder Füße aufgrund von gekennzeichnet ist. Unter Umständen können auch Nase und Ohren betroffen sein. Etwa 3% der Bevölkerung leiden an einem primären M. Raynaud (siehe weiter unten). Frauen sind fünfmal häufiger betroffen als Männer. Bei stillenden Frauen können auch die Brustwarzen betroffen sein, während des Stillens verfärbt sich die jeweilige Brustwarze weiß. Manifestationsalter des primären M. Raynaud meist zwischen dem 20-40 Lj. Die Erkrankung ist nach ihrem Entdecker – dem französischen Arzt(1834–1881) – benannt. Umgangssprachlich wird sie auch als Weißfingerkrankheit oder Leichenfinger bezeichnet, andere Bezeichnungen hinsichtlich der Symptome sind Digitus mortuus (Totenfinger) oder Reilscher Finger.







