Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Powerpoint 프레젠테이션
Topical Immunomodulator
Department of Dermatology
Seoul National University College of Medicine




Topical Calcineurin Inhibitors
Tacrolimus
Protopic: 822 Da 0.1%, 0.03% oint
epi-chloro
double bond
double bond
Calcipotriol: 313
Tretinoin: 300 Da)
Clinical Efficacy of TCIs: already proven
tacrolimus(Protopic), pimecrolimus(Elidel)
-used effectively for AD since early 1990s -No need to worry about the side effects of long-term
use of topical corticosteroid
(esp. useful in sensitive skin areas) -Indications for dermatologic field widened
Tacrolimus (Protopic)
-extracted from Streptomyces tsukubaensis, a soil microbe found in Tsukuba, Japan
-hydrophobic macrolide lactone (MW 822.05 Da)
-10-100 times higher immunosuppressive than cyclosporine
-initially used for systemic immunosuppression of patients with allograft transplantation
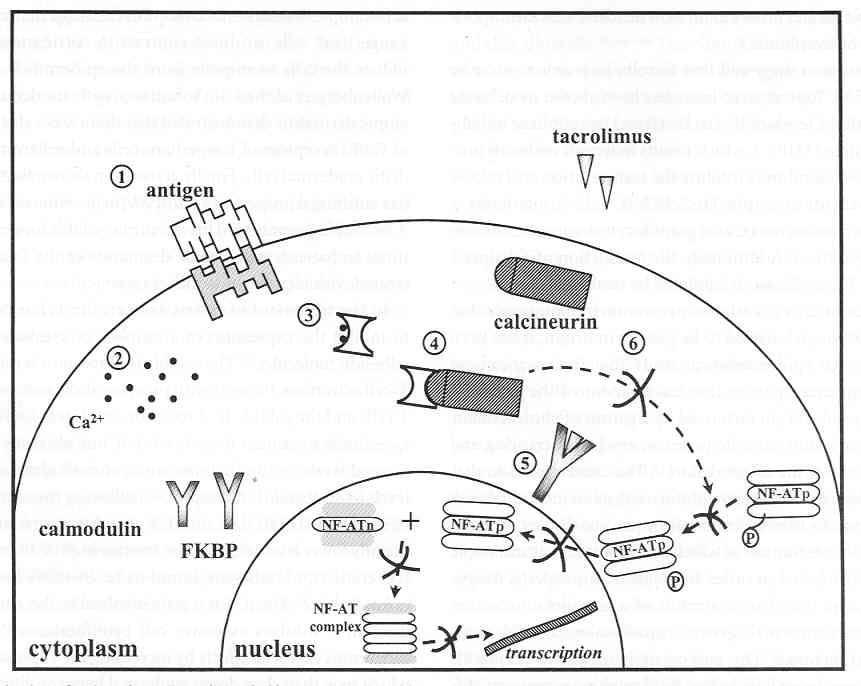
Mechanism of action
Inhibition of T cell activation: primary mechanism
inhibit calcineurin
Absorption properties of tacrolimus
MW of 822 Da (cf. CSA 1202 Da), absorbed topically
dermal penetration study on intact human skin:
1) relatively low percutaneous absorption
2) average rate of percutaneous penetration from 0.03%, 0.1% and 0.3% tacrolimus ointment: 3.1, 4.9, and 6.8 ng/cm2/hr, respectively
3) higher rates in damaged skin (40 ng/cm2)
4) absorption higher in facial lesions than in lesions on trunk and limbs
5) occlusion did not affect percutaneous penetration
• 3-wk course of twice daily application (0.03, 0.1, 0.3%) resulted in blood conc. below 0.25 ng/mL
(in renal TPL: 30-60 ng/mL)
• The mean time to attain the highest blood conc. is between 5 and 6 h after application in adults and
2.5 h in children.
• does not accumulate, either in skin or blood, following repeated application.
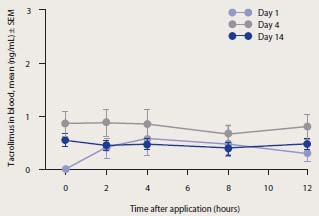
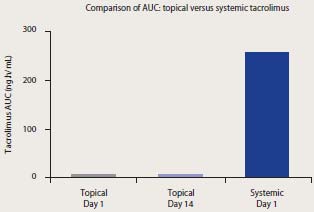
Pharmacokinetics – Patients with AD
Blood concentration-time profile of
Comparison of AUC values in pediatric
tacrolimus in adult patients using 0.1%
patients administered topical versus systemic
tacrolimus ointment with treated body
surface area up to 10,000 cm²
Cited from T. Ruzicka, and S. Reitamo. Tacrolimus ointment: a topical immunomodulator for AD (eds): Berlin:Springer, 2004.
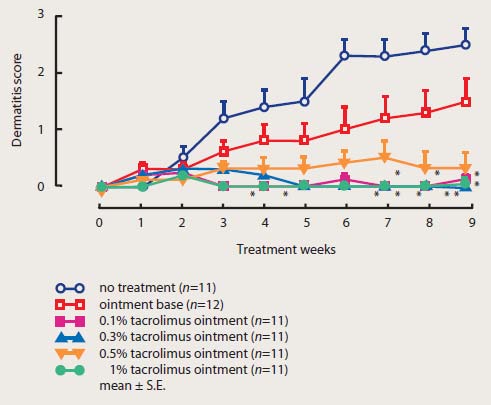
Effect of tacrolimus ointment on the development of spontaneous dermatitis in NC mice
Cited from T. Ruzicka, and S. Reitamo. Tacrolimus ointment: a topical immunomodulator for AD (eds): Berlin:Springer, 2004.
• not metabolized in human skin
• Systemically available tacrolimus: metabolized by hepatic isoenzyme CYP 3A4
• Since systemic levels of tacrolimus are so low following topical application, interactions with agents which inhibit CYP 3A4 (e.g. erythromycin, itraconazole and ketoconazole) are unlikely.
Topical use in AD (early reports): randomized, double-blind vehicle controlled studies
No of Pt Treatment
>90% improvement in 36.8%(0.1%)
27.5%(0.03%), 6.6%(vehicle)
>90% improvement in 40.7%(0.1%)
35.9%(0.03%), 6.9%(vehicle)
both group significantly improved
0.03, 0.1, 0.3%
decrease in score by 75%(0.3%)
for 3 wk 83.3%(0.1%), 66.7%(0.03%), 22.5%(vehi)
0.03, 0.1, 0.3%
marked improvement in 86%(0.3%)
for 3 wk 100%(0.1%), 0%(0.03%), 0%(vehi)
Clinical Experience in Adults
Vehicle-controlled 12-week studies: patients achieving at least 90% and at least 50% improvement according to the physician's global evaluation of response
Vehicle-controlled 12-week studies: decline in EASI score, change from baseline to end of Tx.
Cited from T. Ruzicka, and S. Reitamo. Tacrolimus ointment: a topical immunomodulator for AD (eds): Berlin:Springer, 2004.
Clinical Experience in Children
Vehicle-controlled multicentre study: physician's global evaluation of improvement at 12 weeks
Vehicle-controlled 12-week multicentre study: decline in EASI score, change from baseline to end of treatment
Cited from T. Ruzicka, and S. Reitamo. Tacrolimus ointment: a topical immunomodulator for AD (eds): Berlin:Springer, 2004.
1. the most profound difference in symptoms:
-achieved within the 1st or 2nd week of Tx
-esp. on facial lesions
2. adverse effects:
-common (30-50%)--transient skin burning and pruritus
-no abnormal changes in laboratory values
Tacrolimus (Protopic)
-0.03%, 0.1% ointment
-Efficacy and safety already reported in the literatures from Japan, Europe, and USA
-How about in other Asian countries?
So we analyzed the pooled data from 8 Asian countries.
Analysis of the pooled data of AD patients
from 8 Asian countries vs. Europe, Japan and US
(Physician's Global Evaluation of Clinical Response)
Malaysia
Thailand
Singapore
Indonesia
Philippines
Success was defined as a rating of better than moderate improvement (≥ 50% improvement)
(Int J Dermatol 2011 in press)
Physician's Global Evaluation
of Clinical Response
AsiaEurope [1] Reitamo et al. (2002) Japan [3] [4] FK506 Ointment
Study Group (1997)
[6] Hanifin et al. (2001)
Pa
f
t o 40%
Success: defined as a rating of better than moderate
improvement (≥ 50% improvement)
Physician's Global Evaluation
of Clinical Response
AsiaEurope [2] Reitamo et al. (2002) Japan [5] Otsuki et al. (2003)
[8] Paller et al. (2001)
Pa
f
t o 40%
Success: defined as a rating of better than moderate
improvement (≥ 50% improvement)
Common Adverse Events
Asia 0.1% or 0.03%
Europe 0.1% [1]
Europe 0.03% [1]
US 0.1% [7]
US 0.03% [7]
Asia 0.1% or 0.03%
Europe 0.1% [2]
Europe 0.03% [2]
US 0.1% [8]
US 0.03% [8]
*1:Including stinging, pain and soreness. *2:Including itch and worsening of pruritus.
[1] Reitamo et al. (2002)
[2] Reitamo et al. (2002)
[7] Soter et al. (2001)
[8] Paller et al. (2001)
Conclusion about the efficacy and safety Of tacrolimus ointment in Asian Study (Int J Dermatol 2011 in press)
effective and well tolerated treatment option in
patients with AD in Asia.
* The efficacy and safety of tacrolimus are similar to
those in the US, European and Japan studies.
New paradigm for use: Proactive treatment of AD with tacrolimus ointment 2 or 3 times application of tacrolimus oint to normal-appearing skin reduces time to relapse -Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 2008: 63: 742–750 -Three Times Weekly Tacrolimus Ointment Reduces Relapse in Stabilized Atopic Dermatitis: A New Paradigm for Use. Pediatrics 2008;122;e1210-e121
a. Flare prevention with tacrolimus ointment, continue with maintenance therapy (1–2 times/week) b. Treatment of flares with topical corticosteroids
Cited from T. Ruzicka, and S. Reitamo. Tacrolimus ointment: a topical immunomodulator for AD (eds): Berlin:Springer, 2004.
Pimecrolimus (Elidel)
derivative of ascomycin
(discovered in a screen for antifungal agents from
Streptomyces hygroscopicus var. ascomyceticus)
pharmacokinetic study
-1% pimecrolimus cream was applied to 15-59% of total body surface in 12 adult patients---majority of blood samples provided nonquantifiable conc.
-in 58 pediatric patient treated on up to 92% of total body surface area, blood conc were consistently low.
-no skin atrophy
-no evidence of systemic accumulation with repeated dosing
Pimecrolimus (Elidel)
Clinical Efficacy
-Study subjects: mild to moderate AD patients
In infants Elidel clears AD signs
Elidel, n=123
Vehicle, n=63
*** P< 0.001
1Investigators' Global Assessment (IGA) of 0 (clear) or 1 (almost clear)
In infants, Elidel clears AD signs
Baseline
12-month old female patient with moderate atopic eczema at baseline
Clinical photographs courtesy of Prof. R. Kaufmann, Frankfurt, Germany
In children, Elidel clears AD signs
Elidel, n=267
Vehicle, n=136
* P 0.05 ** P 0.01
*** P 0.001
1Investigators' Global Assessment (IGA) of 0 (clear) or 1 (almost clear)
In children, Elidel clears AD signs
4-year old male patient with moderate atopic eczema at baseline
Clinical photographs courtesy of Dr D. Pariser, Norfolk, VA, USA
In children, Elidel particularly effective in face and neck area
Vehicle face/neck
Progressive improvement in total body IGA rating in pediatric and adult patients
Lü bbe J et al. Poster presented at EADV 2003, Barcelona
Progressive improvement in facial IGA rating
in pediatric and adult patients
-common (10%)--transient skin burning and pruritus
-no abnormal changes in laboratory values
Naturalistic, open-label, multicenter study of
long-term management in patients ≥ 3 mo
of age with mild or moderate AD
using Elidel (pimecrolimus) cream 1%
3 Months - Main Analysis of Global
ELIDEL bid, on an "as needed" basis
(initiating treatment at first signs and symptoms of AD)
variable treatment period and study
duration: 3 months core phase followed by a
flexible duration of up to 9 months
Patients enrolled / Country
• Region Europe:
• Region LatAm:
• Region Asia Pacific:
LatAm total: 345
Treatment Success (IGA 1)
ITT (n = 2014), LOCF
Treatment Success (IGA 1)
AP Regional DATA
ITT (n = 324), LOCF
Conclusions of NOVIDEL study
• effective and sustained disease control with ELIDEL in AP regions as
well as in Western contries
especially effective;
for children on the face
• excellent safety profile and tolerability • high patient acceptance
Pimecrolimus vs tacrolimus 1. Direct comparison of efficacy--difficult
-pimecrolimus treated for mild to mod AD
-tacrolimus treated for mod to severe AD
2. Direct comparison of local adverse effects
pimecrolimus (10%) < tacrolimus (50%)
3. Pimecrolimus is more lipophilic
-higher affinity to the skin
-less permeation through the skin
4. Tacrolimus: ca 3-fold higher affinity to FKBP than
pimecrolimus and consequently a higher calcineurin-
inhibiting potency
Skin penetration and permeation:
pimecrolimus and tacrolimus are different
Penetration into skin1
Permeation through skin1
1Human skin in vitro
Experiences in SNUH
tacrolimus 0.1% ointment 치료 효과
tacrolimus 0.03% ointment 치료 효과
0.03% tacrolimus 치료 전
0.03% tacrolimus 치료 2주
1% Elidel cream 치료 효과
1% Elidel 치료 효과
Calcineurin Inhibitors
vs Corticosteroids
Cytokines Mediators
Systemic exposure
Protopic (tacrolimus)
Pharmacokinetics of 0.1% Tacrolimus Ointment After First and Repeated Application to Adults or children with Moderate to Severe AD
(J Invest Dermatol 2005;125:68 –71)
(J Invest Dermatol 2005;124:695 –699)
Comparative absorption of tacrolimus: 0.1% tacrolimus oint vs. adult or childhood transplanted patients administered tacrolimus systemically.
Mean maximum blood tacrolimus concentrations in adults and children receiving tacrolimus 0.1% oint, stratified by BSA affected.
Br J Dermatol 2007: 157:861–73
Elidel (pimecrolimus)
Minimal Increase in Blood Concentrations
with Rising % BSA Treated
BSA affected on Day 1 (%)
Difference in mean blood concentration for infants with
90% BSA and 10% BSA is 0.4 ng/ml
Lakhanpaul et al. World Kongr. Ped. Derm. 2001
Pharmacokinetics of TCIs in adult AD: A randomized, investigator-blind comparison: x2/d Tx for 13 d, pimecrolimus cream 1% and tacrolimus ointment 0.1%
(JAAD2005;53:602-9)
ELIDEL does not deplete skin Langerhans'
cells, in contrast to corticosteroids
Number of Ia+ dendritic cells in
murine epidermal sheets after
5 days of topical treatment
Controlsa
Clobetasol
Betamethasone Hydrocortisone
aTreatment with solvent alone
Meingassner JG et al. Br J Dermatol 2003;149:853–7
long-term safety of TCIs
1) Minimal systemic absorption
2) Thousands of patients in many clinical trials with a very impressive safety record
3) No effect on collagen synthesis: no skin atrophy
4) No increased risk of cutaneous infection in AD 5) No decrease in immunocompetence in the skin 6) No clinical evidence of increased risk of malignancy both in adults or children
FDA: updated labeling for Elidel Cream and Protopic Ointment in 2006
Long-term safety of topical calcineurin inhibitors has
not been established
Therefore:
Continuous long-term use of TCIs, including Elidel Cream, and Protopic
Ointment in any age group should be avoided, and application limited to
areas of involvement with AD.
Elidel Cream is not indicated for use in children less than 2 years of age.
Protopic Ointment is not indicated for use in children less than 2 years of
age. Only 0.03% Protopic Ointment is indicated for use in children 2-15
years of age.
Is there clinical evidence for an increased risk of malignancies?
Carcinogenicity Studies with Pimecrolimus and Tacrolimus
MRHD: maximally recommended human dose NOAEL: non-observed adverse event level LOAEL: lowest observed adverse event levels
(Dermatology 2005;211:174–87)
Reports of Malignancies
in Comparative Clinical Trials*
(Worldwide Data as of December 2005)
23 Comparative Clinical Trials
(N 7000)
Tacrolimus ointment
(N 500)
(N 4200)
(N 900)
(N 1400)
Squamous
Basal Cell
Male, 75 yr
Male, 69 yr
Giant Cell
Lymphoma
Male, 26 yr
*Study duration (range: 3 weeks to 7 months) was comparable for all treatment groups
Carroll CL, Fleischer AB Jr. Drugs Today. 2006;7:431-439.
Clinical data show no evidence for an increased risk of malignancies with Protopic or Elidel
insufficient evidence in the epidemiologic literature to infer whether TCIs do or do not cause malignancy. (Br J Dermatol. 2011 Apr 5. doi: 10.1111/j.1365-2133)
Conclusions about TCI in AD
1. Very effective Especially useful in sensitive skin area such as face
2. Excellent safety profile and tolerability 1) no concerns associated with long-term use of TCS 2) no evidence for an increased risk of malignancies 3. Proactive treatment suggested
Source: http://www.allergy.or.kr/journal/abst/2011s/030_ppt.pdf
Enzymatic Immunoassay for the quantitative determination of free Prostata Specific Antigen (PSA) in human serum The standards are calibrated against NIBSC (WHO) Prostate cancer is the most frequent type of cancer found in the man and the second cause of death due to the cancer in man, Until recently, digital rectal examination was the most accepted Zero Standard/Diluent:
The World Anti-Doping Code JU-JITSU FEDERATION Anti-Doping Rules Version 2.0 (Based upon the 2009 revised Code) December 2008 TABLE OF CONTENTS Fundamental Rationale for the Code and JJIF's Anti-Doping Rules . 3 Scope . 3 DEFINITION OF DOPING . 4 ANTI-DOPING RULE VIOLATIONS . 4











