Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Model based drug development - what is it good for?
LIFE SCIENCE TECHNICAL BULLETIN
MODEL BASED DRUG DEVELOPMENT – WHAT IS IT
GOOD FOR?
AUTHOR: JANET R WADE, PHD, SENIOR CONSULTANT, SGS EXPRIMO
The FDA ‘‘critical path'' document characterizes model-based drug development (MBDD) as the development and application of pharmaco-statistical models of drug efficacy and safety from preclinical and clinical data to improve both drug development knowledge management and decision-making (1), (2). A less formal description of MBDD would be the development and use of mathematical models to aid answer existing and future project team questions that arise during drug development. As an analogy, many of us probably played with Lego bricks during our childhood. We could make many different models out of those bricks, but we made what we wanted to make at that point in time. In MBDD, the Lego bricks represent pieces of information and the models they are used to create (castle, house, farm, etc.), depends on what is needed (a place to defend yourself from an attack, somewhere warm to sleep, somewhere to raise cows and chickens, respectively).
More seriously, MBDD brings data from many sources together and describes it (the biomedical situation) as a whole, thus maximizing the information and understanding that can be gained from the data. All of this is done to provide answers to questions. Some examples of the different types of models that may be built are listed below.
POPULATION PK MODELING
pharmacology study done to assess the
Traditional statistical analysis of dose
Population pharmacokinetic (PK)
influence of renal impairment on the PK
response usually involves an analysis
models are frequently built during the
of a compound. When appropriate, a
of covariance (ANCOVA), followed by
development of new medicines. The
further study may look at the influence
multiple pair-wise comparisons. These
population PK model can bring together
of metabolic status (such as poor and
analyses are often unable to reconcile
information from the single ascending
extensive metabolizers of CYP2D6).
the not infrequent occurrence in a
dose (SAD) and multiple ascending dose
While no pharmaceutical company would
parallel group study when a lower dose
(MAD) studies to provide an integrated
attempt to study the combination of poor
of a new compound gives rise to a
description of the new medicines'
renal function in poor metabolizers of
better response than a higher dose. By
pharmacokinetics. For example, such
CYP2D6, these patients do infrequently
analyzing the data using a single model,
an approach has greater power to
exist and their prescriber needs to decide
the observed response disharmony
characterize any non-linear PK situations
what dose they should start treatment
between the different dose groups can
due to saturable absorption or elimination
with. In this situation, the population
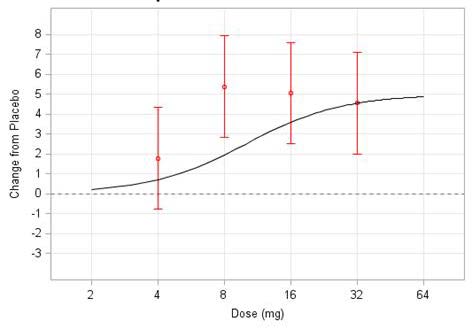
be resolved. A simulated example is
PK model could be used to propose a
presented in the figure below (Figure
reasonable starting dose and this model
1). The black line depicts the true dose
Population PK models built using later
predicted information could well be
response curve; the red points and
stage data often provide information
included in the drug label.
vertical lines are a set of simulated
about the influence of demographic
possible point estimates with their
or pathophysiological factors on the
DOSE RESPONSE CHARACTERISATION
associated 95% confidence intervals
pharmacokinetics of the compound,
under the variability described by the
in the target population. These
Building models that describe dose-
underlying model. If this was a real life
models can also be used to predict
response or dose-concentration-
example, it would not be surprising if
unstudied situations. An example of
response relationships is one of the
the project team decided that the 8 mg
this is a frequently performed clinical
more important MBDD activities.
clients (Roche), developed a comprehensive hepatitis C viral kinetic model that explainedcure (4). A representation of the disease part of the model is shown in Figure 2; the assorted different viral response profiles that the model can explain are shown in Figure 3.
Representation of the extended HCV viral kinetic model. Infectious HCV virions (VI)
infect target cells (T), creating productively infected hepatocytes (I). Uninfected hepatocytes (T) are produced at rate s and die at rate d. Infected hepatocytes die at rate δ. A density-dependentproliferationof hepatocytes (r) is assumed. Infectious (VI) and non-infectious (VNI) virions are
LIFE SCIENCE TECHNICAL BULLETIN
produced at rate p and cleared at rate c. Peginterferon α-2a dose-dep2endently inhibits the
production of new virions (ε), and ribavirin dose-dependently renders a fraction of newly produced virions non-infectious (ρ). SVR, defined as the status of having an undetectable viral load at 24 weeks after completion of treatment, is the primary clinical end point desired to be predicted in the treatment of hepatitis C. HCV, hepatitis C virus; SVR, sustained virologic response.
dose is the best dose to take forward
first child is dosed. The model and the
to Phase III. The model based analysis
simulated virtual trials are part of an
model, the observed response disharmony between the different dose groups can be resolved. A simul act
atedually indicates that the 8 mg response
example is presented in the figure below (Figure 1). The iterativ
black lin e process to design the best
depicts the true is higher than w
dose response curv ould be e
e; the red p xpected, purely
oints and vertical lines ar possible st
simulated possible point estimates with their associated 95% confidence intervals under
udy. This aids the goal to
the variability de due to the random v
scribed by the underlyin
g model. If t y that is
his was a real life exampl collect sufficient dat
a in the children
not be surprising if the project team decided that the 8 mg dose is the best dose to take forward to Phase present in all clinical st
III. The model based analysis udies.
actually indicates that the
8 mg response er the study question while
is higher than would be expected, purely due to the random variability that is present in all clinical studies.
ensuring that no more children than necessary are included. The models can also be used to ensure that a minimal, yet sufficient, number of blood samples are taken.
The viral kinetic model characterizes the complexity and diversity of clinically
observed HCV viral kinetics in patients with hepatitis C virus infection treated with peginterferon α-2a, alone or in cFigure 3:
ombination The viral kinetic model c
with ribavirin, and links thharacteriz
e kinetics es the
to clinical outcome. This is
achieved by the implementation of a viral-eradication cure boundary and incorporation of left-
MBDD IN VIRAL INFECTION
censored data, larcomple
gely exclxit
u y and div
analysi y of clinically obser
s in earlier studies, in ved
a simultaneous analysis of a
wide spectrum of HCV viral kinetics in patients with hepatitis C
peginterferon α-2a ±ribavirin treatment regimens in 2,100 patients. EVR, early
The models that can be built vary from
virologic response; HCV, hepatitis C virus; LLOQ, lower limit of quantification; RVR, rapid virologicresponse.
virus infection treated with peginterferon δ-2a,
reasonably straight forward (population
alone or in combination with ribavirin, and links
PK) to very complex. An example of a
the kinetics to clinical outcome. This is achieved
The true dose response is shown in black and the observed change from placebo
by the implementation of a viral-eradication cure
(with 95% CI) is shown in red.
complex model is one that describes
Figure 1: The true dose response is shown in
boundary and incorporation of left-censored data,
If the (virtual in this
blaccase) project tea
k and the obserm
ed c take the 8 mg dose forward to
hange from placebo (with
the underlying disease process and
largely excluded from analysis in earlier studies,
they could face a nasty disappointment later on. The MBDD approach of build
95% CI) is shown in red.
then integrates it with a PK-biomark
to describe dose response, performed alongside traditional statistical multiple testing
in a simultaneous analysis of a wide spectrum of
procedures where each dose level is compared to placebo, helps the project t clinical endpoint model. Suc
peginterferon δ-2a ±ribavirin treatment regimens
an informed choice about which dose(s) to study in Phase III. MBDD model building thus helps to maximize the probabilit
If the (virt y of trial success.
ual in this case) project
has enormous potential to support drug
in 2,100 patients. EVR, early virologic response; HCV, hepatitis C virus; LLOQ, lower limit of
MBDD in paediatrics
team did take the 8 mg dose forward
development decisions. SGS Exprimo's
Used in paediatrics, MBDD mitigates the practical and ethical constraints present when
to Phase III, they could face a nasty
quantification; RVR, rapid virologic response.
performing studies in children. A MBDD approach allows for less data to be c Managing Director Eric Snoec
fewer subjects which is helpful since the underlying variability across the age
disappointment later on. The MBDD
with other Exprimo colleagues and
to 18 years can be far more considerable than the variability observed in adults. Further,
The developed viral kinetic model
while reasonable assumpti
approacons can be made about scaling adult
h of building models to describe pharmone of our clients (R
oche), developed
provided a framework for mechanistic
parameters into children using well defined size and maturation factors, scaling biomarker or clinical endpoint responses is fra
dose response, perf ught with questions. Imp
ormed alongside lementina comprehensiv
e hepatitis C viral
exploration of treatment outcome and
approach allows the influence of the assumptions made to be tested by running virtual
traditional statistical multiple testing
clinical trials before the first child is dosed. The model and the simulated virtua kinetic model that e
xplained cure ( ). A
facilitated evaluation of alternative
part of an iterative process to design the best possible study. This aids the goal to collect
procedures where each dose level is
sufficient data in the children to answer the study question while ensuring tharepresent
ation of the disease part of the
chronic hepatitis C treatment options;
children than necessary are included. The models can also be used to ens
compared to placebo, helps the project
minimal, yet sufficient, number of blood samples are taken.
wn in Figure 2; the assorted
the ultimate aim was to develop and test
team make an informed choice about
clients (Roche), dif
dev ferent viral response profiles that the
eloped a comprehensive hepatitis C viral kinetic model th h
or personalizing treatments
MBDD in viral infectionThe models that can be
whic built vary from re
h dose(s) to st asonably straight forw
udy in Phase III.
assorted differe model can e
nt viral response xplain are sho
e disease part of
profiles that the wn in Figure 3.
the model is shown in F in the disease.
The disease part of the
very complex. An example of a complex model is one that describes the underlying
MBDD model building thus helps to
model can explain are shown in Figure
disease process and then integrates it with a PK-biomarker-clinic
. l endpoint model. Such
model is drug independent (Figure 2)
a model has enormous potential to support drug development decisions. SGS Exprimo's
maximize the probability of trial success.
Managing Director Eric Snoeck, together with other Exprimo colleagues and one of our
and can be applied to different or new compounds being developed to treat
MBDD IN PAEDIATRICS
Used in paediatrics, MBDD mitigates the practical and ethical constraints present when performing studies in children. A MBDD approach allows for less data to be collected in fewer subjects which is helpful since the
Representation of the extended HCV viral kinetic model. Infectious HCV virions (VI)
infect target cells (T), creating productively infected hepatocytes (I). Uninfected hepatocytes (T) are produced at r Figure 2: R
ate s and die epresent
at rate d. ation of the e
Infected hepato xtended HCV
underlying variability across the age
cytes die at rate δ. A density-dependent
proliferationof hepproduced at rate viral kinetic model. Inf
atocytes (r) is assumed.
p and cleared at rate cectious HCV virions (VI)
Infectious (VI) and non-infectious (VNI) virions are
range of 0 to 18 years can be far more
. Peginterferon α-2a dose-dependently inhibits the
production of new inf
ions ( arget cells (T), creating productiv
ε), and ribavirin dose-dependently ren ely
ders a fraction of newly produced
considerable than the variability obser
non-infectious (ρ). SVR, defined as the status of having an undetectable viral load at 24
weeks after comp inf
leti ected hepatocytes (I). Uninf
on of treatment, is the primary c ected hepatocytes
linical end point desired to be predicted in the
in adults. Further, while reasonable
treatment of hepa (T) are produced at rate s and die at rate d.
titis C. HCV, hepatitis C virus; SVR, sustained virologic response.
assumptions can be made about scaling
Infected hepatocytes die at rate δ. A density-dependent proliferationof hepatocytes (r) is
adult pharmacokinetic parameters into
assumed. Infectious (VI) and non-infectious (VNI)
children using well defined size and
virions are produced at rate p and cleared at rate
maturation factors, scaling biomarker or
c. Peginterferon δ-2a dose-dependently inhibits
clinical endpoint responses is fraught
the production of new virions (δ), and ribavirin
with questions. Implementing a MBDD
dose-dependently renders a fraction of newly produced virions non-infectious (δ). SVR, defined
approach allows the influence of the
as the status of having an undetectable viral load
assumptions made to be tested by
at 24 weeks after completion of treatment, is the
running virtual clinical trials before the
primary clinical end point desired to be predicted in the treatment of hepatitis C. HCV, hepatitis C virus; SVR, sustained virologic response.
The viral kinetic model characterizes the complexity and diversity of clinically
observed HCV viral kinetics in patients with hepatitis C virus infection treated with peginterferon α-2a, alone or in combination with ribavirin, and links the kinetics to clinical outcome. This is achieved by the implementation of a viral-eradication cure boundary and incorporation of left-censored data, largely excluded from analysis in earlier studies, in a simultaneous analysis of a wide spectrum of peginterferon α-2a ±ribavirin treatment regimens in 2,100 patients. EVR, early virologic response; HCV, hepatitis C virus; LLOQ, lower limit of quantification; RVR, rapid virologicresponse.
LIFE SCIENCE TECHNICAL BULLETIN
The MBDD activities performed by SGS
Exprimo efficiently aid in bringing the
1. Kola I & Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev.
right dose of new, safe, and effective
Drug Discov. 2004; 3:711–715.
medicines to the patients who need them. We at SGS Exprimo are extremely
2. US Department of Health and Human Services, Food and Drug Administration.
proud of our track record—we have
Innovation or stagnation? Challenge and opportunity on the critical path to new
performed over 300 MBDD projects,
medical products (2004). Accessed 12 Oct 2013.
all delivered on time and to the highest
3. Jonsson EN, Wade JR, Karlsson MO. 2000. Nonlinearity Detection: Advantages of
scientific standards. Global feedback
Nonlinear Mixed-Effects Modeling. AAPS Pharmsci; 2000; 2(3); article 32.
received from regulatory assessors who
4. Snoeck E, Chanu P, Lavielle M, Jacqmin P, Jonsson EN, Jorga K, Goggin T, Grippo J,
read the reports of our MBDD analyses
Jumbe NL, Frey N. A comprehensive hepatitis C viral kinetic model explaining cure.
has been consistently good and always
Clin. Pharmacol. Ther. 2010; 87(6): 706-713.
supported clients drug development decision at the right time.
In conclusion, the answer to the question ‘What is MBDD good for?' is ‘Better drug development decisions, made at the right time!'.
ABOUT SGS
SGS Life Science Services is a leading
ISO guidelines and directives and local
can also count on SGS's large database
contract service organization providing
regulatory bodies. For optimized early
of investigators and key opinion leaders
clinical research, analytical development,
phase clinical trials, SGS features sample
with therapeutic expertise in Infectious
biologics characterization, biosafety,
tracking for safety lab data interfaced
Disease & HIV/HCV, Vaccines, Oncology
and quality control testing. Delivering
with Oracle for PK samples, full
and Respiratory. Clients benefit from the
solutions for bio-pharmaceutical
eSource clinic automation (EDC), a GMP
favorable regulatory environment in the
companies, SGS provides Phase I-IV
pharmacy for on-site formulation, and a
two countries with very short phase I
clinical trial management services
Biosafety Level 2 quarantine facility.
trial approval.
encompassing clinical project
SGS has a wealth of expertise in: First-
SGS also offers GMP/GLP contract
management and monitoring, data
In-Human studies, QT/QTc prolongation,
laboratory services that include analytical
management, biostatistics, and
radio-labeled 14C ADME & PET scan
chemistry, microbiology, stability
regulatory consultancy. SGS's clinical unit
trials, viral challenge testing, biosimilars,
studies, bioanalysis, virology, and protein
located in Antwerp, Belgium has a total
and complex PK/PD studies. For a
of 92 beds, and has successfully met
qualitative and faster patient recruitment
the standards of the US FDA, GCP, ICH,
across Americas and Europe, clients
To receive future articles on current trends and regulatory updates, subscribe to SGS' Life Science Ne Read more about SGS'.
ved - SGS is a registered trademark of SGS Group Management S
+ 1 877 677 2667
SGS Group Management S
Source: http://www.sgs.com.tw/~/media/Global/Documents/Technical%20Documents/Technical%20Bulletins/SGS%20LSS%20Model%20Based%20Drug%20Development%20A4%20EN%2013.pdf
5255 E. Stop 11 Road, Suite 405Indianapolis, IN 46237 Page 1 of 5 A Division of Otolaryngology Associates Pre-Appointment Packet Balance Point Assessment Insurance Information Your doctor has recommended Balance Function Testing to help determine the cause of your symptoms of dizziness, motion sickness, and/or unsteadiness. The findings help guide your doctor in choosing the best form of treatment.
Your link to osteoarthritis research in Canada "State of the Art" Treatment of OsteoarthritisBy John M. Esdaile, MD, MPH, FRCPC, Co-Principal Investigator Research by scientists in the Tooling Up for Exercise by New Emerging Team scientists, is looking Early Osteoarthritis: Measuring What Matters




