Transport and bioavailability studies of astragaloside iv, an active ingredient in radix astragali
C
Basic & Clinical Pharmacology & Toxicology 2004,
95, 295–298.
Printed in Denmark . All rights reserved
Copyright C
ISSN 1742-7835
Transport and Bioavailability Studies of Astragaloside IV,
an Active Ingredient in Radix Astragali
Yongchuan Gu1, Guangji Wang1, Guoyu Pan1, J. Paul Fawcett2, Jiye A1 and Jianguo Sun1
1Center of Pharmacokinetics, China Pharmaceutical University, Nanjing, China and
2School of Pharmacy, University of Otago, Dunedin, New Zealand
(Received April 13, 2004; Accepted August 23, 2004)
Abstract: Astragaloside IV is an important constituent of
Radix Astragali, a herbal remedy widely used in traditionalChinese medicine.
Radix Astragali is administered orally but little is known about the transport properties and bioavail-ability of astragaloside IV. In this paper we report studies of the absorption of astragaloside IV in the perfused ratintestinal model, transport and uptake in Caco-2 cell monolayers and
in vivo bioavailability in rat after an oral dose. Inthe perfused rat intestinal model, absorption of astragaloside IV was low from an aqueous solution but was significantlyhigher from a solution of
Radix Astragali. Absorption was not affected by bile duct ligation. Transport through Caco-2cells gave a very low permeability value (mean
Papp of 6.7∫1.0¿10ª8 cm/sec.) and uptake was unaffected by P-glycoproteininhibitors. The absolute bioavailability of astragaloside IV in rat was 2.2%. The correlation between low permeability
invitro and poor bioavailability
in vivo indicates
in vitro absorption studies are useful in the evaluation of traditional Chinesemedicines.
Radix Astragali had been widely used as an effective herbal
China) and housed six to a plastic walled cage with unlimited access
remedy in traditional Chinese medicine for its antiinflam-
to food and water except for 12 hr before and during an experiment.
The protocols for animal experiments were reviewed and approved
matory, antimicrobial and immune stimulating activities
by the China Pharmaceutical University Animal Ethics Committee.
(Rios & Waterman 1997; Pharmacopoeia of P.R. China2000). Astragaloside IV (fig. 1), a cycloartane-type triter-
Cell culture materials. Dulbecco's modified Eagle's medium and
pene glycoside, is regarded as a characteristic and active
non-essential amino acids were obtained from GIBCO Invitrogen
constituent of
Radix Astragali and the content of astragalo-
Co. (CA, USA). Foetal calf serum, L-glutamine and penicillin/strep-
side IV is the main criterion of its quality assurance and
tomycin (10,000 U/ml, 10,000 mg/ml) were obtained from TBD Co.
(Tianjin, China). Caco-2 cells were obtained from ATCC (Rock-
control. Although astragaloside IV has relatively high lipo-
ville, MD, USA) and used between passages 40–65. All other cell
philicity (ClogP of 2.0), the high molecular weight (785) and
culture materials were obtained from Costar (Cambridge, MA,
low solubility predict it would be poorly absorbed and have
low bioavailability. We were interested to confirm this andestablish whether
in vitro methods of drug absorption
Absorption in the in situ perfused rat intestinal model. Experiments
would correctly predict bioavailability
in vivo. Accordingly,
were carried out as described previously (Ikumi
et al. 1997; Stewart
et al. 1997). Rats were divided into 4 groups (nΩ6), two of which
we examined transport properties of astragaloside IV in the
were subjected to bile duct ligation before an experiment. After an
in situ perfused rat intestinal model, uptake and transport
overnight fast, they were anaesthetized with urethane (1 g/kg by
in monolayers of the human colon carcinoma cell line Caco-
intraperitoneal injection) and affixed supine on a surface under suit-
2 and absolute bioavailability in rat.
able lighting to maintain body temperature. The small intestine wasthen exposed by a midline abdominal incision, and two polyethylenecannulae (outer diameter, 5 mm; inner diameter, 3 mm) were in-
Materials and Methods
serted through small slits at the proximal and distal ends (about 90cm apart) and secured by ligation with silk suture. The intestine
Compounds and animals. Astragaloside IV, cyclosporin A and vera-
was returned to the abdominal cavity to aid in maintaining its integ-
pamil were purchased from the National Institute for the Control
rity. Using a syringe, saline at 37 æ was slowly flushed through the
of Pharmaceutical and Biological Products (Beijing, China).
Radix
gut until the effluent solution was clear after which the remaining
Astragali Oral Solution (containing 0.5 mg/ml astragaloside IV) was
solution was carefully expelled by means of air pumped from the
obtained from the Jiangsu Provincial Hospital (Nanjing, China).
syringe. Then a solution (50 ml) containing astragaloside IV (50 mg/
All other reagents were analytical grade. Unless otherwise indicated,
ml) or
Radix Astragali Oral Solution (5 ml) in Krebs-Ringer solu-
astragaloside IV was dissolved in dimethyl sulfoxide (DMSO) and
tion was circulated at 2.0 ml/min using a peristaltic pump from a
diluted to the desired concentration with water (final concentration
reservoir maintained at 37 æ with bubbling O2/CO2 (95/5). At half
of DMSO⬍0.1%). Sprague-Dawley rats (male, 190–210 g) were pur-
hourly intervals up to 6 hr, aliquots of perfusion solution (1 ml)
chased from Sino-British Sippr/BK Lab Animal Ltd (Shanghai,
were removed from the reservoir and replaced with 5 ml Krebs-Ringer solution. Solutions were filtered (0.22 mm) and analysed forastragaloside IV. The concentrations were corrected for the change
Author for correspondence: J. Paul Fawcett, School of Pharmacy,
in volume of circulating fluid on the basis of the change in concen-
University of Otago, P.O. Box 913, Dunedin, New Zealand (fax
tration of phenolsulfophthalein in the perfusate (initial concen-
64 3 4797034, e-mail njguyc/hotmail.com).
tration 100 mM) as determined by UV spectroscopy. After an ex-
YONGCHUAN GU
ET AL.
Absorption parameters of astragaloside IV in the perfused rat intestinal model with and without bile duct ligation (data are mean∫S.D. for6 rats).
Astragaloside IV aqueous solution
Radix Astragali Oral Solution
Absorption rate* (mg/g/hr)
* Balanced ANOVA shows this is significantly lower from aqueous solution than from
Radix Astragali Oral Solution (PΩ0.02); no interaction
between solution and bile duct ligation.
periment, the part of the small intestine under study was removed,
1.5, 2, 3, 4, 6, 8 and 12 hr after intravenous injection and 0.25, 0.5,
dried at 40 æ and weighed. Absorption rate constant (ka), percent
0.75, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 hr after oral administration.
astragaloside IV absorbed in 6 hr (F) and absorption rate (mg/gintestine/hr) were calculated from the linear log concentration ver-
Assay of astragaloside IV. Astragaloside IV was analysed as pre-
sus time plots and corrected for differences in dry weight of the
viously described (Gu
et al. 2004). Plasma samples were subjected
to solid phase extraction before analysis. HPLC was carried outon a reversed phase C18 column with mass spectrometric detection
Uptake and transport in Caco-2 cell monolayers. Uptake of astra-
(selective ion monitoring at m/z 807.5).
galoside IV by cultured monolayers of Caco-2 cells was examinedas previously described (Pan
et al. 2002). Experiments were per-
Data analysis. All data are given as mean∫standard deviation
formed with confluent epithelial cell monolayers grown on 6-well
(S.D.). The effects of solution and ligation in the
in situ perfused
plates. The culture medium was renewed and 3 hr later cells were
rat intestinal model were analyzed using balanced ANOVA with
washed three times with 1 ml aliquots of HBSS at 37 æ and then
solution, ligation and their interaction included in the model. Dif-
preincubated in HBSS for 20 min. Uptake was initiated by adding
ferences were considered to be statistically significant when P⬍0.05.
2 ml HBSS containing astragaloside IV (50 mg/ml) alone or with P-
Pharmacokinetic parameters (maximum plasma concentration
glycoprotein inhibitors (cyclosporin A 5 mM, verapamil 200 mM) at
(Cmax) and the corresponding time (tmax), absorption rate constant
37 æ. After various times (up to 40 min.), solutions were removed
(ka), elimination half-life (t1/2), clearance (CL), volume of distri-
and the monolayers washed three times with 1 ml aliquots of ice-
bution (Vd), area under the curve (AUC0»¬) and absolute bioavail-
cold HBSS and scraped into 1 ml HBSS solution. Cells were rup-
ability) were obtained from concentration-time curves by non-linear
tured by three freeze-thaw cycles and, after centrifugation, astra-
least-squares regression using the program PKAnalystA for Win-
galoside IV was assayed in the supernatant and expressed per milli-
dows (MicroMath, USA).
gram of protein. Protein concentration was determined by the Coo-massie Brilliant Blue method.
Transport experiments were performed with confluent epithelial
cell monolayers grown on Collagen coated Transwells as previously
described (Bartrakova
et al. 1998). Before an experiment, conflu-
Absorption in the in situ perfused rat intestinal model.
ence was established by ensuring transepithelial electrical resistancewas ⬎180 W cm2. Culture medium was renewed and 3 hr later cells
The results of this study are shown in table 1. The absorp-
were washed three times with 1 ml aliquots of Hank's balanced salt
tion rate of astragaloside IV from aqueous solutions was
solution (HBSS) at 37 æ. Transport was initiated by adding 1.5 ml
significantly lower than from
Radix Astragali Oral Solution
HBSS containing different concentrations of astragaloside IV (10,
(PΩ0.02). Absorption rate was also lower in ligated rats
20, 30 mg/ml) to the apical chamber of the Transwell and 2.6 mldrug-free HBSS to the basolateral chamber. Aliquots (7¿100 ml)
than in non-ligated rats but the effect was not significant
were removed from the basolateral chamber between 15 and 120
(PΩ0.053). There was no interaction between solution and
min. and replaced with drug-free HBSS. Solutions were centrifuged
bile duct ligation.
and analysed for astragaloside IV. Apparent permeability coef-ficients (
Papp, cm/s) were calculated using the equation
PappΩ(dQ/
Uptake and transport in Caco-2 cell monolayers.
dt)C0/A where C0 is the initial concentration on the apical side andA is the surface area of the Transwell (Hilgers
et al. 1990).
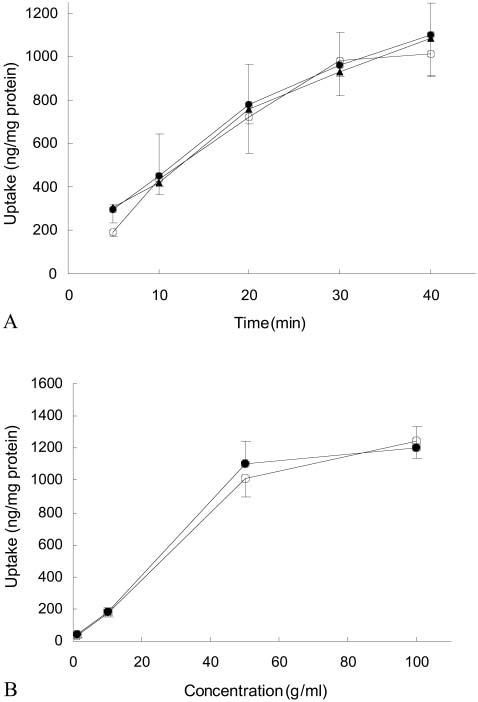
Uptake of astragaloside IV was time-dependent and ap-peared to be approaching steady state at 40 min. (fig. 2A).
Pharmacokinetics in rat. Groups of rats (nΩ6) were administered
Using astragaloside IV uptake in 40 min., increasing initial
either an intravenous (2.5 mg/kg via the tail vein) or oral dose (20.0
concentration of astragaloside IV (1–100 mg/ml) was ac-
mg/kg) of astragaloside IV (1 mg/ml) dissolved in ethanol and di-luted with normal saline. Blood samples were obtained for analysis
companied by an increase in uptake approaching saturation
of astragaloside IV from the femoral vein at 0.03, 0.17, 0.3, 0.5, 1,
at 50 mg/ml (fig. 2B). The effect of P-glycoprotein inhibitors
Pharmacokinetic parameters of astragaloside IV after single intravenous (2.5 mg/kg) and oral (20.0 mg/kg) doses of astragaloside IV in rat.
AUC0»¬ (mg ¡ hr/ml)
ABSORPTION OF ASTRAGALOSIDE IV
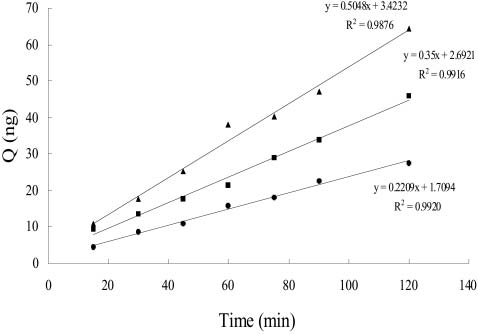
(cyclosporin A and verapamil) on the uptake of astragalo-side IV was not significant. Apical to basolateral transportof astragaloside IV was linear between 15 and 120 min. (fig.
3) and Papp values at concentrations of 10, 20 and 30 mg/mlwere 7.82, 6.19 and 5.95¿10ª8 cm/sec. respectively.
Pharmacokinetics in rat.
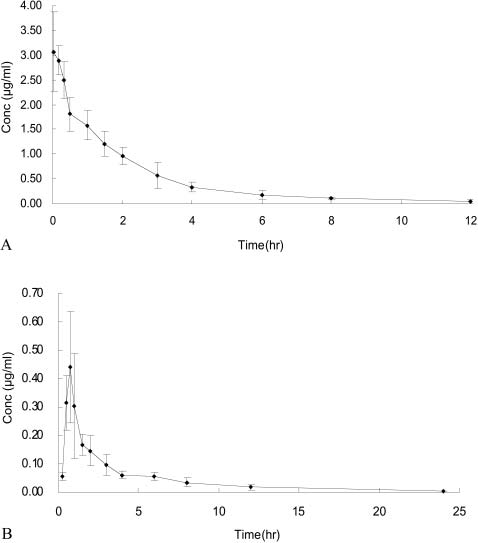
The concentration-time profiles for plasma astragaloside IVfollowing administration of 2.5 mg/kg intravenous and 20.0mg/kg oral doses are shown in fig. 4. Pharmacokinetic par-ameters are given in table 2. The disposition of astragalo-side IV was adequately described by a three-compartmentmodel for the intravenous dose and a two-compartmentmodel for the oral dose. The absolute bioavailability of as-tragaloside IV in rat was found to be 2.2%.
The absorption of astragaloside IV in the in vitro models oftransport studied here was found to be low. This is probablydue to its large molecular weight and poor solubility bothin water (⬍1 mg/ml) and in lipid. The trend of decreasingPapp for transport across Caco-2 cell monolayers with con-centration suggests astragaloside IV may inhibit its owntransport and that the transport is predominantly via theparacellular route (Lee & Thakker 1999). Uptake by Caco-2cells in the presence of cyclosporin A or verapamil indicatesastragaloside IV is not a substrate for P-glycoprotein andthat its poor absorption is not due to the action of thisefflux protein.
Fig. 2 A. Effect of time on uptake of astragaloside IV (initial con-
The low bioavailability of astragaloside IV in rat is con-
centration 50 mg/ml) by Caco-2 cells alone (S) and in the presence
sistent with the extremely low permeability constant for
of 5 mM cyclosporin A (O) or 200 mM verapamil (g). B. Effect ofinitial concentration of astragaloside IV on its uptake over 40 min.
alone (S) and in the presence of 5 mM cyclosporin A (O) (Data aremean∫S.D. for 3 determinations).
transport through Caco-2 cells (Artursson & Karlsson1991). Although there is no simple relationship between
Fig. 3. Transport of astragaloside IV through Caco-2 cell mono-layers at three concentrations of 30 mg/ml (g), 20 mg/ml (H) and 10
Fig. 1. Chemical structure of astragaloside IV.
mg/ml (O) (Data are means of 3 determinations).
YONGCHUAN GU ET AL.
The authors wish to thank Miss Jue Song, Ms Haitang
Xie and Ms Zhengzhong Cao. We are also grateful to Jiang-su Pharmaceutical Research Institute for financial assist-ance.
Akao, T., K. Kawabata, E. Yanagisawa, K. Ishihara, Y. Mizuhara,
Y. Wakui, Y. Sakashita & K. Kobashi: Balicalin, the predominant
flavone glucuronide of Scutellariae Radix, is absorbed from the
rat gastrointestinal tract as the aglycone and restored to its orig-
inal form. J. Pharm. Pharmacol. 2000, 52, 1563–1568.
Artursson, P. & J. Karlsson: Correlation between oral drug absorp-
tion in humans and apparent drug permeability coefficients in
human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res.
Commun. 1991, 175, 880–885.
Bartrakova, E. V., H. Y. Han, V. Y. Alakhov, D. W. Miller & A. V.
Kobanov: Effects of pluronic block copolymers on drug absorp-
tion in Caco-2 cell monolayers. Pharm. Res. 1998, 15, 850–855
Conradi, R. A., K. F. Wilkinson, B. D. Rush, A. R. Hilgers, M. J.
Ruwart & P. S. Burton: In vitro/in vivo models for peptide oral
absorption: Comparison of Caco-2 cell permeability with rat in-
testinal absorption of renin inhibitory peptides. Pharm. Res.
1993, 10, 1790–1792.
Fig. 4. Mean plasma concentration-time curves in rat after single
Gu, Y.C., G. J. Wang & J. P. Fawcett: Determination of Astragalo-
doses of astragaloside IV. A. Intravenous dose of 2.5 mg/kg, B. oral
side IV in rat plasma by liquid chromatography-electrospray
dose of 20.0 mg/kg. Data are mean∫S.D. for 6 rats.
ionization mass spectrometry. J. Chromatogr. B 2004, 801, 285–
288.
Hilgers, A. R., R. A. Conradi & P. S. Burton: Caco-2 cell mono-
layers as a model for drug transport across the intestinal mucosa.
gastrointestinal absorption in vivo and Papp in Caco-2 cell
Pharm. Res. 1990, 7, 902–910.
monolayers (Ren & Lien 2000), our results support the view
Ikumi, T., A. Saheki, R. Saitoh, Y. Sai, I. Yamada & A. Tsuji:
that compounds with P
Nonlinear intestinal absorption of 5-hydroxytryptamine receptor
1¿10ª7 exhibit very poor oral
absorption. The k
antagonist caused by absorptive and secretory transporters. J.
a values in the rat perfused intestinal
Pharmacol. Exp. Therap. 1997, 283, 108–115.
model, Caco-2 cell model and in vivo are in the order in
Kim, D. C., P. S. Burton & R. T. Borchardt: Correlation between
vivo⬎intestinal⬎Caco2. The most likely explanation for the
the permeability characteristics of a series of peptides using an
difference between the perfused intestinal and Caco-2 cell
in vitro cell culture model (Caco-2) and those using an in situ
models probably relates to differences in permeability by
perfused rat ileum model of intestinal mucosa. Pharm. Res. 1993,
the paracellular route and to differences in the absorptive
Lee, K. & D. R. Thakker: Saturable transport of H
surface areas (Conradi
et al. 1993; Kim et al. 1993; Len-
ranitidine and famotidine across Caco-2 cell monolayers. J.
nernäs et al. 1996).
Pharm. Sci. 1999, 88, 680–687.
The absorption of astragaloside IV from Radix Astragali
Lennernäs, H., K. Palm, U. Fagerholm & P. Artursson: Compari-
Oral Solution was found to be greater than from a simple
son between active and passive drug transport in human intesti-nal epithelial (Caco-2) cells in vitro and human jejunum in vivo.
astragaloside IV aqueous solution in the in situ perfused rat
Int. J. Pharm. 1996, 127, 103–107.
intestinal model. This is presumably due to a synergistic
Pan, G. Y., G. J. Wang, X. D. Liu, J. P. Fawcett & Y. Y. Xie: The
effect with other constituents of this herbal remedy. While
involvement of P-glycoprotein in berberine absorption. Pharma-
this may partially explain the efficacy of oral Radix Astra-
cology & Toxicology 2002, 91, 193–197.
Pharmacopoeia of P. R. China (English Edition 2000) Volume I.
gali in man, the fact that the aglycone form of astragaloside
The State Pharmacopoeial Commission, Chemical Industry
IV was found in rat bile after both intravenous and oral
Press, Beijing, China. 2000, pp. 161–162.
administration (data not shown) suggests it may also result
Ren, S. & E. J. Lien: Caco-2 cell permeability vs human gastrointes-
from absorption of the aglycone and subsequent restoration
tinal absorption: QSPR analysis. Prog. Drug Res. 2000, 54, 1–23.
to an active form. Absorption of baicalin, a glucuronide
Rios, J. L. & P. G. Waterman: A review of the pharmacology and
toxicology of Astragalus. Phytother. Res. 1997, 11, 411–418.
contained in Scutellaria baicalensis, has been shown to re-
Stewart, B. H., O. H. Chan, N. Jezyk & D. Fleisher: Discrimination
sult from absorption of the aglycone and subsequent refor-
between drug candidates using models for evaluation of intestinal
mation of the active glucuronide (Akao et al. 2000).
absorption. Adv. Drug Deliv. Rev. 1997, 23, 27–45.
Source: http://terraternal.es/Files/Ag4Bioavailability.pdf
Summer 201 Blastocystis Friend of Foe? Blastocystis Research Foundation By Dr Paul Froomes What is Blastocystosis? or animals. You can become and being exposed to the infected after accidentally parasite as described in the sis-TOS-is) is an illness caused by swallowing the parasite; you Essendon
Pharmacological upregulation of h-channels reduces the excitabilityof pyramidal neuron dendrites Nicholas P. Poolos1–3, Michele Migliore4,5 and Daniel Johnston1 1 Division of Neuroscience and 2Department of Neurology, Baylor College of Medicine, One Baylor Plaza, Houston, Texas 77030, USA 3 Current address: Department of Neurology and Regional Epilepsy Center, University of Washington, 325 9th Avenue, Seattle, Washington 98104, USA4 Yale University School of Medicine, Section of Neurobiology, New Haven, Connecticut 06520, USA5 Permanent address: National Research Council, Institute of Advanced Diagnostic Methodologies, Palermo 90146, Italy







