Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Lawarencepress.com
Stability of Levothyroxine
Sodium Tablets Marketed
NLM Title
J Pharm Biomed Sci
Ishraqa Mohamed1, Siham Abdallah2*
1 National Drug Quality Control Laboratory,
Stability of medicinal products is the extent to which a product retains, within specified
limits throughout its period of storage and use (i.e. its shelf life), the same properties and
2 Qassium University, Faculty of Pharmacy,
characteristics that it possessed at the time of its manufacture.
Buraydah 52571, Saudi Arabia
The aim of this study is to evaluate real-time stability and photo-stability of levothy-
Address reprint requests to:
roxine sodium tablets marketing in Sudan. Levothyroxine sodium tablets from different
*Siham Abdallah, Qassium University,
manufacturers were kept at control room temperature (23–25°C); All the samples were
Faculty of Pharmacy, Buraydah 52571,
analysed every month using the British Pharmacopoeia (BP) HPLC method.
All tablets were kept in a closed glass dish and exposed to direct sunlight for 10 days
to evaluate photo-stability using BP and HPLC methods.
The results revealed that thyroxine tablets had become out of specification (88.0, 87.0
Article citation: Mohamed I, Abdallah S.
and 87.0%) after 15, 20 and 19 months, respectively, from the date of manufacturing
Stability of levothyroxine sodium tablets marketed in Sudan.
and lost more than 5% from initial concentration after 8–9 months; and lost about 40%
J Pharm Biomed
Sci 2016;06(05):328–332.
of its potency after exposure to sunlight.
The shelf life of levothyroxine sodium dosage form should be <2 years to ensure that
Available at www.jpbms.info
the dosage form was containing the correct dose when dispensed for use. It is evident
Statement of originality of work: The
from the analysis, sunlight has measurable effect on the stability of levothyroxine sodium
manuscript has been read and approved by all
even in solid dosage forms.
the authors. The requirements for authorship have been met, and that each author believes that the manuscript represents honest and original work.
Source of funding: None.
The World Health Organisation (WHO) has defined quality assurance as a Competing interest / Conflict of interest: The wide-ranging concept covering all matters that either individually or collec-
author(s) have no competing interests for financial
tively influence the quality of a product; the stability of product through-
support, publication of this research, patents, and royalties through this collaborative research.
out distribution channels until it reaches the user is one of the quality All authors were equally involved in discussed
research work. There is no financial conflict with the subject matter discussed in the manuscript.
Stability of medicinal products
Disclaimer: Any views expressed in this paper
are those of the authors and do not reflect the
United State Pharmacopeia (USP) has defined the stability of medicinal official policy or position of the Department of products as the extent to which a product retains within specified limits and
throughout its period of storage and use (i.e. its shelf life), the same proper-ties and characteristics that it possess at the time of manufacture
The stability of a product is related to its resistance to the various chem-
ical, physical and microbiological reactions that may change the original properties of the preparation during transport, storage and use. Other crite-ria of stability are the effects of such changes on the fitness of the product for use as a medicine. The stability is often expressed in quantitative term as shelf life that is the time during which the medicinal product is predicted to remain fit for its intended use under specified stora
Shelf-lifeShelf-life of medicinal products kept in its closed container under specified conditions is commonly defined from the date of manufacture or prepa-ration until the original potency or content of active constituent has been reduced by 10%. This time is known as the t10% or (t90%). Although it is
often convenient to express shelf-life solely in terms of the chemical stability of the active constituent, it is essential that the other desirable properties of the product are retained during storage.
Copyright 2016
Received Date: 04 April 2016 – Accepted Date: 10 May 2016 – Published Online: 20 May 2016
Stability of levothyroxine sodium tablets
could expire before a course of treatment is completed.
Factors affecting product stability
However, stock with shelf life of 18 months will soon
J Pharm Biomed Sci
Each ingredient, whether therapeutically active or phar-
be available; this formula requires refrigera.
maceutically necessary, can affect the stability of drug substance and dosage forms. Factors affecting stability of medicinal products include
ObjectivesLevothyroxine sodium has narrow therapeutic index.
Environmental factors
Therefore, it is particularly important that the available amount of the active drug should be consistent for a given
The primary environmental factors that can reduce sta-
strength in tablets; hence careful titration of dose is needed.
bility include exposure to adverse temperature, light,
The objectives of this study are to:
humidity, oxygen and carbon dioxide.
1. Evaluate real time stability of levothyroxine sodium
Dosage form factor
2. Study photo-stability of levothyroxine sodium
In dosage forms, reactions usually cause drug instabil-
ity and loss of active drug content, and they usually do
3. Assess the quality of levothyroxine sodium tablets
not provide obvious visual or olfactory evidence of their
from different manufacturer marketing in Sudan.
MATERIALS AND METHODS
Chemistry of levothyroxine sodium
Materials and equipments
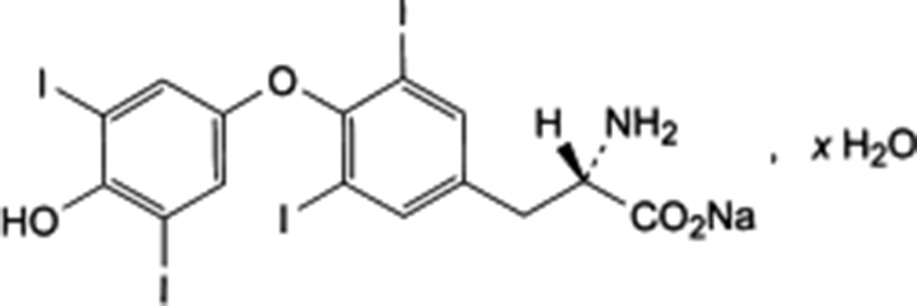
Levothyroxine sodium (formerly called thyroxine sodium in United Kingdom) is a sodium (2S)-2-amino-3-[4-(4-
Reference standard and chemicals
a. Reference standard
Optical rotation of levothyroxine sodium ranges
• Levothyroxine sodium reference standard; batch no.
between +16 and +20.
A010445003, potency was 99.99%, manufactured
Melting point for thyroxine sodium is 235–236°,
by Acros Organic/Belgium.
• Liothyronine standard; batch no. A010434001,
potency was 99.95%, manufactured by Acros Organic/Belgium.
Stability of levothyroxine sodium
Thyroxine is stable in dry air, but unstable in the pres-
• Acetonitril, HPLC grade manufactured by Scharlau;
ence of light, heat and humidity. In some cases over-
seas, thyroxin tablets were found unstable even at room
• Methanol analytical grade manufactured by
temperature, and storage temperatures of 8–15°C were
required to maintain potency. In the USA, the food and
• Phosphoric acid BDH Laboratory England
drug administration has recognised the stability and
• Sodium hydroxide manufactured by Barcelona; Spain
potency problems with oral thyroxine.
The preparation could potentially have adverse effects
on health. It is therefore very important that thyroxin tab-
lets should be kept in their original container and stored
• Eknuer high performance liquid chromatography
in a cool dry place.
(HPLC): instrument equipped with pump K1001,
The expiry date for Australian manufacture thyroxin
UV detector 2600 and injection loop 20 μl and
tablets is 1 year from the date of manufacture. There
connected to software programmed as recorder.
are 200 tablets in a bottle, so it is possible that tablets • Ultra sonic bath
1. Thyorin 100 µg (L-thyroxine sodium 100 µg, batch
no. Ty 02, date of manufacture: 9/05, date of expiry: 9/08. Manufactured by Pharmedic Laboratories Ltd, Lahore, Pakistan).
2. Eltroxin 50 µg (L-thyroxine sodium 50 µg, batch no.
Fig. 1 Structure of levothyroxine sodium: C H I NNaO , xH O
050897A, manufacture date: 3/05, expiry date: 3/07.
Manufactured by GlaxoSmithKline, New Cairo, Egypt).
J Pharm Biomed Sci Vol. 06 No. 05 328–332
3. Eltroxin 100 µg (L-thyroxine sodium 100 µg, batch
no. 051238A, manufacture date: 4/05, expiry date: 4/07. Manufactured by GlaxoSmithKline, New Cairo,
A calibration curve was constructed for levothyroxine
4. Eltroxin tablet 100 µg (L-thyroxine sodium 100 µg,
sodium standard solution against the corresponding
batch no. 071425A date of manufacture: 4/07, date
peak area. It gave linearity in the concentration range
of expiry: 4/09. Manufactured by GlaxoSmithKline,
(10–100 µg/ml) with a regression of 0.999 (
New Cairo, Egypt).
Results of analysis of pharmaceutical
Calibration curve
Assay and uniformity of content of levothyroxine
Concentrations of 10–100 μg/ml were obtained from sodium tablets
freshly prepared solution (A) by serial dilutions using methanol:water (1:1) as solvent. Quantitative analysis of
The three pharmaceutical preparations were assayed
these solutions was carried out using the HPLC method,
and their uniformity of content were tested using BP
described before. Each sample was injected two times HPLC method; the assay was done three times in dif-and their area under the peak corresponding to each ferent days and the relative standard deviation of all injection of each concentration was obtained. This pro-
the results were found <2%. The results obtained are
cedure was repeated three times.
Analysis of Levothyroxine sodium tablets
Photo-stability of levothyroxine sodium tablets
The assay and uniformity of content were carried out Levothyroxine sodium tablets kept in a closed glass dish using HPLC method, described in the BP for the levothy-
that were exposed to direct sunlight for 10 days showed
roxine sodium tablets
that there was a great effect of sun on the stability of levothyroxine even in solid dosage form; the results obtained by the HPLC method are giv
Study the stability of levothyroxine sodium tablets
These results confirm the recommendation men-
Two types of stability studies were conducted on levothy-
tioned in BP and USP for the storage of levothyroxine
roxine sodium tablets.
sodium tablets in a tight container protected from light. Thyroxin sodium is stable in dry air, but it may turn to
Photo-stability of levothyroxine sodium tablets: Photo-stability of levothyroxine sodium tablet was carried out using Table 1 HPLC results of levothyroxine sodium standard
Eltroxin tablet of 100 µg (batch no. 071425A man-
solution in methanolic sodium hydroxide.
ufacturing date 4/07, expiry date 4/09). The tablets were kept in a closed glass dish and exposed to direct
sunlight for 10 days. Then analysis was done as fol-
lows; 10 tablets were accurately weighed and crushed
(using mortar and pestle). A weight of powder equiv-
alent to about 100 µg of levothyroxine sodium was transferred quantitatively to 10 ml amber volumetric
flask, dissolved in a solvent containing methanol and
sodium hydroxide 0.1M (1:1) by the aid of ultrasonic and then shaked for 15 min. The volume was com-pleted using the same solvent, as a control solution, a solution was prepared in the same manner using ten tablets from the same batch stored at room tem-perature; then analysis was done against levothyroxine sodium standard solution.
Real-time stability study of levothyroxine sodium tablets:
Pharma ceutical preparations were kept at control room
temperature (23–25°C) and a real-time stability study
was done. The samples were analysed periodically using
Fig. 2 Calibration curve for levothyroxine sodium standard solution
the BP HPLC method.
in methanolic sodium hydroxide using HPLC.
J Pharm Biomed Sci Vol. 06 No. 05 328–332
Stability of levothyroxine sodium tablets
Table 2 Linearity data for the levothyroxine sodium
Table 5 Results of real-time stability study of
standard solution in methanolic sodium
levothyroxine sodium tablets market in Sudan.
hydroxide by the HPLC method.
Consistency of slope
(%w/w) ± SD (%w/w) ± SD (%w/w) ± SD
Correlation coefficient
Table 3 Assay and uniformity of content of
levothyroxine sodium tablets.
93.3 ± 1.2 93.7 ± 0.88 94.1 ± 0.92
Table 4 Results of sunlight effect on levothyroxine
sodium tablets.
% w/w Content at
% w/w Content after 10 days of
exposure to sunlight ± SD
pink on exposure to light. Exposure of thyroxin in diluted aqueous solution to light energy, gamma radiation, causes
deiodination and transformation into other iodinated or Following the extraction of thyroxin
from sodium (during stability indicating HPLC analysis), results indicated that 200 µg pink tablet from one man-
Fig. 3 Comparative graph of HPLC results of levothyroxine
ufacturer contained an excipient or excipients that accel-
sodium tablets.
erated degradation of thyroxin. This catalytic effect was suggested to require the presence of light
than 5% from initial concentration after 8 months from initial concentration; which indicated instability accord-
Real-time stability study of levothyroxine
ing to WHO guidelines, while EltroxinR (50 and 100 µg)
sodium tablets
became out of specification (87.0%) after 20 and 19 months, respectively, from the date of manufacture and
Pharmaceutical preparations were kept at control room
both lost more than 5% from initial concentration after
temperature (23–25°C) and a real-time stability study 9 months; which reveals that 24 months is not suitable was done. The samples were analysed periodically using
shelf life for levothyroxine sodium tablets in Sudan. These
the BP HPLC method. The study was not started at first
results confirm with the food and drug administration
month from the date of manufacture. The study was per-
(FDA) announcement; that orally administered drug
formed as follows:
products containing levothyroxine sodium are new drugs and there is new information showing significant stability
• For Thyorin 100 µg, studies were conducted on and potency problem with orally administered levothy-
samples after 7 months from date of manufacture.
roxine sodium products. These products fail to maintain
• For Eltroxin 50 µg, the studies were conducted on potency through the expiration date).
samples after 11 months from the date of manufacture.
Orally administered levothyroxine sodium products,
• For Eltroxin 100 µg, the studies were conducted on including the market leader, have reported recalls that
samples after 10 months from the date of manufacture.
are the result of potency or stability problems.
Since 1991, there have been no <10 firm-initiated
From the above study, it is observed that Tyrosine recalls of levothyroxine sodium tablets involving 150 lots
100 µg has become out of specification (88.0%) after and more than 100 million tablets. At one firm, potency 15 months from the date of manufacture and lost more problems with levothyroxine sodium tablets resulted in
J Pharm Biomed Sci Vol. 06 No. 05 328–332
destruction of products and repeated recalls from 1990 to
Sunlight had measurable effect on the stability of
1992. The firm destroyed 46 lots of levothyroxine sodium
levothyroxine sodium even in solid dosage forms.
tablets that failed to meet potency or content uniformity specifications during finished product testing.
Between 1987 and 1994, FDA received 58 adverse REFERENCES
drug experience reports associated with the potency of
1. World Health Organization. Quality assurance. Quality assurance
orally administered levothyroxine sodium products.
of pharmaceutical. 2007;2:16.
2. Rockville MD. United State Pharmacopeia, Convention; 2004.
3. Walter L. Pharmaceutical codex. London: Pharmaceutical Press;
1994. pp 278–282,284.
Unless the manufacturing process can be carefully and 4. Becket AH, Stenlake JB. Practical pharmaceutical chemistry; consistently controlled, orally administered levothyrox-
2004. pp (99–112,127,152–155).
ine sodium products may not be fully potent through the
5. British Pharmacopeia. The stationery office, levothyroxine sodium,
labeled expiration date, such variation in product potency
London: British Pharmacopoeia; 2005.
presented actual safety and effectiveness concerns.
6. Anthony CM, David OM, Widdop B, Galichet LY. Clarke's analysis
of drugs and poisons, 3rd ed., London: Pharmaceutical Press;
The shelf life of levothyroxine sodium dosage form
2004. p 480.
should be <2 years to ensure that the dosage form was
7. Roberts GW. Taking care of thyroxine. Aust Prescr. 2004;27:75–6.
containing the correct dose when dispensed for use.
8. Waston DG. Pharmaceutical analysis: a textbook for pharmacy
Levothyroxine sodium dosage form should not be
students and pharmaceutical chemists. Edinburgh: Churchill
supplied in large pack. As well, type of container should
Livingstone. 1999. pp 97–98,207–208,237–238.
be considered to ensure protection from light.
9. Federal Register. Vol. 62(157). 1997. pp 43535–43538.
J Pharm Biomed Sci Vol. 06 No. 05 328–332
Source: http://lawarencepress.com/ojs/index.php/JPBMS/article/download/233/pdf_110
September 19, 2014 Board of Directors* Vice President Joseph R. Biden, Jr. The White House Steven H. Schulman, President Akin Gump Strauss Hauer & Feld LLP 1600 Pennsylvania Avenue NW Washington, DC 20500 Harlene Katzman, Vice President Simpson Thacher & Bartlett LLP Dear Mr. Vice President: Latonia Haney Keith, Immediate Past
Yonsei Med J 53(4):842-848, 2012 pISSN: 0513-5796, eISSN: 1976-2437 Fluoxetine Protects against Big Endothelin-1 Induced Anti-Apoptosis by Rescuing Kv1.5 Channels in Human Pulmonary Arterial Smooth Muscle Cel s FeiFeng Dai, ZhiFu Mao, Jun Xia, ShaoPing Zhu, and ZhiYong Wu Department of Cardiothoracic Surgery, Renmin Hospital of Wuhan University, Wuhan, China.




