Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Agimed.com.ar
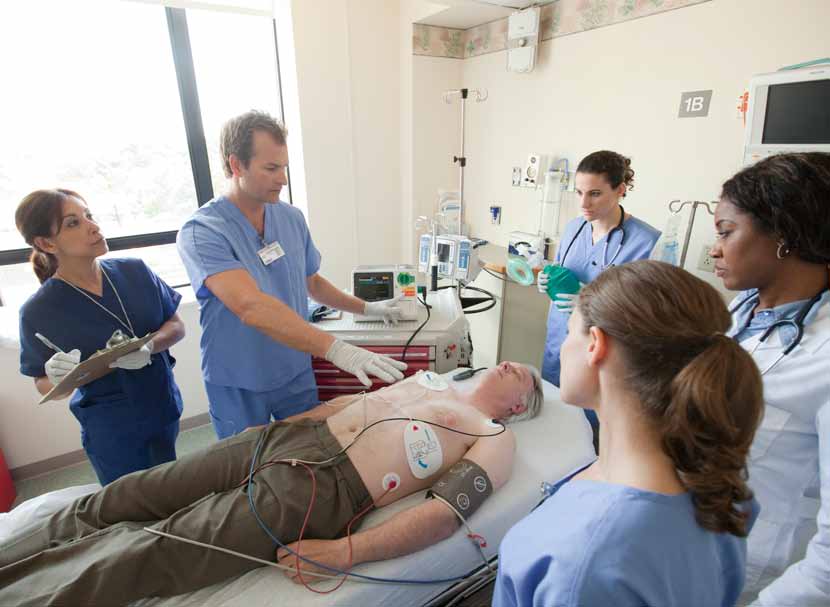
Ready to save lives
Philips HeartStart XL+ Defibril ator/Monitor
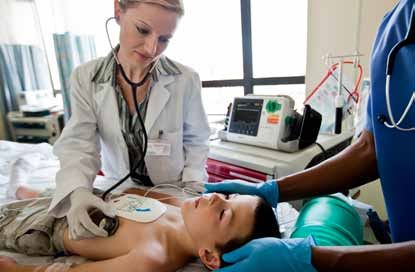

Ready to respond, revive, improve
Hospital cardiac emergencies are typical y stressful and chaotic events. The last thing you want to worry about is whether your defibril ator is ready. Designed for Resuscitation Teams and Rapid Response Teams, the Philips HeartStart XL+ Defibril ator/Monitor contains meaningful innovations that can help you confidently and effectively respond to patients throughout the hospital.
The HeartStart XL+ has a similar user interface as other
Ready to respond – The HeartStart XL+ has a fast
Philips defibril ator/monitors and Philips industry-leading
battery recharge time and a highly visible "ready-for use"
AEDs. The HeartStart XL+ also uses similar alarms, as
indicator, as well as LEDs on the front of the device that
well as identical cables and accessories as the HeartStart
signal power status, so clinicians can quickly see that
MRx, and Philips patient monitors. This means enhanced HeartStart XL+ is ready. ease of use and "plug and play" patient hand-off, as well as simplified inventory management and reduced costs.
Ready to revive – The easy-to-use HeartStart XL+
delivers Philips proven biphasic therapy for defibril ation
The HeartStart XL+ is always ready to meet the needs
and synchronized cardioversion, and is the only
of your organization. Because it is built on a scalable
defibril ator that in AED mode can defibril ate any
platform, you can choose the functions that best meet
patient – adult, child, or infant – with no special pads or
the needs of your staff today - from BLS responders to
accessories required.
ALS clinicians - and in the future as your needs change.
Ready to improve – The HeartStart XL+ and Philips
data management solutions are designed to help support
a culture of continuous improvement and excel ence
among hospitals.
Philips HeartStart XL+ Defibril ator/Monitor

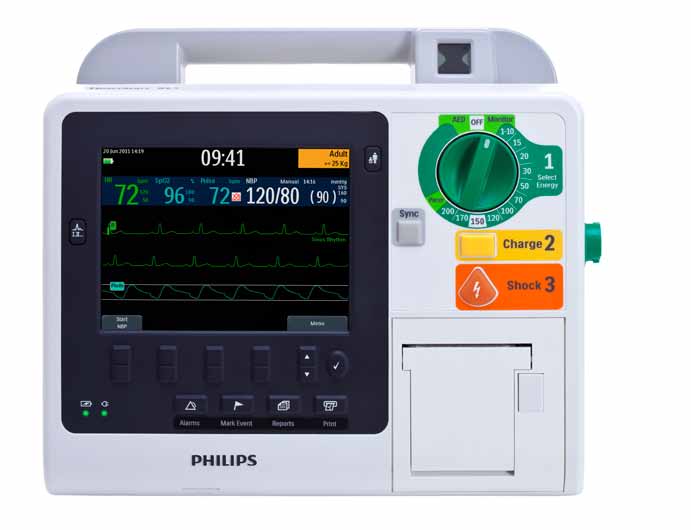
Ready to respond, revive, improve
• Active ready-for-use visual
• Automated External
• HeartStart Adult/Child or
• Green front panel lights
indicator flashes, signaling the unit
Defibrillator (AED) mode
Infant defibrillation pads
indicate AC and battery power.
has passed its most recent self-test
is easy to use for virtual y any
are designed for manual or AED
No need for staff to check that the
and is ready.
responder. HeartStart XL+
mode, pacing, cardioversion,
AC power cord is connected or
automatically analyzes and charges
and monitoring. No special pads
charge batteries separately.
• Automated self-tests run
for a potential y lifesaving shock
required for infant/child AED mode.
hourly, daily, and weekly to help
within seconds, guiding the user
• Large multi-color display for
simplify defibril ator shift checks
with clear, concise voice and on-
easy viewing of up to 3 waves,
and free up clinician time.
screen prompts.
numeric values, and alarm limits in the parameter bar, as well as patient condition information.
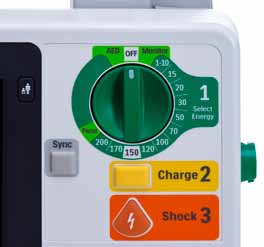
• Single therapy knob similar to
other Philips defibril ator/monitors to turn device on/off, as well as select mode and energy.
• Defibril ation as easy as 1-2-3.1
1. Select energy.
2. Press Charge button to charge
the defibrillator.
3. Press Shock button to deliver
• Integrated strip chart
recorder (printer) documents
ECG rhythm strips, clinical events,
event summary reports, vital
signs trending, operational checks,
configuration, status logs, 3-lead
and 5-lead ECG reports, and
other device information. 50 mm
standard paper used by all Philips
defibrillators.
Philips HeartStart XL+ Defibril ator/Monitor
Ready to save lives
Ready to reviveThe HeartStart XL+ is designed to be ready to help save the life of any patient. And the option of easily switching between AED mode and manual mode makes it possible for all levels of trained responders to use the HeartStart XL+.
• Philips SMART Biphasic therapy uses real-time
impedance compensation technology to adjust and deliver
HeartStart XL+ simplifies
personalized electric medicine for each patient on each shock. Philips biphasic therapy has been rigorously studied
and is supported by substantial peer-reviewed, published data. It has been clinical y proven to deliver high first
In AED mode, the HeartStart XL+ is the only
shock efficacy for long-downtime SCA patients, as well as
defibril ator/monitor that can defibril ate any
ef ectively defibril ate across the full spectrum of patients,
patient, of any weight without the need for
including those considered "difficult-to-treat."1-5
special accessories, which can help save valuable
• Quick Shock in AED mode and one of the fastest
time when responding to an emergency. Press
charge times to the standard adult dose in manual mode
the Patient Category button to Infant/Child
(3 seconds) help minimize interruptions to CPR and speed
to quickly and automatical y decrease the
shock delivery.
defibril ation energy.
• Customizable energy settings and defibril ation
protocols that are consistent with the HeartStart MRx
monitor/defibril ator in AED mode and HeartStart AEDs,
as well as flexibility should protocols change in the future.
• Patient monitoring measurements, including 3- and
5-lead ECG, heart rate, SpO , and non-invasive blood
pressure, provide continuity of care from the cardiac emergency to patient monitoring at the bedside in a single device. Measurements can be trended over time, as well as displayed and printed.
In addition to defibril ation, the HeartStart XL+ delivers
ef ective synchronized cardioversion and noninvasive pacing.
• Synchronized cardioversion – Peer-reviewed evidence
supports the ef ectiveness of Philips biphasic synchronized cardioversion capabilities, which are activated by the user
Patient category button that is close to the AED position
with the push of a button.1,6,7 At a glance, the user can see
on the control dial. The display will also show Infant/Child
that the Sync mode is active, as indicated by a Sync label on
< 8 years old.
the device display and a back lit Sync button.
• Noninvasive pacing – With rate and output controls
visible on the front panel, the HeartStart XL+ makes it easy to train and perform transcutaneous pacing.
Philips HeartStart XL+ Defibril ator/Monitor
Ready to save lives
training solutions
The HeartStart XL+ and Philips data management solutions are
Philips has created a variety of education
designed to help support a culture of continuous improvement
and training solutions using sound
and excel ence within your hospital.
instructional design principles to help ensure competent device operation, as well as assist with your training and implementation needs in a cost-effective
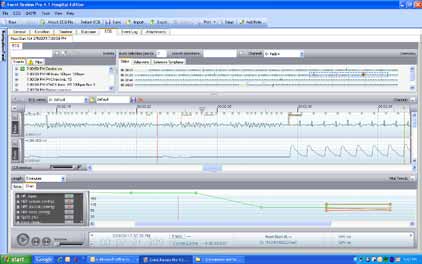
Event Summaries can be copied to a standard USB drive
for easy transfer to HeartStart Event Review Pro,
Philips data management program. HeartStart Event
Interactive web-based training
Review Pro captures and stores an entire code for
Use the free, self-paced, interactive,
post-event review by the Resuscitation Team or Rapid
web-based training program to learn
Response Team. Post-resuscitation reports can be easily
device features, simulate hands-on
shared with other clinicians as needed.
procedures, and test your understanding.
Instructor toolkit
An Instructor Guide, User Training
Workbook, and Skil s Checklist combine
to help you deliver live HeartStart XL+
education in an effective and efficient
manner. These tools also can help
facilitate refresher training.
Instructor-based training
Customized on-site, instructor-based
training delivered by Philips clinical
educators in a realistic critical care
context is also available.
Instructional video
View the video to get an overview of
HeartStart Event Review Pro provides a robust, insightful view of a resuscitation event,
important device features and related
along with built-in, easy-to-use navigation to pinpoint key areas in a specific patient's code
device functionality. Available on the
event for retrospective review.
web and in a DVD format.
Application notes
Application notes explain the theory
behind Philips therapeutic and monitoring
technologies, as well as provide support
for their clinical efficacy and intended
interpretation.
Philips HeartStart XL+ Defibril ator/Monitor

Standardize with Philips
Ease-of-use is the hal mark of the Philips family of defibril ators, including the HeartStart XL+, the
HeartStart defibril ation
HeartStart MRx, and HeartStart AEDs. Standardizing with Philips can make training and delivery of care more
efficient and give users confidence.
• All Philips defibril ators have similar user interfaces and
HeartStart multifunction defibril ation pads
AED prompts.
come in Adult/Child, Infant, and specialty
• The HeartStart XL+ and the HeartStart MRx use the
choices to fit the needs of a variety of
same ready-for-use indicator.
departments, clinicians, patients, and therapies.
• The HeartStart XL+ uses the same ECG algorithm and
alarms as those in Philips bedside patient monitors.
If external paddles are preferred, the
• The HeartStart XL+ in AED mode uses the same SMART
HeartStart XL+ can be equipped with a set of
Analysis algorithm as Philips industry-leading AEDs.
external paddles with unique Paddle Contact Indicators. The new external paddles have
Standardizing with Philips can help streamline patient
flashing shock buttons on each paddle that
hand-off by eliminating re-cabling, simplifying inventory
are the same as the shock button on the
management, and reducing inventory costs.
front panels of the HeartStart XL+ and the
• The HeartStart XL+ has the same ports as Philips
HeartStart MRx. For open-heart and other
patient monitors, so no time is wasted re-cabling
intrathoracic procedures, the HeartStart XL+
the patient.
can be used with Philips sterilizable switched
• The HeartStart XL+ supplies and accessories
or switchless internal defibril ation paddles.
are compatible with earlier generation Philips monitor/ defibril ators.
• The HeartStart XL+ patient monitoring cables
and sensors are the same ones used with Philips patient monitors.
Philips HeartStart XL+ Defibril ator/Monitor
HeartStart XL+ Defibril ator/Monitor specifications
9˝ high x 11.6˝ wide x 10.9˝ deep
Rechargeable, lithium ion; see battery label for
(23 cm x 29.6 cm x 27.9 cm)
capacity information
14.7 lbs (6.6 kg), includes one battery, one new
1˝ high x 4.5˝ wide x 5.7˝ long
rol of paper, one pads cable. Incremental weight
(23.6 mm high x 116 mm wide x 146 mm long)
of external standard paddles and paddle tray is
less than 3 lbs (1.3 kg)
Approximately 1.5 lbs (.68kg)
Standard operator Within one meter (3 feet) of the device
With the temperature between 0-35° C
(32-95° F), less than 3 hours to 100% capacity;
less than 2 hours to 80% capacity
Power supply
Approximately 3 years
Rechargeable lithium ion battery; AC power
using a protectively grounded outlet
With a new ful y charged battery, at 20 °C
(68 °F), one of the fol owing: At least 3 hours
of monitoring (ECG and SpO monitored
Biphasic Truncated Exponential.
continuously and NBP sampled every 15 minutes)
Waveform parameters adjusted as a function of
fol owed by 20 ful -energy charge/shocks; OR
patient impedance
at least two hours of pacing (180ppm at 140mA
with 40 msec pulse width) while monitoring
Via multifunction electrode pads or paddles
(ECG and SpO monitored continuously and
Configurable energy escalation in a series
NBP sampled every 15 minutes) fol owed by 20
ful -energy charge/shocks; OR at least 175 full
Leads off sensing Apply 500nA rms (571Hz); 200uA rms (32KHz)
energy charge/shocks
Battery gauge on battery, capacity indicator on
display, power indicators on front of device;
• 3 seconds to the recommended adult energy
flashing RFU indicator, chirp and Low Battery
level (150 Joules) with a new ful y-charged
messages on the display for low battery condition.
battery instal ed
When a low battery message first appears there
• Less than 5 seconds to the selected energy
is still enough energy for at least 10 minutes of
level (up to 200 Joules) with a new ful y
monitoring and 6 maximum energy discharges
charged battery instal ed
• Less than 15 seconds to the selected energy
Storing the battery for extended periods at
level while connected to AC power only
temperatures above 40° C (104° F) reduces
• The device powers on in manual defibril ation
battery capacity and degrades battery life
mode ready to deliver shock in less than 8
seconds plus applicable charge time, assuming
an immediate selection of an energy and
Approximately 6.5 in (16.5 cm) diagonal viewing
initiation of a charge, even at 90V AC and after
15 maximum energy discharges
• The device powers on in AED mode ready
to deliver shock in less than 17 seconds plus
640 x 480 pixels (VGA) with 32 brightness
applicable charge time
Minimum: 25 ohm (external defibril ation);
20 mm/s nominal (stationary trace; sweeping
impedance range 15 ohm (internal defibril ation); Maximum:
erase bar) for ECG and SpO2
250 ohm. Actual functional range may exceed
Wave viewing time 5.2 sec
0°C to 45°C (32°F to 113°F) operating;
Continuous ECG The Print button starts and stops the strip.
20°C to 70°C (-4°F to 158°F) storage
The printer can be configured to be run real
time or with a 10-second delay. The strip
Up to 95% relative humidity
prints the primary ECG lead and a second wave
Operating and storage - 1014 mbar to 572 mbar
with event annotations and measurements
(0 to 15,000 ft; 0 to 4,500 m)
The printer can be configured to automatical y
Half-sine waveform, duration ≤11 ms,
print on Mark Events, Charge, Shock and Alarm
acceleration ≥ 15.3 G, 3 shocks per face
The fol owing can be printed: Event Summary
Trapezoidal waveform, acceleration 30G,
(Long or Short), Vital Signs Trends, Operational
velocity change 7.42 m/s ±10% 1 shock per face
Check, Configuration, Status Log, Device
Meets Ingress Protection level IP21
ingress resistance
25 mm/s with an accuracy of ±5%
Complies with the requirements of standard
5% for offset voltages of ± 300 mV at 5Hz
EN 60601-1-2:2002
Meets UL 60601-1 (1st edition),
50 mm wide x 30 m long
EN 60601-2-4:2003, EN 60601-1:1990
Mode of operation ContinuousAC Line powered 100-240 VAC, 50 or 60 Hz, 1-0.46A, Class I
Battery powered Minimum 14.4 V, rechargeable lithium ion
Philips HeartStart XL+ Defibril ator/Monitor
Philips Healthcare is part of
Philips HeartStart Battery Recycling
1 Schneider T, Martens PR, Paschen H, et al. Multicenter,
Royal Philips Electronics
randomized, controlled trial of 150-J biphasic shocks
Help protect and preserve the environment
compared with 200- to 360-J monophasic shocks in the
resuscitation of out-of-hospital cardiac arrest victims.
for future generations – at no cost to you –
with the Philips HeartStart Battery Recycling 2 Santomauro M, Borrelli A, Ottaviano L, et al. Transthoracic
cardioversion in patients with atrial fibrillation:
Program. When a HeartStart XL+ battery
comparison of three different waveforms. Ital Heart J.
needs to be replaced, simply order a new
Suppl. 2004;5(1 Suppl):36-43.
3 White RD, Blackwell TH, Russell JK, et al. Body weight
one from Philips. When it arrives, place the
does not affect defibrillation, resuscitation or survival
+49 7031 463 2254
old battery in the same packaging and use
in patients with out-of-hospital sudden cardiac arrest
treated with a non-escalating biphasic waveform
the pre-paid shipping label to send it to a
defibrillator. Crit Care Med. 2004;32(9) Supplement:
Europe, Middle East, Africa
recycling center. No more storing piles of
4 White RD, Blackwell TH, Russell JK, et al. Transthoracic
+49 7031 463 2254
used batteries in your biomed or repair
impedance does not affect defibrillation, resuscitation
shop. And no risk of your old battery ending
or survival in patients with out-of-hospital cardiac
arrest treated with a non-escalating biphasic waveform
up in a landfil . Join Philips in making the
world a greener, cleaner place.
5 Hess EP, Russell JK, Liu PY, et al. A high peak current 150-J
fixed-energy defibrillation protocol treats recurrent
ventricular fibrillation (VF) as effectively as initial VF.
* This program is valid only in the U.S.A.
Resuscitation. 2008;79(1):28- 33.
6 Page RL, Kerber RE, Russell JK, et al. Biphasic versus
monophasic shock waveform for conversion of atrial
800 285 5585 (toll free, US only)
fibrillation. The results of an international randomized,
double-blind multicenter trial. J Am Coll Cardiol.
2002;39:1956-1963.
7 Glover BM, Walsh SJ, McCann CJ, et al. Biphasic energy
selection for transthoracic cardioversion of atrial
fibrillation. The BEST AF Trial. Heart. 2008;94:884–887.
Please visit www.philips.com/xlplus
2012 Koninklijke Philips Electronics N.V.
All rights are reserved.
Philips Healthcare reserves the right to make changes in specifications and/or to discontinue any product at any time without notice or
obligation and will not be liable for any consequences resulting from the use of this publication.
Printed in The Netherlands.
4522 962 74671 * JAN 2012
Source: http://www.agimed.com.ar/catalogo/cardiodesfibrilador.pdf
Customer Name (MetaCondNormal-Roman 26pt/2 Managing clinical evidence at the speed of change Business overviewBased in Boston, Massachusetts, Partners HealthCare is an integrated health systemfounded by Brigham and Women's Hospital and Massachusetts General Hospital in 1994.Partners HealthCare is one of the nation's leading biomedical research organizations and
Neurocomputing 70 (2007) 1977–1982 A model of the illusory contour formation based on dendritic computation Drazˇen Domijan�, Mia Sˇetic´, Domagoj Sˇvegar Department of Psychology, Faculty of Philosophy, University of Rijeka, I. Klobucˇaric´a 1, 51000 Rijeka, Croatia Available online 9 November 2006 We proposed a new model of illusory contour formation based on the properties of dendritic computation. The basic elements of the











