Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Clinical relevance of igg antibodies against food antigens in crohns disease: a double-blind cross-over diet intervention study
Original Paper
Digestion 2010;81:252–264
Received: September 4, 2009
Accepted: November 26, 2009 Published online: January 30, 2010
Clinical Relevance of IgG Antibodies against
Food Antigens in Crohn's Disease: A Double-Blind
Cross-Over Diet Intervention Study
S. Bentz a M. Hausmann a H. Piberger d S. Kellermeier a S. Paul c L. Held b
W. Falk d F. Obermeier d M. Fried a J. Schölmerich d G. Rogler a
a Division of Gastroenterology and Hepatology, University Hospital Zurich, and b University of Zurich,
Institute of Social and Preventive Medicine, Biostatistics Unit, Zurich , Switzerland; c Evomed MedizinService
GmbH, Darmstadt , and d Department of Internal Medicine I, University of Regensburg, Regensburg , Germany
Key Words
clusion: A nutritional intervention based on circulating IgG
Crohn's disease ⴢ Immunoglobulin G antibodies ⴢ Food
antibodies against food antigens showed effects with re-
antigen ⴢ Interferon
spect to stool frequency. The mechanisms by which IgG an-tibodies might contribute to disease activity remain to be elucidated.
Copyright 2010 S. Karger AG, Basel
Abstract
Background: Environmental factors are thought to play an
important role in the development of Crohn's disease (CD).
Immune responses against auto-antigens or food antigens
Introduction
may be a reason for the perpetuation of inflammation.
Meth-
ods: In a pilot study, 79 CD patients and 20 healthy controls
Genetic influences [1, 2] , cytokine activation [3] and
were examined for food immunoglobulin G (IgG). There-
various specific and nonspecific environmental factors
after, the clinical relevance of these food IgG antibodies
like hygiene, social standard, climatic factors, environ-
was assessed in a double-blind cross-over study with 40 pa-
mental pollution, smoking, stress and nutrition [4–7]
tients. Based on the IgG antibodies, a nutritional interven-
have been considered to be associated with the induction
tion was planned. The interferon (IFN) ␥ secretion of T cells and/or exacerbation of inflammatory bowel disease was measured. Eosinophil-derived neurotoxin was quanti-
(IBD), such as Crohn's disease (CD).
fied in stool.
Results: The pilot study resulted in a significant
Serologic markers for IBD, like anti-
Saccharomyces
difference of IgG antibodies in serum between CD patients
cerevisiae antibodies and atypical perinuclear antineu-
and healthy controls. In 84 and 83% of the patients, respec-
trophil cytoplasmic antibodies, remain to play a role in
tively, IgG antibodies against processed cheese and yeast
the pathophysiology of IBD. There is a wide range of
were detected. The daily stool frequency significantly de-
other antibodies including outer-membrane porin C,
creased by 11% during a specific diet compared with a sham
anti-
Pseudomonas fluorescens and antiglycan antibodies
diet. Abdominal pain reduced and general well-being im-
(anti-laminaribioside carbohydrate antibody, anti-chito-
proved. IFN ␥ secretion of T cells increased. No difference for
bioside carbohydrate antibody, anti-mannobioside car-
eosinophil-derived neurotoxin in stool was detected.
Con-
bohydrate antibody), and anti-CBir1. The latter is the
2010 S. Karger AG, Basel
Gerhard Rogler, MD, PhD
Division of Gastroenterology and Hepatology
Fax +41 61 306 12 34
Department of Internal Medicine University Hospital of Zurich
E-Mail
[email protected]
Accessible online at:
Rämistrasse 100, CH–8091 Zurich (Switzerland)
www.karger.com/dig
Tel. +41 44 255 9579, Fax +41 44 255 9497, E-Mail gerhard.rogler @ usz.ch
first bacterial antigen found to induce colitis in animal active disease and 8 were in remission. Patients were recruited models of IBD and also leads to a pathological immune from the German IBD Competence Network serum bank and ex-
amined for food specific IgG by the ImuPro300 test (Evomed,
response in IBD patients [8] .
Darmstadt, Germany). Disease activity was assessed by the pa-
Frequently, IBD patients report that dietary intoler-
tient's medical record.
ance significantly contributes to their symptomatology. The benefit from eliminating certain foods [9] from dai-
Study Design 2: Following Intervention Study
ly diet was refocused in the present study. Attempts to test
Consecutively in a randomized, double-blind, cross-over in-
tervention study, the clinical relevance of IgG antibodies against
for food intolerance in IBD have largely focused on classic
food antigens in 40 CD patients was tested. Not all patients from
food allergies based on the presence of immunoglobulin the previous pilot study were willing to participate in the follow-E (IgE)-mediated antibody responses, although these re-
ing intervention study; therefore, new patients were also tested for
actions appear probably quite rare in IBD [10] . It is there-
food IgG antibodies in serum. Patients were not selected for IgG
fore possible that adverse reactions in IBD might be due levels in serum. A sample size calculation was not performed. Fi-
nally, the specific antibody pattern of 40 patients was determined
to some reactions mediated by IgG antibodies, which in serum samples by the ImuPro300 test system. The reactivity of
characteristically give a more delayed response following
T regs and CD4+CD25– T cells to the patient-specific food antigens
exposure to a particular antigen [11] and have been im-
was determined in vitro (mixed lymphocyte stimulation assay)
plicated in some cases of food hypersensitivity [12–14] .
and correlated with in vivo changes on the basis of a nutritional
However, this mechanism is controversial and is consid-
record and a patient diary. The diary contained questions about stool frequency, abdominal pain and general well-being. Patients
ered to be physiological [15–17] , as IgG food antibodies validated their pain perception with scores of 0, 1, 2 and 3 which
can be present in apparently healthy individuals [18, 19] .
represented no pain, slight pain, moderate pain and severe pain,
It has been assumed that chronic inflammation in IBD is
respectively. The values were accumulated after each week. Ad-
due to an imbalance between inflammatory and anti-in-
ditionally, the patients rated their general well-being. The patients
flammatory mechanisms like regulatory CD4+CD25+ T assessed general well-being by a score of 0, 1, 2, 3 and 4, which
represented good, worse, bad, very bad and terrible, respectively.
cells (T regs ) [20–22] .
To get an overall impression of the symptoms, stool frequency,
High IgG levels against certain food components in abdominal pain and general well-being, a total score was calcu-
blood and the inflammatory response of T cells to food lated. Each subject recorded his eating habits and disease symp-antigens and the regulatory effect of T
toms over a period of 12 weeks and followed a specific or sham
regs in vitro was as-
sessed. As IgG food antibodies may play a role during the
diet. Each diet was followed for 6 weeks ( fig. 1 ). The definition of specific and sham diet was based on similarity of excluded food
initiation or perpetuation of IBD, we first investigated the components. If, for example, IgG against hazelnut was detected,
presence of IgG antibodies in CD patients and healthy then almond was excluded in the sham diet; if cauliflower IgG was controls. In a second approach, the therapeutic potential found, broccoli was excluded. Patients were concealed and allo-of an elimination diet based on the presence of IgG anti-
cated to one of the two diet sheets based on a randomization
bodies to food in patients with CD in a randomized con-
schedule using a random computer number generator. Thus, pa-tients received either an elimination diet based on their true sen-
trolled trial was investigated. Primary outcome parame-
sitivity results (specific diet) or a sham diet. Baseline demograph-
ters were stool frequency, abdominal pain and general ic and clinical characteristics of the two groups, including the use well-being. The possible activation of T-effector cells of concomitant medication, were found to be similar. All patients through IgG antibodies was measured by interferon and clinical staff in the gastroenterology research department (IFN) ␥ secretion. For the evaluation of disease activity, were blinded to the group assignment of all patients for the dura-
tion of the study. Patients were given their allocated diet sheet by
eosinophil-derived neurotoxin (EDN) was quantified in staff at the gastroenterology department and asked to eliminate
stool. A secondary outcome parameter was the total score
the indicated foods from their diet for a period of 12 weeks. They
built from stool frequency, abdominal pain and general also received a booklet with advice on how to eliminate the dif-well-being.
ferent foods (recipes and menus). This was explained by an expe-rienced nutritionist. Furthermore, the telephone contact details of a free nutritional advisor who they could contact for further advice if necessary was given to each patient.
Subjects
Study Design 1: Pilot Study
There were 16 male and 24 female subjects. Ultimately, data
Initially, 20 healthy volunteers without history of food intoler-
analysis of 23 patients was performed. Patients between the ages
ance and 79 CD patients with different disease status were in-
of 18 and 60 years were considered eligible. In this study, the pa-
cluded in a pilot study. Forty-seven of them had clinical and en-
tients were between 21 and 59 (mean 41 8 11) years of age. Both
doscopic signs of acute inflammation (i.e. diarrhea and mucosal
active and inactive patients were included, and diagnosis was
ulcerations). Twenty-four CD patients presented with chronically
manifested at least 6 months before onset of the trial. Duration of
Food Antigens in Crohn's Disease
Assessed for eligibility from the
German IBD Competence Network,
Ratisbona, Germany (n = 100)
Excluded (n = 60)
Not meeting inclusion criteria
Enrollment
Refused to participate
Randomized (n = 40)
Allocated to intervention 1 (specific diet, 6 weeks)
Allocated to intervention 2 (sham diet, 6 weeks)
Received allocated intervention
Allocation
Received allocated intervention
Did not receive allocated intervention
Did not receive allocated intervention
Allocated to intervention 2 (sham diet, 6 weeks)
Allocated to intervention 1 (specific diet, 6 weeks)
Received allocated intervention
Received allocated intervention
Did not receive allocated intervention
Did not receive allocated intervention
diet too restrictive: 4
diet too restrictive: 6
low compliance: 2
low compliance: 1
other reasons: 2
other reasons: 2
Analyzed (n = 12)
Analyzed (n = 11)
Analysis
Excluded from analysis (n = 0)
Excluded from analysis (n = 0)
Fig. 1. Study flow of the intervention trial. Patients were allocated to one of the two diets: either an elimination
diet based on their true sensitivity results (specific diet) or a sham diet and followed for 6 weeks. 17 patients did
not finish the trial.
disease was between 2 and 39 years (mean 14.9 8 10.6). Patients
comitant medication provided it had been constant for the 12
were excluded from participating if they had any significant co-
weeks of intervention ( table 1 ). The constant medication over
existing disease. During screening some patients had to be ex-
time was requested to determine a specific effect of nutritional
cluded due to a lack of cooperation (n = 3), severe concomitant
intervention and not of higher doses of medication. This study
disease (n = 10), abscesses (n = 15) or for C-reactive protein 1 150
was approved by the ethics committee of the University of Re-
(n = 4). Twenty-eight of the patients refused to participate. During
gensburg and performed according to the declaration of Helsinki.
the intervention phases the patients were allowed to take con-
Informed consent was obtained from all patients.
Table 1. Baseline characteristics of the patients: 16 males (m) and 24 females (w) participated in the study
Disease localization
terminal ileum, cecum
mesalazine, dimeticone, diltiazem
colon, terminal ileum
terminal ileum, cecum
budesonide, azathioprine, mesalazine, hydro-cortisonacetate, loperamide
terminal ileum, proximal colon, proctitis
terminal ileum, cecum
ileum resection, multiple perianal fistulae
terminal ileum, cecum
neoterminal ileum, colon, sigma, anal stenosis
esophagus lesions
infliximab, azathioprine, prednisolone
stenosis terminal ileum, ileocecal resection
sigma segment resection, stenosis colon descendens
colon, sigma, rectum, stenosis terminal ileum
mesalazine, azathioprine, hydrocortisone
azathioprine, azulfidine
neoterminal ileum, colon
neoterminal ileum
colon, distal ileum
terminal ileum, caecum, sigma, rectum
budesonide, azathioprine
infliximab, azathioprine, azulfidine, mesalazine, cholestyramine
colon, ileocecal resection
azathioprine, loperamide
prednisolone, mesalazine, hydrocortisone
colon, neoterminal ileum
terminal ileum, colon
neoterminal ileum
rectum, sigma, fistulae
prednisolone, ciprofloxacine, metronidazole
terminal ileum, colon
terminal ileum, sigma
terminal ileum, colon
Patients were between 21 and 59 years of age (42 8 12), treated with diverse drugs (treatment) and had different disease localization.
ImuPro300 Test
strated in table 2 . Only high values (score 3) and very high values
Sera from 40 patients was examined for food specific IgG by
(score 4) of IgG were excluded from the diet of the patients. Di-
an enzyme-linked immunosorbent assay (ELISA) ImuPro300 test
luted human serum was incubated in three different 96-well
according to the manufacturer's recommendations (R-Biopharm,
plates, each well coated with a different food extract. After wash-
Darmstadt, Germany). Specific IgG antibodies against 271 food
ing the plates 3 times with diluted washing buffer, a polyclonal
allergens (online suppl. table 1, for all online suppl. material see
anti-human IgG antibody (sheep; R-Biopharm) conjugated to al-
www.karger.com/doi/10.1159/000264649) are possible to deter-
kaline phosphatase was added. After washing with phosphate-
mine in human serum. The content of IgG antibodies is demon-
buffered saline (PBS), substrate solution (pnpp, R-Biopharm) was
Food Antigens in Crohn's Disease
Table 2. IgG antibodies in patients' serum by ImuPro300
nol (final concentration 3 ! 10 –5 M ; Gibco, Invitrogen, Karlsruhe, Germany), and 10% AB-serum (Cambrex Corporation, Europe).
Cells were stimulated with food antigens (20 g/ml; HAL Allergie
GmbH, Düsseldorf, Germany), negative control solution (diluent without antigen, HAL Allergie GmbH) or Dynabeads 쏐 CD3/
CD28 T Cell Expander (Dynal
쏐 , Hamburg, Germany). These an-
tigen solutions (as well as negative control solution) are common-
7.5–12.49 g/ml
ly used for prick test analysis as previously described by Van Den
12.5–19.99 g/ml
Bogaerde [25] . The cells were cultured at 37 ° C, 5% CO
20.0–49.99 g/ml
72 h, respectively.
Fluorescence-Activated Cell Sorting (FACS)
In patient-specific diets, only foods with score 3 (IgG content
To determine the purity of the isolated T cell fractions, cells
20–49.49 g/ml) and score 4 (IgG content ≥50 g/ml) were ex-
were stained with CD25-PE and CD4-FITC. The following anti-
bodies were used: 10 l of anti-human CD4-FITC, clone M-T466, isotype mouse IgG1 and 10 l anti-human CD25-PE, clone 7D4, isotype mouse IgG2b (Miltenyi Biotec) according to the manufac-turer's instructions. The antibodies were incubated for 15 min on
added to reveal the presence of IgG in the serum. Color develop-
ice and washed twice with PBS (PAA Laboratories GmbH). Sub-
ment is proportional to the quantity of bound antibodies. After
sequently, the cells were fixed with 3.7% paraformaldehyde, cen-
addition of a stop solution (NaOH; R-Biopharm), optical densities
trifuged at 300
g for 5 min and resuspended in 100 l of PBS.
were measured photometrically (405/620 nm, Tecan Sunrise; Te-
Stimulation of T cells with food antigens results in cell divi-
can GmbH, Crailsheim, Germany). IgG concentrations were cal-
sion with distinct fluorescence peaks, allowing determination of
culated using a standard curve.
the number of cell divisions calculated by carboxyfluorescein di-acetate succinimidyl ester (CFSE) fluorescence (Sigma-Aldrich
Collection of Peripheral Blood and Isolation of T regs and
Chemie, Taufkirchen, Germany). CFSE is a fluorescein derivative
CD4+CD25– T Cells
which passively diffuses through the cell membrane and binds
50 ml of peripheral blood were obtained from each patient at
irreversibly to cytoplasmatic proteins. 24 and 72 h after in vitro
the beginning of the trial and after 6 and 12 weeks. Blood was di-
stimulation, cells were analyzed by FACS. Proliferation is shown
luted with RPMI 1640 (Sigma-Aldrich Chemie, Steinheim, Ger-
as a decrement of fluorescence because CFSE is distributed among
many) in a ratio of 1: 2. Peripheral blood mononuclear cells the daughter cells. The cells were examined with an EPICS XL-
(PBMCs) were isolated from the diluted blood by lymphocyte sep-
MCL (Coulter Immunotech, Hamburg, Germany).
aration medium (PAA Laboratories GmbH, Pasching, Austria). 20 ml of lymphocyte separation medium were carefully covered
Determination of IFN ␥
with a layer of diluted blood and centrifuged at 400
g for 20 min
IFN ␥ secretion in cell supernatants was quantitatively mea-
at room temperature.
sured by ELISA (IFN ␥ -ELISA Set; Biosciences, San Diego, Calif.,
CD4+ T cells were isolated from PBMC using AutoMACS USA) according to the manufacturer's protocol.
(Miltenyi Biotec, Bergisch Gladbach, Germany) with a CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec) follow-
Determination of EDN
ing the manufacturer's instructions. 0.4–1 ! 10 6 T regs were iso-
For the evaluation of a potential food allergy and disease activ-
lated from 50 ml with the AutoMACS programs Depl05 and Pos-
ity, EDN was detected in 80 g of stool by ELISA (Immundiagnos-
seld2. Normally, T regs make up to 0.7–5.5% of PBMCs and 5–10%
tik, Bensheim, Germany) according to the manufacturer's proto-
of T-helper cells [20, 23, 24] . CD4– cells in the remaining negative
fraction were used to isolate antigen presenting cells (APC). CD4– cells were allowed to adhere to 96-well tissue culture plates (Fal-
Data Analysis
con, Becton Dickinson, Heidelberg, Germany) for 2 h in an incu-
Statistical analysis was carried out using SPSS, R and Sigma
bator at 37 ° C, 5% CO
Stat. Weekly counts of stool frequency were analyzed with Pois-
2 (Heraeus 6000, Sepatech, Osterode, Ger-
many). Nonadherent cells were removed by washing the wells son regression using generalized estimating equations (GEEs, repeatedly with prewarmed RPMI 1640. Adherent cells were used
[26] ) to account for correlations between observations made from
the same individual. This method provides a robust standard er-ror for the treatment effect which was used to calculate confi-
Assay of Suppressor Function by T regs
dence intervals and p values. Tests for cross-over effects were also
For suppression assays, 0.5–1 ! 10 5 CD4+CD25– T cells were
performed by GEEs. Moreover, GEEs with normal outcomes have
cultured in the absence or presence of 0.5–1 ! 10 5 autologous
been used to analyze the total score. Data were not analyzed ac-
T regs /well in 96-well plates and in the presence of 2 ! 10 5 adher-
cording to the intention-to-treat principle.
ent APC in RPMI 1640 medium with 1% nonessential amino ac-
The application of all tests was verified by normality tests
ids (100 ! ) and 1 m M sodium pyruvate (PAA Laboratories GmbH,
(Kolmogorov-Smirnov test, Shapiro-Wilk test). For statistical
Pasching, Austria), 1% MEM vitamins (100 ! ; Biochrom, Berlin,
analysis of the pilot study, the quantity of patients and healthy
Germany), 25,000 U penicillin and 25 mg streptomycin (Gibco
controls with IgG antibody levels (in percent) was assessed by a t
BRL Life Technologies, Eggenstein, Germany),  -mercaptoetha-
test. Statistical analysis of IFN ␥ secretion was performed by the
Fig. 2. Progression of stool frequency. Stool frequency was moni-
Fig. 3. Progression of total score. An average reduction of the total
tored per week. Only those patients who first followed the spe-
weekly score of 6.5 points was estimated for the specific diet group
cific diet had a significant reduction in stool frequency. Subjects
compared with the sham diet group (GEE analysis).
who first followed the sham diet had no significant change in their stool frequency (GEE analysis).
Kruskal-Wallis test based on non-normally distributed data. Val-
patients had IgG antibodies against hazelnut in contrast
ues were significantly different when the obtained difference in
to only 15% of healthy controls. This was even more pro-
mean ranks was greater than the 2 value (in all figures indicated
nounced in IgG antibodies against linseed, where 70% of
with #). Values are expressed as the mean [minimum, maximum]. Statistical analysis of EDN in stool was performed by analysis of
CD patients and only 10% of healthy controls showed IgG
variance (ANOVA) for normally distributed data. Statistical sig-
antibodies. The same was seen with processed cheese
nificance was based on a p value smaller than 0.05.
(60% of healthy controls vs. 84% of CD patients).
The most frequently detected IgG antibodies in healthy
controls were against yeast (66%),
Aspergillus niger (60%),
whey (60%), processed cheese (60%), bamboo sprouts (55%), paprika spice (55%), crawfish meat (50%), cottage
Pilot Study-IgG Antibodies in CD Patients and
cheese (45%), yoghurt (45%) and zander (45%).
Healthy Controls The pilot study resulted in a significant difference of
Effects of the Nutritional Intervention on Stool
IgG antibodies in serum between CD patients and healthy
Frequency, Abdominal Pain and General Well-Being
controls (p ! 0.0001, t test). All detected IgG antibody
There was no evidence for a cross-over effect in the
reactions are presented online (online suppl. table 2). The
analysis of the weekly stool frequency counts (p = 0.08).
ten most frequently measured IgG antibodies in CD pa-
In the specific diet group, a significant reduction in the
tients were against processed cheese (84%), yeast (83%), daily stool frequency by 11% was achieved compared to agave syrup (78%), camembert cheese (76%), poppy seeds
the sham diet group (p = 0.004, 95% CI: 4%, 18%). How-
(74%), aloe vera (74%), bamboo sprouts (73%), kamut (du-
ever, the effect was confounded by a significant increase
rum wheat, 70%), unripe spelt grain (69%) and wheat in stool frequency of 9% in the second intervention phase (60%). More CD patients showed reactions against the of the study, regardless of type of diet (p = 0.025, 95% CI: evaluated food components than healthy controls, i.e. 1%, 18%; fig. 2 ). The comparison of loose stools during 35% of healthy controls had IgG antibodies against wheat
the specific and sham diets of each patient demonstrated
in contrast to 60% of CD patients. Moreover, 39% of CD that, surprisingly, only those patients who first followed
Food Antigens in Crohn's Disease
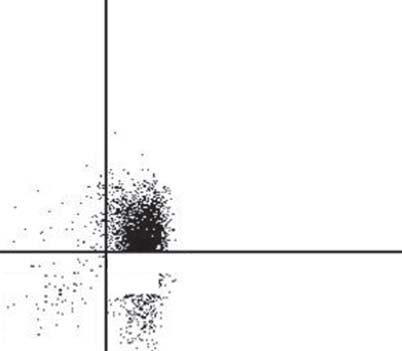
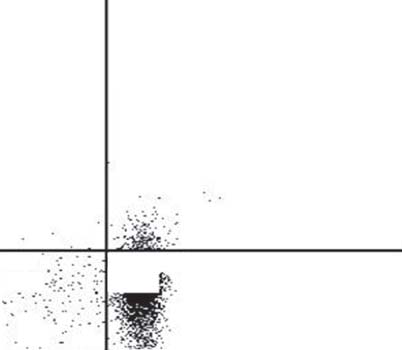
Fig. 4. FACS analysis of CD4+CD25– T cells and T regs . a Staining of T regs . b Staining of CD4+CD25– T cells
(effector T cells). The purity of CD4+CD25– effector T cells was 90.8% and of T regs 62.6%.
the specific diet had a significant reduction in stool fre-
cell activation was evaluated by quantification of IFN ␥ in
quency. The group of subjects who first followed the sham
cell supernatants by ELISA ( fig. 5–7 ). All obtained IFN ␥
diet and then specific diet had no significant change in values were not normally distributed; therefore, a Krus-their stool frequency.
kal-Wallis test was performed.
Patients were asked to rate their pain perception and
general well-being. The given points were accumulated
Stimulation of CD4+CD25– and APC with Food
after each week. To obtain an overall impression of stool
Antigens and Negative Control Solution
frequency, abdominal pain and general well-being, a total
The incubation of CD4+CD25– T cells and APC with
score was calculated. There was neither evidence for a food antigens caused an increase in IFN ␥ secretion ( fig. 5 , cross-over effect nor an intervention phase in the analysis
left panel). The amount of IFN ␥ at base value (time point
of the total score. An average reduction of the total week-
zero) was 170.5 pg/ml [16.5; 495.3] and increased during
ly score ( fig. 3 ) of 6.5 points was estimated for the spe-
specific diet (411.1 pg/ml [16.9; 1,117.0]) and sham diet
cific diet group compared with the sham diet group (95%
(481.5 pg/ml [1.5; 1,234.0]). Unfortunately, there was no
CI: –0.6, 13.6 points). The estimated effect seems to have significant difference between the three time points.
a clinically relevant effect, but is not significant (p =
Stimulation of CD4+CD25– T cells and APC with
negative control solution (
, right panel) showed
IFN ␥ secretion comparable to food antigen solution. The
IFN ␥ Secretion of CD4+CD25– Effector T Cells and
amount of IFN ␥ at base value was 153.1 pg/ml [5.8; 587.6]
and increased during specific diet (308.3 pg/ml [61.0;
Isolation of CD4+CD25– effector T cells and T regs was
1,220.0]) and sham diet (681.6 pg/ml [126.0; 1,347.0]).
controlled by FACS analysis. The purity of CD4+CD25– There was a significantly higher IFN ␥ secretion during effector T cells was 90.8 and 62.6% for T regs ( fig. 4 a, b).
sham diet than during specific diet or base value, when
CD4+CD25– effector T cells and APC were incubated in cells were stimulated by negative control solution.
the absence or presence of T regs and stimulated with food
In summary, CD4+CD25– T cells were not clearly
antigens, negative control solution or antiCD3/antiCD28
more stimulated by food antigen solution than by nega-
solution. The effect of food antigens on CD4+CD25– T tive control solution.
Fig. 5. Stimulation of CD4+CD25– T cells
and APC with food antigens or negative
control solution. Comparison of IFN ␥ se-
cretion (pg/ml) between base value (be-
ginning of intervention), specific diet and sham diet (after 6 weeks of intervention, re spectively). Stimulation with food anti-
gens (left panel) increased IFN ␥ secretion of CD4+CD25– T cells cultivated with APC during intervention. Stimulation with negative control solution (right pan-el) also increased IFN ␥ secretion during intervention compared with food antigen stimulation. Kruskal-Wallis test; # indi-
cates significance (obtained difference in
mean ranks was greater than the 2 val-ue); u indicates outliers.
Fig. 6. Stimulation of CD4+CD25– T cells
and APC in the presence of T regs with food
antigens or negative control solution.
Comparison of IFN ␥ secretion (pg/ml) be-
tween base value (beginning of interven-
␥ (pg/ml) 1,000
tion), specific diet and sham diet (after 6
weeks of intervention, respectively). Stim-ulation with food antigens (left panel): CD4+CD25– T cells co-cultivated with APC and T
regs secreted more IFN ␥ during
specific diet and sham diet in contrast to base value. Stimulation with negative con-trol solution (right panel): less IFN ␥ secre-tion between base value and the specif -
ic and sham diets, respectively. Kruskal-
Wallis test; # indicates significance (ob
tained difference in mean ranks was great - er than the 2 value); uindicates outliers.
Food Antigens in Crohn's Disease
CD4+CD25– and APC
Mixture CD4+CD25–, APC, Tregs
Fig. 7. Stimulation of CD4+CD25– T cells,
APC and T regs with antiCD3/antiCD28 so-
lution. Comparison of IFN ␥ secretion (ng/ml) between base value (beginning of in-tervention), specific diet and sham diet
(after 6 weeks of intervention, respective-
ly). There was 1,000-fold higher IFN ␥ se-
cretion when cells were stimulated with antiCD3/antiCD28 solution than with food antigen/negative control solution. There was higher IFN
CD4+CD25– T cells (left panel) between base value and specific diet. The mixture of CD4+CD25– T cells, APC and T
showed a significant increase only between base value and the sham diet. Kruskal-
Wallis test; # indicates significance (ob-
tained difference in mean ranks was great-er than the 2 value); uindicates outliers.
Stimulation of the Co-Culture of CD4+CD25–, APC er IFN ␥ secretion in contrast to the stimulation with food
and T regs with Food Antigens and Negative Control Solu-
antigens or negative control solution. Therefore, cells
were more effectively stimulated with antiCD3/antiCD28
The co-culture of CD4+CD25– T cells and APC with solution than with food antigen/negative control solu-
T regs and stimulation with food antigens ( fig. 6 , left panel) tion, as seen in 1,000-fold higher IFN ␥ secretion.
also resulted in an increase in IFN ␥ secretion from base
In case of CD4+CD25– T cells ( fig. 7 , left panel), a sig-
value (169.5 pg/ml [42.7; 543.1]) to specific diet (683.8
nificant increase in IFN ␥ secretion was detected between
pg/ml [37.4; 1,311.0]) or sham diet (609.8 pg/ml [34.0; base value (48.2 ng/ml [0.8; 151.0]) and specific diet (169.3 1,209.0]). Unfortunately, there was no significant differ-
ng/ml [19.6; 307.0]). There was no significant difference
ence between the three time points.
between base value and sham diet (204.6 ng/ml [12.10;
There was lower IFN ␥ secretion when co-cultivated 791.0]).
CD4+CD25– T cells, APC and T regs were stimulated with
The mixture of CD4+CD25– T cells, APC and T regs
negative control solution ( fig. 6 , right panel). Significant-
( fig. 7 , right panel) showed only a significant increase be-
ly lower IFN ␥ secretion was only received between base tween base value (51.9 ng/ml [0.1; 271.0]) and sham diet value (294.4 pg/ml [10.1; 1,587.0]) and sham diet (21.2 pg/
(135.9 ng/ml [49.20; 309.0]). The increase during specific
ml [1.9; 53.8]). Specific diet (71.6 pg/ml [2.9; 231.4]) re-
diet (157.9 ng/ml [26.20; 621.0]) was not significant.
sulted in no significant difference.
In summary, none of the three different stimulation
In summary, there was no clear difference between
methods led to a significant difference in IFN ␥ secretion
the culture of CD4+CD25– T cells and the culture of of CD4+CD25– T cells or the mixture of CD4+CD25– T CD4+CD25– T cells with T regs .
cells and T regs .
Stimulation with AntiCD3/AntiCD28 Solution
Time Course of Cell Division after Cell Stimulation
The stimulation with antiCD3/antiCD28 beads of
The proliferative response of the isolated T lympho-
CD4+CD25– T cells and APC, or the co-cultivation of cytes to stimulation with food antigens, negative control CD4+CD25– T cells, APC and T regs ( fig. 7 ) showed high-
solution and antiCD3/antiCD28 solution was investigated
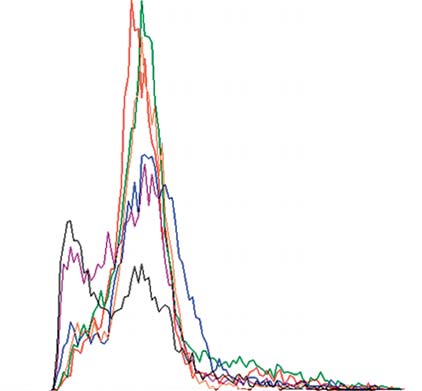
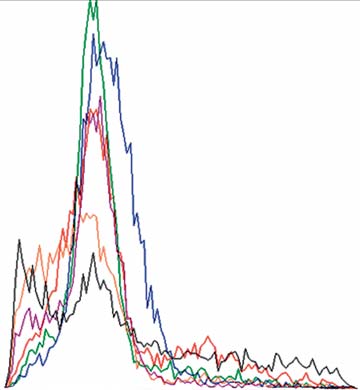
Fig. 8. CFSE proliferation in the mixture of CD4+CD25– T cells,
Fig. 9. CFSE proliferation of CD4+CD25– T cells and APC mea-
APC and T regs measured by FACS analysis. CD4+CD25– T cells
sured by FACS analysis. CD4+CD25– T cells and APC in the
and APC in the presence of T regs did not demonstrate any in vitro
absence of T regs did not demonstrate any in vitro proliferative
proliferative responses to food antigens. After 24 h: stimulation
responses to food antigens. After 24 h: stimulation with food
with food antigens (red), negative control solution (green) and
antigens (red), negative control solution (green) and antiCD3/
antiCD3/antiCD28 solution (blue); after 72 h: stimulation with
antiCD28 solution (blue); after 72 h: stimulation with food anti-
food antigens (orange), negative control solution (purple) and an-
gens (orange), negative control solution (purple) and antiCD3/
tiCD3/antiCD28 solution (black); graphs were recorded separate-
antiCD28 solution (black). Graphs were recorded separately and
ly and are shown in overlay mode.
are shown in overlay mode.
by CFSE ( fig. 8 , 9 ). Each of the curves reflects a measure-
Discussion
ment of the fluorescence intensity caused by cell division. CD4+CD25– T cells and APC in the absence or presence
In the present study, we have shown that IgG antibod-
of T regs did not demonstrate any in vitro proliferative re-
ies against food antigens are elevated in patients with CD
sponses to food antigens. Hence, the isolated T cells do not
in contrast to healthy controls. A clinically significant
proliferate in vitro after stimulation with food antigens.
improvement in IBD symptoms was observed in patients eliminating foods to which they were found to exhibit
Quantification of EDN in Stool
sensitivity. IFN ␥ secretion by T cells was increased after
The disease activity was evaluated by the quantifica-
specific diet, but also after sham diet. There was a reduc-
tion of EDN in stool samples ( fig. 10 ). The samples were tion of EDN concentration in stool during specific diet normally distributed and, therefore, ANOVA was per-
and sham diet, but no significant difference between the
formed. The concentration of EDN at the beginning of two diets.
intervention (base value) was 1,536 8 405 ng/ml. The
Forty-eight percent of patients in the present interven-
concentration declined during specific diet (1,228 8 530
tion study had an improvement in stool frequency and
ng/ml) but also during sham diet (1,355 8 373 ng/ml). general well-being (total score). Only 9% of patients de-There was no significant difference between the three scribed opposite effects.
time points. EDN concentration dropped in the same de-
The study results are encouraging; however, they have
gree under specific diet and sham diet. No difference for to be interpreted with care as results may have been in-EDN in stool indicated an absence of eosinophil-medi-
fluenced by several confounders: The daily reporting of
ated reactions.
consumed foods and the attachment to the diet recom-
Food Antigens in Crohn's Disease
cific and sham diet was based on the similarity of exclud-ed food components. If for example IgG against hazelnut
was detected, then almond was excluded in the sham diet; if cauliflower IgG was found, broccoli was excluded.
There may be some cross-reactivity of the respective an-tigens, which could explain some effects of the sham diet
on IBD symptoms, T cell cytokine secretion and stool EDN levels.
In addition, we did not have a washout phase at the
cross-over point, which may have led to some transmis-sion of effects into the sham arm of the study.
More than 80% of CD patients in the pilot study and
more than 30% of CD patients in the intervention study
had IgG antibodies against yeast. The IgG-antibody
reactions against food antigens were also investigated in
CD patients by Van Den Bogaerde [25] . Increased sensi-tization against yeast was demonstrated in vivo and in vitro as in the present study. Additionally, a study from
Fig. 10. Detection of EDN in stool samples. Comparison between
Darroch et al. [31] pointed out that antibodies against
base value (beginning of intervention), specific diet and sham diet
Saccharomyces cerivisiae are significantly increased in
(after 6 weeks of intervention, respectively). EDN concentration (ng/ml) declined during intervention in comparison to base val-
patients with CD in comparison to patients with ulcer-
ue. There was no significant difference between the two diets.
ative colitis and that they play a role in the function of T
and B cells in patients with CD. In the present study, the difference in T cell function is shown by higher IFN ␥ se-cretion. Elevated IFN ␥ levels after nutritional interven-tion were detected in supernatants of cultures of
mendations required much time and discipline from the CD4+CD25– T cells and APC in the presence or absence study subjects. Therefore, the problem of under-report-
of T regs . The mucosa of CD patients is dominated by T
ing (consumed foods and beverages were not listed cor-
cells of the T-helper cell phenotype 1 [32] .
rectly) is evident. We tried to reduce this problem by ex-
These cells are characterized by the secretion of IFN ␥ .
plaining the list of foods to be avoided in great detail to A greater number of cells secreting IFN ␥ in CD in con-each patient and showing ‘hidden sources'. This was done
trast to ulcerative colitis and healthy controls was found
by one well-trained person.
[33] . IFN ␥ is involved in specific cell-mediated immunity
Basically, the main limitation of this cross-over study and causes the secretion of IgG2 antibodies during de-
was the high dropout rate (n = 17), which was a result of layed-type hypersensitivity. Van Den Bogaerde et al. [25] the length of the study, the changes in CD and voluntary illustrated, both in vitro and in vivo, a more pronounced withdrawals.
reaction of T cells against food antigens. They tested the
In addition, the 40 patients initially included in this proliferation of peripheral blood lymphocytes after stim-
study were on different medications. Due to low numbers
ulation with different food antigens like cereal, cabbage,
of individuals in this study, stratification according to the
citrus fruits, yeast and nuts in 10 CD patients and 10
different treatments would have caused inability to do a healthy controls [25] . They used commercial prick solu-statistical analysis. However, strong immunosuppres-
tion (just like in the present study) for cell stimulation.
sants certainly could have influenced the study results, The authors concluded that in vivo sensitization against especially with respect to T cell function. We tried to re-
food antigens exists. The mechanism has to be further
duce this problem by keeping the medication constant for
elucidated, perhaps due to a defective epithelial barrier
the time of intervention. In addition, many of the patients
function, which might allow infiltration of food antigens
with CD already kept to some individual forms of diet, to the mucosa. IFN ␥ secretion of T regs after stimulation avoiding bloating foods such as onions or garlic [27–30] .
with antiCD3/antiCD28 solution was also analyzed by
In addition, it may be argued that the sham diet was Earle et al. [34] . In this issue, T regs secreted no IFN ␥ in
too similar to the specific diet, as the definition of spe-
contrast to PBMC which secreted a concentration of 500
pg/ml. In addition, a co-cultivation of PBMC and T regs claimed that the raised IgG antibodies are due to a failure resulted in reduced IFN ␥ production. In contrast to the of intestinal barrier function. This circumstance could be findings of Earle, Nakamura et al. [35] postulated that one reason for the effectiveness of an IgG-based diet in-T regs produce significantly less IFN ␥ than CD4+CD25– T tervention.
cells when stimulated adequately with antiCD3/antiCD28
In conclusion, a nutritional intervention diet based on
solution. However, a limitation of the current study was circulating IgG antibodies against food antigens showed that no FoxP3 staining was performed.
effects with respect to stool frequency, abdominal pain
In the context of this study, a significant reduction of and general well-being in this double-blind cross-over
EDN concentration in stool during intervention could be
study with 40 CD patients. Stool frequency and total
detected. Values up to 1,300 ng/ml were examined. These
score during the specific diet were significantly lower in
findings are in line with results from Saitoh et al. [36] who
contrast to the sham diet. Stimulation of T cells with the
found values up to 3,500 ng/ml in active CD and 910 ng/
specific antigens was followed by an increase in IFN ␥ se-
ml in inactive CD.
cretion. However, there was no difference in T cell prolif-
Similar to our data, an elimination diet in patients eration in response to different antigens. The concentra-
with irritable bowel syndrome was effective. One hun-
tion of EDN in stool declined during the specific diet, but
dred fifty irritable bowel syndrome patients were tested also during sham diet. The mechanisms by which IgG and got a ‘true' or a ‘sham' diet. The true diet excluded antibodies might contribute to disease activity remain to foods against which IgG antibodies were found, the sham
be elucidated.
diet excluded foods against which no IgG antibodies were found (as in the present study). After 12 weeks of inter-vention, patients with the true diet had a 10% greater im-
Acknowledgements
provement of their symptoms than those patients with sham diet. Most of the patients within this study had IgG
The authors thank the study nurses and the blood bank of the
German Competence Network for Inflammatory Bowel Disease
antibodies against yeast, followed by milk, chicken, egg, (KN-CED) for providing us with serum samples and we are grate-
wheat and cashew nuts [37] . This study lacks a cross-over
ful for the patients' contribution of the blood samples. This study
design in contrast to the present study. Moreover, immu-
was supported by the German Competence Network for Inflam-
nohistochemical research found IgG producing B cells in
matory Bowel Disease (KN-CED) and the Evomed MedizinSer-
the colon and ileum of CD patients [38, 39] . The authors
References
1 Hugot JP, et al: Prevalence of CARD15/
7 Hafner S, et al: The role of domestic hygiene
12 Awazuhara H, Kawai H, Maruchi N: Major
NOD2 mutations in Caucasian healthy peo-
in inflammatory bowel diseases: hepatitis A
allergens in soybean and clinical signifi-
ple. Am J Gastroenterol 2007;
and worm infestations. Eur J Gastroenterol
cance of IgG4 antibodies investigated by
Hepatol 2008; 20: 561–566.
IgE- and IgG4-immunoblotting with sera
2 Ogura Y, et al: A frameshift mutation in
8 Papp M, Altorjay I, Lakatos PL: Relevance of
from soybean-sensitive patients. Clin Exp
NOD2 associated with susceptibility to
serologic studies in inflammatory bowel dis-
Allergy 1997; 27: 325–332.
Crohn's disease. Nature 2001; 411: 603–606.
eases (in Hungarian). Orv Hetil 2007; 148:
13 el Rafei A, et al: Diagnostic value of IgG4
3 Rogler G, Andus T: Cytokines in inflamma-
measurements in patients with food allergy.
tory bowel disease. World J Surg 1998; 22:
9 Ferguson LR, et al: Genes, diet and inflam-
Ann Allergy 1989; 62: 94–99.
matory bowel disease. Mutat Res 2007; 622:
14 Host A, et al: Prospective estimation of IgG,
4 Regueiro M, et al: Cigarette smoking and age
IgG subclass and IgE antibodies to dietary
at diagnosis of inflammatory bowel disease.
10 Mekkel G, et al: Increased IgE-type antibody
proteins in infants with cow milk allergy.
Inflamm Bowel Dis 2005; 11: 42–47.
response to food allergens in irritable bowel
Levels of antibodies to whole milk protein,
5 Kane SV, Flicker M, Katz-Nelson F: Tobacco
syndrome and inflammatory bowel diseases
BLG and ovalbumin in relation to repeated
use is associated with accelerated clinical re-
(in Hungarian). Orv Hetil 2005; 146: 797–
milk challenge and clinical course of cow
currence of Crohn's disease after surgically
milk allergy. Allergy 1992; 47: 218–229.
induced remission. J Clin Gastroenterol
11 Crowe SE, Perdue MH: Gastrointestinal
15 Barnes RM, et al: Human serum antibodies
2005; 39: 32–35.
food hypersensitivity: basic mechanisms of
reactive with dietary proteins. IgG subclass
6 Picco MF, Bayless TM: Tobacco consump-
pathophysiology. Gastroenterology 1992;
distribution. Int Arch Allergy Appl Immu-
tion and disease duration are associated with
103: 1075–1095.
nol 1988; 87: 184–188.
fistulizing and stricturing behaviors in the
16 Lessof MH, Kemeny DM, Price JF: IgG anti-
first 8 years of Crohn's disease. Am J Gastro-
bodies to food in health and disease. Allergy
enterol 2003; 98: 363–368.
Proc 1991; 12: 305–307.
Food Antigens in Crohn's Disease
17 Husby S, et al: Oral tolerance in humans. T
25 Van Den Bogaerde J, et al: Gut mucosal re-
34 Earle KE, et al: In vitro expanded human
cell but not B cell tolerance after antigen
sponse to food antigens in Crohn's disease.
CD4+CD25+ regulatory T cells suppress ef-
feeding. J Immunol 1994; 152: 4663–4670.
Aliment Pharmacol Ther 2002;
fector T cell proliferation. Clin Immunol
18 Haddad ZH, et al: Detection and kinetics of
2005; 115: 3–9.
antigen-specific IgE and IgG immune com-
26 Zeger SL, Liang KY: Longitudinal data anal-
35 Nakamura K, Kitani A, Strober W: Cell
plexes in food allergy. Ann Allergy 1983; 51:
ysis for discrete and continuous outcomes.
contact-dependent immunosuppression by
Biometrics 1986; 42: 121–130.
CD4(+)CD25(+) regulatory T cells is medi-
19 Husby S, et al: Humoral immunity to dietary
27 Jones VA, et al: Food intolerance: a major fac-
ated by cell surface-bound transforming
antigens in healthy adults. Occurrence, iso-
tor in the pathogenesis of irritable bowel syn-
growth factor beta. J Exp Med 2001; 194: 629–
type and IgG subclass distribution of serum
drome. Lancet 1982; 2: 1115–1117.
antibodies to protein antigens. Int Arch Al-
28 Nanda R, et al: Food intolerance and the ir-
36 Saitoh O, et al: Fecal eosinophil granule-de-
lergy Appl Immunol 1985; 77: 416–422.
ritable bowel syndrome. Gut 1989; 30: 1099–
rived proteins reflect disease activity in in-
20 Jonuleit H, Adema G, Schmitt E: Immune
flammatory bowel disease. Am J Gastroen-
regulation by regulatory T cells: implications
29 Niec AM, Frankum B, Talley NJ: Are adverse
terol 1999; 94: 3513–3520.
for transplantation. Transpl Immunol 2003;
food reactions linked to irritable bowel syn-
37 Atkinson W, et al: Food elimination based
drome? Am J Gastroenterol 1998; 93: 2184–
on IgG antibodies in irritable bowel syn-
21 Spoettl T, et al: Serum soluble TNF receptor
drome: a randomised controlled trial. Gut
I and II levels correlate with disease activity
30 Burden S: Dietary treatment of irritable bow-
2004; 53: 1459–1464.
in IBD patients. Inflamm Bowel Dis 2007; 13:
el syndrome: current evidence and guide-
38 Baklien K, Brandtzaeg P: Comparative map-
lines for future practice. J Hum Nutr Diet
ping of the local distribution of immuno-
22 Podolsky DK: Inflammatory bowel disease.
2001; 14: 231–241.
globulin-containing cells in ulcerative coli-
N Engl J Med 2002; 347: 417–429.
31 Darroch CJ, Barnes RM, Dawson J: Circulat-
tis and Crohn's disease of the colon. Clin Exp
23 Beissert S, Schwarz A, Schwarz T: Regulatory
ing antibodies to Saccharomyces cerevisiae
Immunol 1975; 22: 197–209.
T cells. J Invest Dermatol 2006; 126: 15–24.
(bakers'/brewers' yeast) in gastrointestinal
39 Baklien K, Brandtzaeg P: Immunohisto-
24 Wing K, et al: CD4 T cell activation by my-
disease. J Clin Pathol 1999; 52: 47–53.
chemical characterization of local immuno-
elin oligodendrocyte glycoprotein is sup-
32 Elson CO, et al: Immuno-bacterial homeo-
globulin formation in Crohn's disease of the
pressed by adult but not cord blood CD25+ T
stasis in the gut: new insights into an old
ileum. Scand J Gastroenterol 1976; 11: 447–
cells. Eur J Immunol 2003; 33: 579–587.
enigma. Semin Immunol 2001; 13: 187–194.
33 Breese E, et al: Interleukin-2- and interferon-
gamma-secreting T cells in normal and dis-eased human intestinal mucosa. Immunol-ogy 1993; 78: 127–131.
Source: http://amodomedical.se/wp-content/uploads/2014/Gastro/2010_Bentz_Morbus_Crohn_Digestion.pdf
aller Nº 1: Encuentros Nacionales de MujeresLos Encuentros Nacionales de Mujeres en la Argentina. Impacto social y políticode los Encuentros, del XXXI en particular. Forma de convocatoria,funcionamiento y organización. Autogestión y organización. Rol y objetivos dela Comisión Organizadora. Relación de los movimientos nacionales de mujerescon los Encuentros. Niveles de interrelación y coordinación de los movimientosde mujeres con otros movimientos sociales. Encuentros regionales y locales;iniciativas. Balances y desafíos. Historia y logros.
SOMMAIRE G.MENTHA (Genève) F.-R. PRUVOT (Lille) J.-Y. MABRUT (Lyon) Kyste biliaire simple et formes compliquées P. PESSAUX (Strasbourg) Polykystose hépatique J. HARDWIGSEN (Marseille) Cystadénome biliaire, cystadénocarcinome et autres tumeurs malignes kystiques O. SCATTON (Paris)







