Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
E.smith
Cellular and molecular effects
of steroid hormones on CNS excitability
SHERYL S. SMITH, PHD, AND CATHERINE S. WOOLLEY, PHD
■
ABSTRACT
has been shown to have activating effects on mood
The steroid hormones 17β-estradiol (estradiol) and
(euphoria, anxiety, or antidepressant effects), cogni-tion, sensory response, motor behavior, and seizure
progesterone not only regulate the reproductive
activity.1,2 Progesterone produces effects that are
system but have other central nervous system
generally opposite to those produced by estradiol.1
effects that can directly affect a variety of behav-
Increases in circulating levels of progesterone have
iors. Generally, estradiol has been shown to have
been correlated with depressant effects, including
activating effects, including the ability to increase
anxiolytic3 and anticonvulsant4,5 effects. At higher
seizure activity, while progesterone has been
doses, this hormone is sedative and can act as a gen-
shown to have depressant effects, including anti-
eral anesthetic,6 an effect first demonstrated by
convulsant properties. Because levels of these hor-
Hans Selye in the 1940s.7
mones fluctuate across the menstrual cycle, it isimportant to understand how changes in these hor-
Hormones and epilepsy across the menstrual cycle
Despite the fact that these hormones have very well-
mone levels may influence levels of excitability in
characterized effects on nuclear receptors, many of
the brain, especially in women who have seizure
these nontraditional effects in the brain may be due
patterns that are related to their menstrual cycle, a
to nonclassic actions of the hormones on conven-
phenomenon known as catamenial epilepsy. This
tional neurotransmitters. Estradiol and progesterone,
paper reviews the effects of estradiol and proges-
or their metabolites, acutely potentiate responses to
terone on excitatory and inhibitory neurotransmit-
excitatory (estradiol) or inhibitory (progesterone
ters, respectively, and the possible cellular and mol-
metabolites) neurotransmitters in a rapid fashion
ecular mechanisms underlying the changes in brain
(seconds to minutes) and, after chronic exposure or
excitability mediated by these hormones.
withdrawal (days to weeks), produce structural,synaptic, or molecular effects by which both hor-
In addition to their well-known effects on repro- mone systems increase CNS excitability.
ductive actions mediated through classic nucle-
Across the menstrual cycle, estradiol is elevated
ar receptors, the ovarian hormones 17β-estradi-
in the second half of the follicular phase and
ol (estradiol) and progesterone can also exert
increases to a peak at midcycle, while progesterone
nonclassic effects on the central nervous system
is primarily elevated during the luteal phase and
(CNS) that alter a variety of behaviors. Estradiol
declines before menstruation begins. The contrast-ing effects of these hormones in activating ordepressing CNS function, respectively, may have
From the Department of Physiology and Pharmacology, SUNY
implications for behavior or perhaps even epilepsy
Downstate Medical Center, Brooklyn, N.Y. (S.S.S.), and the
across the cycle.
Department of Neurobiology and Physiology, Northwestern
Catamenial epilepsy is a change in seizure frequen-
University, Evanston, Ill. (C.S.W.).
cy or severity across the menstrual cycle;8 increases
Address: Sheryl S. Smith, PhD, Associate Professor, Depart-
in seizure severity have been reported at the mid-
ment of Physiology and Pharmacology, SUNY Downstate
cycle peak in ovarian hormones and also during the
Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203;e-mail:
[email protected].
late luteal phase, during the decline in ovarian hor-
mones—a period that may be a time of hormonewithdrawal.
■
THE BASIS OF ESTRADIOL'S EXCITATORY EFFECTS
One cellular mechanism contributing to the excita-tory effects of estradiol is its ability to rapidlyincrease responses of neurons to the excitatory neu-
rotransmitter glutamate
(Figure 1A).9–11 Gluta-
mate, in turn, can activate a number of receptorsubtypes, including those selective for AMPA andkainate, the typical glutamate receptors responsiblefor fast synaptic transmission at excitatory synapses
in the brain. These receptors are composed of four
subunits, and once bound, the transmitter gatesopen a channel that allows Na+ in to depolarize theneuron, thereby increasing its activity. TheNMDA-selective subtype of glutamate receptor,however, requires extensive depolarization beforechannel gating occurs, owing to a Mg2+ block that is
unblocked by depolarization. The NMDA receptoris permeable to both Na+ and Ca2+. The Ca2+ influxthat accompanies NMDA channel activation maycontribute to neuronal plasticity, as well as neuraldegeneration under excessive activation, as is some-times seen during seizure states.
This potentiating effect of estradiol on excitato-
ry synaptic transmission influences both the non-NMDA12–15 and the NMDA16 types of glutamate
10 nM 3α, 5α-THP + GABA
receptors, the former due to a G-protein–dependentmechanism involving protein kinase A activation.13
This rapid effect of estradiol may underlie the obser-vation that direct application of estradiol to the cor-
tex of an animal can produce de novo ictal dis-
FIGURE 1. Acute application of
(A) 17β-estradiol or
(B) a proges-
terone metabolite exerts opposite effects on neuronal responses to
In contrast to estradiol's rapid effect, more pro-
neurotransmitters.
(A) Local acute application of 17β-estradiol (E2)
longed exposure to estradiol results in structural and
increases cerebellar Purkinje cell responses to iontophoretically
functional changes at excitatory synapses that selec-
applied glutamate, an excitatory transmitter (bars above histogram).
tively enhance neurons' sensitivity to NMDA
Both individual responses (left) and averaged response (right) ofextracellular discharge from a representative neuron are presented.
receptor–mediated synaptic input. These effects
(B) In contrast, physiologic concentrations of the progesterone
have been mostly studied in the hippocampus,
metabolite 3α-OH-5α-pregnan-one (THP) increase GABA-gated cur-
which is often a site for the initiation and propaga-
rent recorded from acutely isolated CA1 hippocampal pyramidal
tion of limbic seizure activity. Excitatory synapses
neurons using whole cell patch-clamp recording techniques.
on neurons in the hippocampus are formed by pre-synaptic axonal varicosities, from which neuro-
spines
(Figure 2) and excitatory synapses on hip-
transmitter vesicles are released, and postsynaptic
pocampal neurons.18 Notably, increases in the num-
dendritic spines, which are small thornlike protru-
ber or density of spines and synapses occur not only
sions that densely cover the dendrites of neurons.
with estradiol treatment but also as hormone levels
Anatomic studies in animals such as rats have
fluctuate naturally across the reproductive cycle.18
shown that 3 days' exposure to elevated estradiol
Further anatomic analysis of axonal varicosities has
levels increases the number and density of dendritic
shown that estradiol increases the number of vari-
Because NMDA receptors have been shown to be
important in experimental models of epilepsy, thisfunctional effect of estradiol also could contributeto its proconvulsant effects. Consistent with thisprediction, estradiol has been shown to increasehippocampal seizure susceptibility in several animalmodels: direct measurement of electrographicseizure threshold in the hippocampus,23 hippocam-pal kindling,24 and chemically induced seizures thatdepend upon the hippocampus, such as kainate-induced behavioral seizures.2
■ THE BASIS OF PROGESTERONE'S
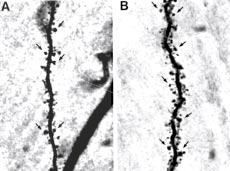
FIGURE 2. Estradiol increases dendritic spine density, as shown
Another ovarian hormone, progesterone, can exert
in these photomicrographs of representative dendrites on hippo-
numerous effects via a classic nuclear receptor, but
campal neurons from (A) a control female rat that was ovariecto-
can also exert effects via nonnuclear receptors after
mized to remove endogenous ovarian hormones and (B) an ova-
it is readily metabolized via two enzymatic conver-
riectomized female rat treated for 3 days with estradiol. Note that
sions in the brain to a neuroactive steroid, 3α-OH-
the density of dendritic spines, small thornlike protrusions that are
5α-pregnan-one (3α,5α-THP, or allopregnano-
sites of excitatory synaptic contact, is greater in the estradiol-treat-
lone). The primary effect of 3α,5α-THP and its iso-
ed rat. Some dendritic spines are indicated by arrows. Reprinted,
with permission, from reference 21. Copyright 1997 by the Society
α,5β-THP, is to modulate the GABAA recep-
for Neuroscience.
tor,25 which mediates most fast inhibition in the
brain (Figure 1B). Levels of this metabolite in the
cosities that make synaptic connections with multi-
circulation parallel those of progesterone; therefore,
ple dendritic spines, and that these multiple spines
it is increased during the luteal phase and during
arise from different postsynaptic neurons.19
Thus, anatomic studies show that estradiol not
The GABAA receptor is a pentameric structure
only increases the density of excitatory inputs to
composed of varying combinations of 5 subunits
individual neurons in the hippocampus but also pro-
from a pool of 17 genetically distinct subunit sub-
motes divergence of pre- to postsynaptic input. This
types: 6 α, 3 β, 3 γ, and 1 each of δ, ε, π, θ, and ρ.26
change could increase the synchronization of synap-
Each subunit, in turn, is composed of 4 membrane-
tically driven neuronal firing in the hippocampus,
spanning α-helices, with the second transmembrane
and therefore may be important in estradiol's pro-
segment surrounding a central chloride channel.
convulsant effects on limbic seizure activity.
Generally, this receptor contains 2 α, 2 β, and 1 γ
Because the synapses formed on dendritic spines
subunits, but other combinations exist. Different
are glutamatergic, the anatomic data described
subunit isoforms can produce receptors with varying
above predict that estradiol would increase neu-
biophysical and pharmacologic properties.
ronal sensitivity to glutamatergic synaptic input.
When two molecules of GABA bind to the
Indeed, electrophysiologic studies show this to be
GABAA receptor, the central chloride channel is
the case. Interestingly, estradiol selectively increas-
gated open, allowing Cl– influx into the neuron,
es neuronal sensitivity to synaptic input mediated
which hyperpolarizes most neurons of the adult CNS
by the NMDA type of glutamate receptor, while
and results in inhibition of neuronal activity. The
responses mediated by the AMPA receptor are not
GABAA receptor is also the target of most known
affected (Figure 3).20 This electrophysiologic result
depressant sedative drugs, such as benzodiazepines,
is corroborated by receptor-binding autoradiography
barbiturates, and anesthetics, which all bind to
studies showing that estradiol increases glutamate
unique sites on the receptor, as does the steroid
binding to NMDA, but not AMPA, receptors21 and
3α,5α-THP. At physiologic concentrations, this
histologic studies indicating that estradiol increases
steroid rapidly enhances the ability of GABA to
expression of the NMDA receptor subunit that is
allow Cl– into the cell25 by increasing the open time
common to all forms of the NMDA receptor.22
of the channel;27 as a result, this steroid is more
Charge transfer (
Charge transfer (
FIGURE 3. Estradiol increases neuronal sensitivity to glutamatergic synaptic input mediated by the NMDA type of glutamate receptor, with
no effect on the AMPA type. Shown are electrophysiologic recordings of AMPA (A) and NMDA (C) receptor–mediated excitatory postsynaptic
currents (EPSCs) in hippocampal neurons from control and estradiol-treated animals, and stimulus-response curves for AMPA (B) and
NMDA (D) receptor–mediated neuronal responses. Note that estradiol treatment increases neuronal sensitivity to NMDA receptor–mediated
input, with no effect on AMPA receptor–mediated responses. Hormone treatment was identical to that of Figure 1, and AMPA and NMDA
responses were recorded from the same cells. Adapted, with permission, from reference 20. Copyright 2001 by the Society for Neuroscience.
potent as an anxiolytic than the benzodiazepine class
GABAA receptor function. Such withdrawal effects
of tranquilizers, and in fact can act effectively as an
are seen with other sedative drugs, such as alcohol.
anxiolytic, anticonvulsant, and even anesthetic drug.
Using a 21-day-administration paradigm in rats,withdrawal from 3α,5α-THP resulted in a behav-
Progesterone withdrawal and CNS excitability
ioral excitability state characterized by increased
Across the menstrual cycle, circulating levels of this
anxiety and seizure activity triggered by GABAA
steroid are increased for 10 to 12 days before declin-
channel blockers.28–30 Across the entire time course
ing to low levels. It is therefore important to char-
of progesterone exposure, a more complex pattern
acterize not only acute effects but also chronic and
of anxiety behavior has emerged,31 with anxiety lev-
potential withdrawal effects of the steroid on
els increasing after 48 to 72 hours of exposure, then
f=1.7 ms; s=7.5 ms
f=0.76 ms; s=7 ms
PERMISSION NOT GRANTED FOR ONLINE REPRODUCTION
See figure 3 from reference 30.
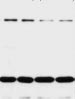
FIGURE 4. Progesterone withdrawal increases CNS excitability, an effect dependent upon GABAA receptor α4 subunit upregulation.
(A) Paired-pulse inhibition is a model of hippocampal circuit excitability, such that response to the second of two paired stimuli ("2") is
smaller than response to the first ("1"). This inhibition is reduced following progesterone withdrawal (ie, response "2" is larger). This effect
was prevented when increases in α4 subunit expression were suppressed by intraventricular administration of α4 antisense oligonu-
cleotide during withdrawal, but not altered in the missense control. Reprinted, with permission, from reference 34. (B) Chronic exposure to
the GABA-modulatory progesterone metabolite 3α,5β-THP for 48 hours increases expression of the GABAA receptor α4 subunit similar to
progesterone withdrawal (67-kDa band on the representative Western blot). (C) Suppression of α4 expression during 48-hour steroid treat-
ment ("Low α4") produced inhibitory synaptic current with a slower decay than normally observed after steroid treatment ("High α4"),
suggesting that increased α4 expression produces reduced inhibition. Reprinted, with permission, from reference 33. (D) Seizure activity
produced by the Cl– channel blocker picrotoxin is increased after progesterone withdrawal, an effect prevented when α4 expression is sup-
pressed by antisense treatment during the withdrawal period. These results suggest that chronic treatment and withdrawal from proges-
terone and its 3α,5β-THP metabolite result in increased excitability, both in vitro and in vivo, because of increased expression of GABAA
receptors containing the α4 subunit. Adapted, with permission, from reference 30. Copyright 1998 Nature Publishing Group.
decreasing by 5 to 7 days after exposure until with-
drawal, when anxiety again increases. This bimodal
effects of
pattern of anxiety response is, in fact, similar to the
effects of
pattern reported in catamenial epilepsy,8 with
exacerbation of seizures reported at midcycle andagain during the late luteal-phase decline in circu-lating levels of progestins.
A similar pattern of change is observed when the
cellular characteristics of hippocampal neurons are
17 β-estradiol (E )
analyzed. Both 2-day progesterone exposure and
progesterone withdrawal result in GABA-gated
current nearly insensitive to modulation by benzo-diazepines, owing to an increase in expression of
novel subtypes of GABA
A receptors containing the
α4 subunit,30,31 which are uniquely insensitive tomodulation by the benzodiazepine class of GABA
250 Serum levels (ng/mL)
The increase in α4 expression also leads to
decreases in inhibition gated by the GABAA recep-
tor, as suggested by several findings (Figure 4). First,
suppression of α4 expression using antisense tech-
Serum levels (ng/mL)
nology prevents the increase in seizure susceptibilityobserved following progesterone withdrawal.30 Under
conditions of suppressed α4 expression, measures ofreduced inhibition seen at the circuit and synaptic
level following progesterone withdrawal are also pre-vented.33,34 At the circuit level, inhibitory feedback
triggered by paired stimuli (paired-pulse inhibition)
is significantly attenuated following progesterone
Day of the menstrual cycle
withdrawal.34 At the synaptic level, unitary currentrecorded from the CA1 region of the hippocampus
FIGURE 5. Potential time course of altered excitability by ovarian
after 48-hour 3α,5α/β-THP exposure exhibits a
steroids across the menstrual cycle. Time course for fluctuations incirculating levels of progesterone (left axis) and 17β-estradiol (E
faster decay than control. If the total integrated cur-
right axis) during a typical 28-day menstrual cycle. The first two
rent is evaluated after hormone exposure, there is a
arrows indicate theoretical time points when acute and chronic
reduction in the total amount of Cl– transferred,
actions of E2 might exert excitatory effects on the CNS via gluta-
leading to a reduction in inhibitory tone.33 Because
mate receptors and increases in excitatory synapse formation,
suppression of α4 expression prevents these effects,
respectively, around the midluteal peak in levels of this hormone.
these findings suggest that substitution of novel α4-
In contrast, potentiation of GABA-mediated inhibition by 3α,5α-
THP during the progesterone-dominant luteal phase (third arrow)
A receptors for the ambient recep-
tor population after progesterone exposure/with-
would produce a potentially anticonvulsant effect until the
drawal leads to reduced inhibition in the brain, thus
decline in steroid levels ("withdrawal," shaded area), whendecreased inhibition may result as a function of lower 3α,5α-THP
permitting increased CNS excitability.
levels and the formation of quickly decaying α4-containing
SUMMARY AND CONCLUSIONS
A receptors. Dotted lines indicate time points for exacerba-
tion of seizure activity associated with catamenial epilepsy.
Across the menstrual cycle, it appears that both theacute and chronic effects of estradiol enhance exci-
receptors' subunit composition would reduce inhibi-
tatory input around the time of the midcycle peak.
tion, leading again to increased excitability (Figure
In contrast, the acute effects of the progesterone
5). Thus, these diverse effects of ovarian hormones
metabolite appear to enhance inhibitory responses
tend to exacerbate seizure activity at midcycle and
of limbic neurons during the luteal phase, until the
during the late luteal phase, a pattern common to
time of hormone decline, when altered GABAA
catamenial epilepsy.
J Neurosci 1992; 12:2549–2554.
Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic
Smith SS. Female sex steroids: from receptors to networks to per-
connectivity between single presynaptic inputs and multiple post-
formance—actions on the sensorimotor system. Prog Neurobiol
synaptic CA1 pyramidal cells: a serial electron microscopic study.
1994; 44:55–86.
Proc Natl Acad Sci U S A 2001; 98:3525–3530.
Woolley CS. Estradiol facilitates kainic acid-induced, but not
Rudick CN, Woolley CS. Estrogen regulates functional inhibi-
flurothyl-induced, behavioral seizure activity in adult female rats.
tion of hippocampal CA1 pyramidal cells in the adult female rat.
Epilepsia 2000; 41:510–515.
J Neurosci 2001; 21:6532–6543.
Bitran D, Shiekh M, McLeod M. Anxiolytic effect of proges-
Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA.
terone is mediated by the neurosteroid allopregnanolone at brain
Estradiol increases the sensitivity of hippocampal CA1 pyramidal
GABAA receptors. J Neuroendocrinol 1995; 7:171–177.
cells to NMDA receptor-mediated synaptic input: correlation
Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the
with dendritic spine density. J Neurosci 1997; 17:1848–1859.
progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur
Gazzaley AH, Weiland NG, McEwen BS, Morrison JH.
J Pharmacol 1989; 166:325–329.
Differential regulation of NMDAR1 mRNA and protein by estra-
Frye CA, Scalise TJ. Anti-seizure effects of progesterone and
diol in the rat hippocampus. J Neurosci 1996; 16:6830–6838.
3alpha,5alpha acid and perforant pathway models of epilepsy.
Terasawa E, Timiras PS. Electrical activity during the estrous
Psychoneuroendocrinology 2000; 25:407–420.
cycle of the rat: cyclic changes in limbic structures. Endocrinology
Harrison NL, Simmonds MA. Modulation of the GABA receptor
1968; 83:207–216.
complex by a steroid anaesthetic. Brain Res 1984; 323:287–292.
Buterbaugh GG, Hudson GM. Estradiol replacement to female
Selye H. Correlations between the chemical structure and pharma-
rats facilitates dorsal hippocampal but not ventral hippocampal
cological actions of the steroids. Endocrinology 1942; 30:437–453.
kindled seizure acquisition. Exp Neurol 1991; 111:55–64.
Herzog A, Klein P, Ransil B. Three patterns of catamenial epi-
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM.
lepsy. Epilepsia 1997; 38:1082–1088.
Steroid hormone metabolites are barbiturate-like modulators of
Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on
the GABA receptor. Science 1986; 232:1004–1007.
extrahypothalamic CNS. I. Estrogen augments neuronal respon-
Hevers W, Luddens H. The diversity of GABAA receptors.
siveness to iontophoretically applied glutamate in the cerebellum.
Pharmacological and electrophysiological properties of GABAA
Brain Res 1987; 422:40–51.
channel subtypes. Mol Neurobiol 1998;18:35–86.
Smith SS, Waterhouse BD, Woodward DJ. Locally applied
Twyman RE, Macdonald RL. Neurosteroid regulation of
estrogens potentiate glutamate-evoked excitation of cerebellar
GABAA receptor-single channel kinetic properties of mouse
Purkinje cells. Brain Res 1988; 475:272–282.
spinal cord neurons in culture. J Physiol 1992; 456:215–245.
Wong M, Moss RL. Patch-clamp analysis of direct steroidal mod-
Frye CA, Bayon LE. Cyclic withdrawal from endogenous and
ulation of glutamate receptor-channels. J Neuroendocrinol 1994;
exogenous progesterone increases kainic acid and perforant pathway
induced seizures. Pharmacol Biochem Behav 1999; 62:315–321.
Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents
Reddy D, Kim H, Rogawski M. Neurosteroid withdrawal model
via activation of the cAMP cascade. J Neurosci 1996; 16:3620–3629.
of perimenstrual catamenial epilepsy. Epilepsia 2001; 42:328–336.
Gu Q, Moss RL. Novel mechanism for non-genomic action of 17
Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen
beta-oestradiol on kainate-induced currents in isolated rat CA1
JMH, Li X. GABAA receptor α4 subunit suppression prevents
hippocampal neurones. J Physiol 1998; 506:745–754.
withdrawal properties of an endogenous steroid. Nature 1998;
Rudick CN, Woolley CS. Selective estrogen receptor modulators
regulate phasic activation of hippocampal CA1 pyramidal cells by
Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to
estrogen. Endocrinology 2003; 144:179–187.
a neuroactive steroid increases α4 GABAA receptor subunit levels
Smith SS. Estrogen produces long-term increases in excitatory
in association with increased anxiety. Brain Res 2000; 910:55–66.
neuronal responses to NMDA and quisqualate. Brain Res 1989;
Wisden W, Laurie DJ, Monyer H, Seeburg P. Cloning, pharma-
cological characteristics and expression pattern of the rat
Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW.
GABAA receptor α4 subunit. FEBS Lett 1991; 289:227–230.
17beta-Estradiol enhances NMDA receptor-mediated EPSPs and
Hsu F-C, Waldeck R, Faber DS, Smith SS. Neurosteroid effects
long-term potentiation. J Neurophysiol 1999; 81:925–929.
on GABAergic synaptic plasticity in hippocampus. J Neuro-
Marcus EM, Watson CW, Goldman PL. Effects of steroids on
physiol 2003; 89:1929–1940.
cerebral electrical activity. Arch Neurol 1966; 15:521–532.
Hsu F-C, Smith SS. Progesterone withdrawal reduces paired-
Woolley CS, McEwen BS. Estradiol mediates fluctuation in hip-
pulse inhibition in rat hippocampus: dependence on GABA-A
pocampal synapse density during the estrous cycle in the adult rat.
receptor alpha-4 upregulation. J Neurophysiol 2003; 89:186–198.
Source: http://www.bodybuilding.dk/files/attachments/4959-EPISmith.pdf
The Journal of Emergency Medicine, Vol. 45, No. 2, pp. e31–e34, 2013 Copyright Ó 2013 Elsevier Inc. Printed in the USA. All rights reserved 0736-4679/$ - see front matter DIGOXIN TOXICITY WITH NORMAL DIGOXIN AND SERUM POTASSIUM LEVELS: BEWARE OF MAGNESIUM, THE HIDDEN MALEFACTOR Mamatha Punjee Raja Rao, MBBSPrashanth Panduranga, MRCP,† Kadhim Sulaiman, FRCPI,† and
J. Med. Toxicol. (2011) 7:205–212DOI 10.1007/s13181-011-0162-6 2,4-Dinitrophenol (DNP): A Weight Loss Agentwith Significant Acute Toxicity and Risk of Death Johann Grundlingh & Paul I. Dargan &Marwa El-Zanfaly & David M. Wood Published online: 8 July 2011 # American College of Medical Toxicology 2011 Abstract 2,4-Dinitrophenol (DNP) is reported to cause Keywords Dinitrophenol . Weight loss . Toxicity. Fatality





