Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Untitled
ARD Online First, published on June 8, 2013 as 10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
Treating spondyloarthritis, including ankylosingspondylitis and psoriatic arthritis, to target:recommendations of an international task force
Josef S Smolen,1,2 Jürgen Braun,3 Maxime Dougados,4 Paul Emery,5Oliver FitzGerald,6 Philip Helliwell,5 Arthur Kavanaugh,7 Tore K Kvien,8Robert Landewé,9,10 Thomas Luger,11 Philip Mease,12 Ignazio Olivieri,13John Reveille,14 Christopher Ritchlin,15 Martin Rudwaleit,16 Monika Schoels,2Joachim Sieper,17 Martinus de Wit,18 Xenofon Baraliakos,3 Neil Betteridge,18Ruben Burgos-Vargas,19 Eduardo Collantes-Estevez,20 Atul Deodhar,21Dirk Elewaut,22 Laure Gossec,23 Merryn Jongkees,18 Mara Maccarone,18Kurt Redlich,1 Filip van den Bosch,22 James Cheng-Chung Wei,24 Kevin Winthrop,25Désirée van der Heijde26
Handling editor Francis
being aware that the evidence base is not strong and
Background Therapeutic targets have been defined for
needs to be expanded by future research. These
For numbered affiliations see
diseases like diabetes, hypertension or rheumatoid
recommendations can inform the various stakeholders
end of article.
arthritis and adhering to them has improved outcomes.
about expert opinion that aims for reaching optimal
Such targets are just emerging for spondyloarthritis
outcomes of SpA.
Correspondence toProfessor Josef S Smolen,
Division of Rheumatology,
Objective To define the treatment target for SpA
Department of Medicine 3,
including ankylosing spondylitis and psoriatic arthritis
The approaches to the diagnosis, therapy and
Medical University of Vienna,
(PsA) and develop recommendations for achieving the
follow-up of patients with ankylosing spondylitis
Waehringer Guertel 18-20,
target, including a treat-to-target management strategy.
Vienna A-1090, Austria; josef.
(AS) and psoriatic arthritis (PsA) have undergone a
Methods Based on results of a systematic literature
number of paradigmatical changes over the last
review and expert opinion, a task force of expert
decade. Especially considerations of the disease
physicians and patients developed recommendations
spectrum of spondyloarthritis (SpA) have recently
Accepted 15 May 2013
which were broadly discussed and voted upon in a
undergone remarkable changes. In addition to AS,
Delphi-like process. Level of evidence, grade and
strength of the recommendations were derived by
changes in the sacroiliac joints, non-radiographic
respective means. The commonalities between axial SpA,
axial SpA (axSpA) has been defined based on the
peripheral SpA and PsA were discussed in detail.
absence of such changes but presence of sacroiliitis
Results Although the literature review did not reveal
(as documented by MRI) and/or human leukocyte
trials comparing a treat-to-target approach with another
antigen B27. The term axSpA, therefore, includes
or no strategy, it provided indirect evidence regarding an
radiographic axSpA (AS) and non-radiographic
optimised approach to therapy that facilitated the
axSpA. On this basis, new classification criteria
development of recommendations. The group agreed on
5 overarching principles and 11 recommendations; 9 of
SpondyloArthritis international Society (ASAS),1
these recommendations related commonly to the whole
novel therapies have proven efficacious,2–6 MRI has
spectrum of SpA and PsA, and only 2 were designed
been increasingly established as an imaging tool in
separately for axial SpA, peripheral SpA and PsA. The
SpA1 7 8 and new indices to assess disease activity
main treatment target, which should be based on a
have been developed.9–14 The novel approach to
shared decision with the patient, was defined as
classification has also differentiated the two pre-
remission, with the alternative target of low disease
dominant manifestations of SpA, axial and/or per-
activity. Follow-up examinations at regular intervals that
ipheral, and their potential parallel occurrence.15
depend on the patient's status should safeguard the
The basis for the new classification lies in the
evolution of disease activity towards the targeted goal.
sharing of characteristic features of SpA, such as
Additional recommendations relate to extra-articular and
sacroiliitis, spondylitis and enthesitis and common
extramusculoskeletal aspects and other important factors,
genetic markers and a positive family history.
such as comorbidity. While the level of evidence was
Furthermore, extramusculoskeletal manifestations
To cite: Smolen JS, Braun J,
generally quite low, the mean strength of
such as psoriasis in PsA, a preceding gastrointestinal
Dougados M, et al. AnnRheum Dis Published Online
recommendation was 9–10 (10: maximum agreement) for
or urogenital infection as in the case of reactive
First: [ please include Day
all recommendations. A research agenda was formulated.
arthritis (ReA), and chronic inflammatory bowel
Month Year] doi:10.1136/
Conclusions The task force defined the treatment
diseases (IBD) like Crohn's disease and ulcerative
target as remission or, alternatively, low disease activity,
colitis, play a role in the definition of a clinical
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Copyright Article author (or their employer) 2013. Produced by BMJ Publishing Group Ltd (& EULAR) under licence.
Clinical and epidemiological research
syndrome as belonging to the concept of SpA. For the classifica-
already at the steering committee meeting, was the question if
tion of patients with PsA the Classification Criteria for Psoriatic
diseases like AS, PsA, ReA and IBD arthritis should be seen as
Arthritis (CASPAR) criteria are well established.16 Since the
an entity or as different diseases. The respective decision would
presence of psoriasis plays a role in both criteria sets, the ASAS
have a bearing to the consensus finding process, since it would
and the CASPAR criteria, there is some overlap between the
mean to develop one, two or more documents. The initial delib-
two. There is no international agreement whether and how they
erations tended toward separating the individual diseases for
can or should be differentiated. Finally, to account for thera-
several reasons: (1) despite many commonalities, some import-
peutic developments, management recommendations have
ant clinical manifestations are distinct between these conditions
recently been presented.17–20
and certain health professionals (such as dermatologists and gas-
Despite all these advances, a variety of challenges exist when
troenterologists) may not be sufficiently aware of the more uni-
considering the management of patients with SpA,21–24 not least
fying concept of SpA or its relevance when dealing with these
because the definition of a clear therapeutic target and strategies
conditions; (2) further, the existing distinction between PsA and
to reach such target are not yet optimally defined.
AS is well known for and accepted by patients and changes in
In many areas of medicine, such as diabetes care or cardi-
terminology may cause confusion regarding the understanding
ology, clear therapeutic targets are available.25–30 More recently,
of their ‘new' diagnosis; (3) to date, clinical trials have been per-
a treatment target has also been advocated for rheumatoid arth-
formed almost entirely in individual subentities (AS, PsA) rather
ritis (RA), namely remission or low disease activity,31 32 a rec-
than SpA, and even most recently trials in a highly specific novel
ommendation based on insights from various clinical trials as
subset, non-radiographic axSpA, have been performed38; (4) the
revealed by systematic literature reviews (SLRs).33 34 Much less
current drug approval process by regulatory agencies is also
information on the value of defining therapeutic targets is cur-
related to the individual diseases rather than SpA; and (5) there
rently available for AS or PsA. Therefore, a task force was
is some overlap between the different subgroups, but there are
formed to discuss and develop a consensus on recommendations
also major distinctions, for example, PsA with symmetric polyar-
aimed at defining a treatment target for, and thus at improving
thritis as the predominant feature would not fit well into either
the management of axial and peripheral SpA in clinical practice.
the axial or peripheral SpA group. Therefore, the provisionalchoice was to develop at least two documents, one for axSpA
and one for PsA. The discussions took place in separate break-
The consensus finding consisted of a three-step process. In a first
out sessions devoted to these topics and in a plenary session. At
step, the first and last author invited leading experts, defined on
the plenary session, certain items were reformulated and reor-
the basis of their citation frequency in the field and previous con-
dered and two provisional sets of recommendations developed,
tributions to similar activities to form a steering committee. This
with decisions made using a modified Delphi technique.32 The
steering committee, which included rheumatologists experienced
group then realised how similar the individual statements in
in the care of patients with, and/or clinical research in axial and/
each of the two documents were, but left further decisions to
or peripheral SpA (several of them Department chairs and thus
the next stage of the process.
in managerial functions), a dermatologist experienced in psoria-
With these two documents prepared and having in mind that
sis, and patients being diagnosed with one of these diseases and/
peripheral SpA (such as ReA) had not yet been dealt with in
or experienced in consensus finding processes, met in March
2011 in Vienna to discuss unmet needs in the therapeutic man-
(Amsterdam); its membership comprised the initial task force
agement of and the potential of using treatment targets in AS and
expanded by consideration of a more international scope to also
PsA. To this end, the debate focused on axial and peripheral SpA
include experts from Latin America and Asia, aside from previ-
separately in two breakout groups with a subsequent common
ous participants from Europe and North America. Again, the
assessment. In the course of these discussions there was unani-
scope and background of this activity was discussed and the pro-
mous agreement that defining therapeutic targets and an appro-
visional recommendations presented. The issue of disease defin-
priate strategic treatment approach would be valuable, but that
ition and the need of developing one, two or three documents
evidence for its validity may be lacking. Therefore it was decided
were addressed. The committee separated into three breakout
to perform a SLR and respective PICO (Patient, Intervention,
groups discussing axSpA, peripheral SpA and PsA. In the course
Control, Outcome) and search terms were formulated, in line
of the breakout discussions and the plenary session, the initial
with European League Against Rheumatism (EULAR) and
Appraisal of Guidelines for Research and Evaluation recommen-
Importantly, when looking at the individual items, the partici-
dations.35 36 In the course of defining the scope of this activity,
pants felt that most of them were very similar and a broad deci-
the target populations were also specified, namely health profes-
sion was then taken to develop a single document comprising
sionals involved in care of and patients affected by axial and/or
overarching principles and items common to SpA in general,
peripheral SpA. In addition, social security officials, hospital
but within that common document to develop a few individua-
managers and policy makers at national and international levels
lised items for axSpA, PsA and peripheral SpA.
were considered potential stakeholders in this activity.
Each statement, which had been formulated as a draft for
At a subsequent meeting in November 2011 (Dusseldorf )
voting in the course of the breakout sessions and by the whole
comprising an expanded task force with increased international
task force, was subjected to voting as ‘yes' (agreement with the
participation, the SLR was presented. These invitations were a
wording) or ‘no' (disagreement). Statements supported by
consequence of the individuals' contributions to the field and
≥75% of votes were immediately accepted while those with
deliberations among members of the steering committee. The
≤25% were rejected outright. Others were subjected to further
literature search had revealed that currently no strategic trials
discussion and subsequent voting, where ≥67% support or, in
addressing a target-oriented, steered therapy were published,
an eventual third round, a majority of ≥50% was needed.
although some indirect evidence on optimal therapeutic
After the face-to-face meeting, the statements were distributed
approaches was available to inform the next stages of the
to the committee members by email for final comments. Only
process.37 A major focus of discussion at this meeting, but also
suggestions for improvements of clarity of wording or
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
addressing redundancies were considered, while any changes to
B. SpA and PsA are often complex systemic diseases; as
the meaning were not accepted.
needed, the management of musculoskeletal and extra-articular
Finally, the group voted anonymously by email on the level of
manifestations should be coordinated between the rheumatolo-
agreement, that is, strength of recommendation, with each of
gist and other specialists (such as dermatologist, gastroenterolo-
the derived bullet points (in the form it was ultimately agreed
upon by the qualified majority of participants) using a 10-point
This item is supposed to inform patients, healthcare profes-
numerical rating scale (1=do not agree at all, 10=agree
sionals with less experience in the care of SpA and non-medical
stakeholders that patients with SpA are frequently sufferingfrom extramusculoskeletal manifestations and are often in need
of multidisciplinary care for optimal therapy. When multiorgan
The evidence base
involvement is present, a harmonised approach among specia-
The SLR, which is published separately,37 revealed that in con-
lists is required which should ideally be coordinated by the
trast to findings in RA33 no randomised controlled clinical trial
rheumatologist, especially if the musculoskeletal involvement
has evaluated a targeted therapeutic approach in comparison
causes major complaints.
with routine therapy. However, several publications had
C. The primary goal of treating the patient with SpA and/or PsA
employed therapeutic targets and respective time requirements
is to maximise long-term health related quality of life and social
as endpoints or before escalating therapy, although this is often
participation through control of signs and symptoms, prevention
the placebo arm of a study that was allowed to escape or then
of structural damage, normalisation or preservation of function,
escalated to active treatment. These comprised 14 studies in AS
avoidance of toxicities and minimisation of comorbidities.
and 7 studies of PsA which were found suitable to inform the
The significant burden of axSpA and PsA in terms of disabil-
task force. Nevertheless, given the lack of studies evaluating
ity, loss of quality of life and work productivity has only
target-steered versus non-steered treatment, the level of evidence
recently been appreciated.47–52 This generally formulated item
for the developed recommendations is low and mainly based on
addresses the importance to control signs and symptoms like
expert consensus.
pain, structural changes such as ankylosis,53 54 comborbid-ities55–57 and the importance of focusing on the totality ofdisease manifestations and complications when determining the
proposal for a treatment target.
The individual statements receiving a positive vote by the major-
D. Abrogation of inflammation is presumably important to
ity of the expert committee members comprise 5 overarching
achieve these goals.
principles and 11 recommendations. The overarching principles
PsA and SpA are inflammatory diseases and inflammation
and 9 of the statements are recommended for SpA in general,
leads to their signs and symptoms, functional impairment as
whereas the last 2 statements have been individualised for
well as structural changes.7 11 58–61 Therefore, stopping inflam-
axSpA, peripheral SpA and PsA. The recommendations are
mation appears to be of key importance to optimise outcome.
shown in table 1. They are discussed in detail below and this
Indeed, in many patients non-steroidal anti-inflammatory drugs
detailed description should be regarded as part and parcel of
(NSAIDs) can lead to cessation of signs and symptoms, normal-
isation of physical function and potentially inhibition of struc-tural damage in the spine.62 63 Interference with the
Overarching principles
proinflammatory cytokine tumour necrosis factor (TNF) sup-
In the Committee's view, a number of elements related to treat-
presses inflammation effectively and can lead to disappearance
ing SpA are so representative of good clinical practice that they
of signs and symptoms and maximal improvement of physical
form a general framework for more specific recommendations.
function. Thus, the task force was convinced that disappearance
These were therefore termed overarching principles, and five
of inflammation conveys the best outcome. However, current
such principles were developed and voted on.
evidence indicates that TNF inhibition does not prevent pro-
A. The treatment target must be based on a shared decision
gression of structural changes in AS.64 65 Moreover, it has not
between patient and rheumatologist.
been determined if a state of remission leads to better long-term
Patient involvement in therapeutic decision-making has
outcome of SpA and/or PsA than low disease activity. Thus, this
become a mandate in patient care, especially when dealing with
item is somewhat more controversial than most other ones.
chronic diseases. This is a general patient right, and has been
Therefore the word ‘presumably' was added. This point consti-
shown to improve mutual understanding and outcome39–42 and
tutes a backbone for some of the subsequent individual
is also increasingly recognised to be important in SpA.43–45 The
committee was convinced that patients must be informed about
E. Treatment to target by measuring disease activity and
the proposed treatment target, therapeutic options to reach the
adjusting therapy accordingly contributes to the optimisation of
target and reasons for recommending the target also in light of
short and/or long-term outcomes.
the risk related to treatment and risk related to the disease; on
The SLR has revealed that patients with AS who do not reach
the other hand, patients should actively participate in this dis-
predefined, measurable treatment targets can achieve further
cussion. This aspect is subsequently reinforced in recommenda-
improvement upon adaptation of their therapy. While for PsA
tion number 8 and these two items received the highest level of
this has not yet been established, the task force regarded the need
agreement among all bullet points. The principle also specific-
to measure disease activity and amend therapy with persistently
ally mentions the rheumatologist, since it is the rheumatologist
active disease as a general necessity and, therefore, as a principle.
who should coordinate treatment of patients with SpA.
The level of agreement with these five principles was very
Evidence regarding RA suggests that patient outcome is better
high, ranging between 9.1 (item D) and 9.7 (item A) on a scale
when care is provided by a rheumatologist,46 and this might
of 10 (table), indicating that this large and quite heterogeneous
also be so for the musculoskeletal manifestations of PsA and
task force had arrived at a quite unanimous view on the princi-
pal importance of certain approaches to treatment of SpA.
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
Recommendations to treat all forms of Spondyloarthritis to target
Overarching principles
The treatment target must be based on a shared decision between patient and rheumatologist
SpA and PsA are often complex systemic diseases; as needed, the management of musculoskeletal and extra-articular manifestations
should be coordinated between the rheumatologist and other specialists (such as dermatologist, gastroenterologist, ophthalmologist)
The primary goal of treating the patient with SpA and/or PsA is to maximise long-term health related quality of life and social
participation through control of signs and symptoms, prevention of structural damage, normalisation or preservation of function,avoidance of toxicities and minimisation of comorbidities
Abrogation of inflammation is presumably important to achieve these goals
Treatment to target by measuring disease activity and adjusting therapy accordingly contributes to the optimisation of short term and/
or long term outcomes
Common items for all forms of SpA
A major treatment target should be clinical remission/inactive disease of musculoskeletal involvement (arthritis, dactylitis, enthesitis,
axial disease), taking extra-articular manifestations into consideration
The treatment target should be individualised according to the current clinical manifestations of the disease
Clinical remission/inactive disease is defined as the absence of clinical and laboratory evidence of significant inflammatory disease
Low/minimal disease activity may be an alternative treatment target
Disease activity should be measured on the basis of clinical signs and symptoms, and acute phase reactants
The choice of the measure of disease activity and the level of the target value may be influenced by considerations of comorbidities,
patient factors and drug-related risks
Once the target is achieved, it should ideally be maintained throughout the course of the disease
The patient should be appropriately informed and involved in the discussions about the treatment target, and the risks and benefits of
the strategy planned to reach this target
Structural changes, functional impairment, extra-articular manifestations, comorbidities and treatment risks should be considered when
making clinical decisions, in addition to assessing measures of disease activity
Specific items for individual types of Spondyloarthritis
Axial Spondyloarthritis (including ankylosing spondylitis)
Validated composite measures of disease activity such as the BASDAI plus acute phase reactants or the Ankylosing Spondylitis Disease
Activity Score, with or without measures of function such as BASFI, should be performed and documented regularly in routine clinicalpractice to guide treatment decisions; the frequency of the measurements depends on the level of disease activity
Other factors, such as axial inflammation on MRI, radiographic progression, peripheral musculoskeletal and extra-articular
manifestations, as well as comorbidities may also be considered when setting clinical targets
Quantified measures of disease activity, which reflect the individual peripheral musculoskeletal manifestations (arthritis, dactylitis,
enthesitis) should be performed and documented regularly in routine clinical practice to guide treatment decisions; the frequency of themeasurements depends on the level of disease activity
Other factors. such as spinal and extra-articular manifestations, imaging results, changes in function/quality of life, as well as
comorbidities may also be considered for decision
Psoriatic arthritis
Validated measures of musculoskeletal disease activity (arthritis, dactylitis, enthesitis, axial disease) should be performed and
documented regularly in routine clinical practice to guide treatment decisions; the frequency of the measurements depends on the levelof disease activity; cutaneous manifestations should also be considered
Other factors, such as spinal and extra-articular manifestations, imaging results, changes in function/quality of life, as well as
comorbidities may also be considered for decision
An asterisk in the LoE column denotes that for this item indirect evidence is available from the literature search which was nevertheless not sufficient for a higher grading of theevidence level.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; GoR, grade of recommendation; LoE, level of evidence; PsA, psoriaticarthritis; SoR, strength of recommendation (level of agreement); SpA, spondyloarthritis.
Common recommendations
As mentioned previously, after intensive deliberations the com-
1. A major treatment target should be clinical remission/inactive
mittee had decided to create just one document covering axial
disease of musculoskeletal involvement (arthritis, dactylitis,
and peripheral SpA, including PsA. To this end, nine unified
enthesitis, axial disease), taking extra-articular manifestations
recommendations and two additional items dealing separately
into consideration (level of evidence (LoE): 5, grade of recom-
with PsA, axial SpA and peripheral SpA were developed in the
mendation (GoR): D).
course of the discussions. These recommendations applied the
Hitherto, no clinical trial has compared outcomes of PsA or
results of the SLR, but given the low available evidence in the
axSpA for progression of structural changes or improvement of
literature, they were mostly based on expert opinion, albeit con-
physical function or quality of life when remission rather than
sensual opinions of a large group of experts. The sequence of
another state was targeted. Definitions for remission (which is
the recommendations follows a logical order, but also reflects
called inactive disease in AS) or at least minimal disease activity
the level of importance considered by the committee for each
(MDA) exist for PsA and AS,12 66 67 but in contrast to RA the
individual bullet point.
long-term benefits of remission have not yet been sufficiently
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
established. Also, no clear definition of remission for extra-
established,66 especially given that in PsA most composite mea-
articular musculoskeletal features, such as enthesitis or dactylitis,
sures have been borrowed from RA while a composite measure
is currently available. Moreover, it is not sufficiently known at
specific for PsA has only recently been validated,10 but criteria
present how remission of musculoskeletal symptoms relates to
for disease activity states have not yet been defined. Importantly,
remission of skin disease in PsA or bowel disease in IBD.
the term ‘significant' was added deliberately, indicating that
Therefore, the formulation of this first bullet point reflects the
the presence of a minute extent of residual activity, such as the
general lack of data, by saying ‘a major' rather than ‘the major'
presence of a tender joint or residual swollen but painless joint,
treatment goal, or by expanding the term ‘clinical remission' to
or residual axial pain that does not appear to relate to inflamma-
the somewhat less stringent term ‘inactive disease'. However,
tion, would still be compatible with remission. On the other
the lack of thorough evidence and the unwillingness of the
hand, the committee wished to have a stringent definition of
group to arrive at a more determined and clear verbalisation of
remission which would not allow significant residual disease
this bullet point must not be mistaken as an uncertainty of the
activity, such as several swollen joints or significant back pain,
task force regarding the necessity of treating patients to become
even if dramatically improved by therapy, to be called remission.
free of signs and symptoms of their peripheral joint or axial
Also, this bullet point speaks of ‘clinical remission' indicating
disease. On the contrary, the task force deemed this approach to
that clinical rather than imaging measures should be used to
be of utmost importance for short-term benefit and long-term
define remission, at least currently. In this respect it should be
outcome. Therefore it was placed as the first recommendation.
noted that MRI shows evidence of inflammation in AS and that
Importantly, the group focussed the terms ‘remission'/‘inactive
a negative MRI can be regarded as imaging remission; however,
disease' to the musculoskeletal manifestations of SpA and not
the relationship between clinical and imaging remission still
the extramusculoskeletal abnormalities, although it clearly stated
needs to be elaborated. Statement 3, which like the previous
in the last part of this item that this must not be neglected in
ones is based on expert opinion, received approval by 83% of
the therapeutic decision making. After several rounds of discus-
the committee members and a SoR of 9.0±1.4.
sion, 83% of the participants agreed with the formulation of
4. Low disease activity/MDA may be an alternative treatment
this bullet point and the strength of recommendation amounted
target (LoE: 5; GoR: D)
to 9.5±0.9.
While remission (inactive disease) constitutes an ideal goal,
2. The treatment target should be individualised according to the
clinical practice, stringently as it was defined in bullet point
current clinical manifestations of the disease (LoE: 5, GoR: D)
number 3 it may be difficult to achieve in many patients, espe-
This item emphasises that every patient should be treated
cially those with established/long-standing disease. Indeed,
according to her or his current clinical manifestations and that
patients with axSpA with longer disease duration are less likely
in light of their heterogeneity each of these manifestations has
to attain partial remission than those with early disease.72 38
to be accounted for when setting the therapeutic target.
Thus, while remission is the ultimate and an ideal goal, low
However, it also implies that at certain points in time the mus-
disease activity constitutes a useful alternative in the opinion of
culoskeletal symptoms may not be in the foreground, such as
the task force, since it is assumed that physical function and
when extra-articular manifestations prevail and need appropri-
quality of life may not be much worse than in remission and
ate attention. Again, no data exist in the literature to support or
progression of structural damage, while possibly not halted,
refute this recommendation which was voted for by 87% of the
would be minimal. Indeed, as included in the bullet point, low
participants and attained a strength of recommendation (SoR)
disease activity can also be regarded as ‘MDA'. Thus, minor
of 9.3±1.0.
residual signs and symptoms may still exist differentiating this
3. Clinical remission/inactive disease is defined as the absence
state from inactive disease. Importantly, by stating that low
of clinical and laboratory evidence of significant inflammatory
disease activity is an alternative goal to remission, the committee
disease activity.
also clearly implies that any other, higher state, even moderate
This bullet point provides a definition for item 1 in order to
disease activity, would not be acceptable and its presence should
clarify the definition of remission. Remission of an inflammatory
elicit therapeutic adaptations. More research will be needed to
rheumatic disease ideally comprises the absence of its signs and
provide information on the optimal time point for achieving the
symptoms, the maximal improvement in physical function and
treatment target. However, given that in clinical trials of AS
halt of structural changes. While there is compelling evidence
maximal improvement was achieved between week 12 and week
that these three characteristics go along with each other in RA,
24 regarding all outcome measures including ASAS partial
this is not yet sufficiently known in AS and PsA. However, in
remission4 73 74 and that similar observations have been made in
PsA, progression of joint damage is correlated with swollen joint
PsA,2 75 76 a maximum of 6 months for reaching the treatment
counts and dactylitis59 68 and, therefore, it may be assumed that
target of low disease activity or remission seems appropriate,
clinical remission will also lead to halt of structural progression.
but it is advisable to adapt therapy earlier if no significant reduc-
This is not quite clear in AS, since progression of syndesmo-
tion in disease activity is observed within 3 months. Recently,
phyte formation has been observed even when patients were in
thresholds for disease activity states including inactive disease
clinical remission on TNF inhibitors and formation of syndes-
(equivalent to remission) have been defined for axSpA using the
mophytes occurred without presence of MRI inflamma-
ASDAS,12 and ASAS definition of partial remission is also avail-
tion.8 64 65 69 On the other hand, elevated levels of acute phase
able67; a measure of MDA has been developed for PsA which is
reactants (APRs) are associated with progression of structural
beginning to be used in clinical trials.77 78 Additional research
changes in AS.70 Further, physical function and quality of life
in this respect will be required, especially for PsA and peripheral
are related to symptoms of these diseases.71 For all these reasons
SpA. This item was accepted as defined by 79% of the partici-
the task force defined remission as stated above. Definitions of
pants and received a SoR of 9.4±0.9.
remission or partial remission are available for AS, when using
5. Disease activity should be measured on the basis of clinical
mere patient reported outcomes67 or the more recently
signs and symptoms, and APRs (LoE: 5, GoR: D).
Traditionally, given that the spine is not as accessible to phys-
ical examination of signs of inflammation like a peripheral joint,
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
disease activity in axSpA has been evaluated by employing the
therapy will lead to reactivation of AS83 and PsA.84 While the
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
present consensus statement is not designed to provide recom-
which comprises only patient reported variables related to
mendations on therapies with particular agents but rather on
symptoms of the disease. However, axSpA is an inflammatory
treatment strategies, the task force nevertheless points to the
disease with involvement of various inflammatory cells and
importance of maintaining the targeted therapeutic state once
cytokines.79 80 Indeed, inhibition of TNF is one of the main-
achieved and advises against stopping a successful therapy based
stays of treatment today, leading to relief of symptoms, and
on the available evidence. However, it has not yet been studied
inhibition of prostaglandin synthesis can reduce clinical symp-
sufficiently if dose reduction or expansion of treatment intervals
toms, and retard progression of structural changes62 63; this
allows maintaining a good clinical state. Approval was given
appears to be particularly prominent in patients with elevated
by 90% of the task force's members and the SoR amounted
APRs, and the latter seem to be associated with progression of
to 9.4±0.8.
syndesmophyte formation.70 81 82 Consequently, disease activity
8. The patient should be appropriately informed and involved
assessment during follow-up and in the course of targeting a
in the discussions about the treatment target, and the risks and
good outcome should comprise the assessment of clinical
benefits of the strategy planned to reach this target (LoE: 5,
aspects of the disease as well as laboratory abnormalities, that is,
APR measurement. This can be done separately by looking at
While this statement has already been partly covered in the
for example, BASDAI and c-reactive protein (CRP), or by using
overarching principles, it was felt important to also raise this
a measure that comprises both aspects, such as ASDAS.11 Along
point in the context of the actual recommendations to bolster
the same line, PsA as an inflammatory and potentially destruc-
the importance of interaction between health professionals and
tive joint disease should be followed using measures that relate
their patients in all regards: setting and agreeing on the treat-
to the joints and the serological inflammatory response; these
ment target, discussing the strategies available to reach that
are contained in RA-related composite measures of disease activ-
target and the time it may take to attain it, and laying out the
ity, such as Disease Activity Score (DAS), DAS28 and Simplified
benefits and risks of the recommended treatment and consider
Disease Activity Index (SDAI), but also in the Disease Activity in
the totality of clinical disease manifestations (including the
PsA and the psoriatic arthritis disease activity score.13 The
extramusculoskeletal ones) and of comorbidities. This point also
current recommendation does not relate to systemic features of
comprises the need to discuss steps to be taken if the treatment
SpA, but solely to the musculoskeletal manifestations. For sys-
target is not achieved, such as adjustment of or switch to a new
temic features, other measures are needed and, according to the
therapy. In this respect patient education programmes or book-
EULAR recommendations for treatment of PsA,18 this should be
lets may provide additional helpful means. This item was
approved by 81% of the participants and the SoR was 9.8±0.5.
Recommendation 5 received approval by 97% of participants
9. Structural changes, functional impairment, extra-articular
and the SoR amounted to 9.4±1.1.
manifestations, comorbidities and treatment risks should be con-
6. The choice of the measure of disease activity and the level of
sidered when making clinical decisions, in addition to assessing
the target value may be influenced by considerations of comorbid-
measures of disease activity.
ities, patient factors and drug related risks (LoE: 5; GoR: D)
This point is focused on considerations involved in thera-
Patients with chronic musculoskeletal diseases are frequently
peutic decision making. Although the last part of this recom-
also suffering from comorbidities that (1) may be related to the
mendation emphasises the importance of regular assessment of
overall spectrum of the disorder (such as IBD, uveitis or psoria-
disease activity with appropriate measures (see items 5 and 6),
sis), (2) may occur as a consequence of chronic inflammation
the first part suggests taking into account results of other investi-
(such as cardiovascular disease), (3) may be related to therapy
gations for treatment decisions, such as by imaging (especially
(such as gastric ulcer or infection), or (4) just may occur con-
structural changes in PsA), physical function and extra-articular
comitantly by chance. The presence of such comorbidities may
manifestations. The latter comprise enthesitis or dactylitis as
alter the level of the treatment target, since the risk of aggravat-
well as extramusculoskeletal disease. This is of importance,
ing the comorbid condition may outweigh the benefit conveyed
since treatment approaches to PsA will differ in the presence of
by more intensive therapy to achieve the treatment target of the
enthesitis compared with patients who do not suffer from
musculoskeletal manifestations. Further, the choice of follow-up
entheseal affection.18 Moreover, organ disease, such as lung
measure may have to be changed under certain circumstances.
involvement, aortitis, intestinal or skin manifestations as well as
For example, a concomitant disease which raises pain levels or
uveitis, may require involvement of other specialists (see over-
APRs may influence the result of measuring disease activity.
arching principle B). In particular uveitis can present across the
Likewise, when following patients on therapies that affect the
spectrum of SpA and may reflect disease activity, and inflamma-
APRs independently of clinical benefit one may have to recon-
tory bowel disease and psoriatic skin involvement must be con-
sider the choice of a measure that contains an APR. Therefore,
sidered in the respective disorders and do not strongly correlate
this point focuses on the application (and sometimes restricted
with the degree or extent of musculoskeletal involvement. Risks
applicability) of particular disease activity measures. This recom-
and comorbidities are also reiterated here in the context of
mendation was approved by 97% of the task force members
treatment decisions; before they were indicated with respect to
and received a SoR of 9.4±1.0.
the choice of measures of disease activity (see recommendation 6)
7. Once the target is achieved, it should ideally be maintained
and in relation to patient information (item 8). This recommen-
throughout the course of the disease (LoE: 5; GoR: D)
dation achieved 100% agreement and a SoR of 9.5±0.8.
It is clear that no patient's successfully targeted disease activ-
ity state should deteriorate during follow-up, since reactivation
Disease specific recommendations
of disease may again lead to reduced quality of life and disabil-
As indicated above, items 10 and 11 have been formulated spe-
ity. There is evidence that on demand NSAID therapy, in con-
cifically for axSpA, PsA and peripheral SpA to account for the
trast to regular NSAID treatment, is associated with progression
differences between certain characteristics of the different
of radiographic changes in AS63 and that stopping TNF-blocker
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
Axial SpA (including AS)
treatment decisions; the frequency of the measurements depends
10. Validated composite measures of disease activity such as
on the level of disease activity; cutaneous manifestations should
BASDAI plus APRs or ASDAS, with or without measures of func-
also be considered.
tion such as Bath Ankylosing Spondylitis Functional Index
Specific mention of skin disease tailors this recommendation
(BASFI), should be performed and documented regularly in
to PsA, although skin may also be involved in axial and periph-
routine clinical practice to guide treatment decisions; the fre-
eral SpA. Further, in addition to arthritis, dactylitis and enthesi-
quency of the measurements depends on the level of disease
tis, axial disease assessment is specifically brought forward here.
Finally, a reminder is provided that the results of the various
This item is an expansion of recommendation numbers 5 and 9.
measures (and also the treatment target) should be documented.
It mentions those disease activity measures which have been
Clearly, with highly active disease patients should be seen fre-
repeatedly validated and are already in use in contemporary
quently, such as monthly to every 3 months, while with low
clinical trials. In line with recommendation 5, when BASDAI is
disease activity or remission, follow-up examinations may be
employed, an APR, such as CRP or erythrocyte sedimentation
done only every 6–12 months. However, skin involvement also
rate, should also be determined. The ASDAS already comprises
has to be taken into account. The voting achieved 92% approval
such a measure among its components.12 In addition, this rec-
and the SoR amounted to 9.4±0.8.
ommendation also suggests the use of a particular functional
11. Other factors such as spinal and extra-articular manifesta-
measure, but other validated measures can also be applied
tions, imaging results changes in function/quality of life, as well
(therefore the tem ‘such as'). With highly active disease,
as comorbidities may also be considered for decision.
follow-up examinations will have to be more frequent than with
As for the other disease entities, this last recommendation
inactive disease/remission. Moreover, the recommendation
expands item 9 and also the preceding one by reiterating the
requests documentation of the measured results. Among task
importance of comorbidities, and axial and soft tissue manifes-
force members, 88% approved this item and SoR amounted to
tations of PsA in the course of making treatment decisions.
Approval was granted by 100% of the participants and the SoR
11. Other factors such as axial inflammation on MRI, radio-
amounted to 9.3±1.0.
graphic progression, peripheral musculoskeletal and extra-
A final anonymous vote on whether the task force members
articular manifestations, may also be considered when setting
felt influenced by the fact that support for this activity was pro-
clinical targets.
vided by a company, the result was 0.4±1.3 (0 meaning no and
Again, this recommendation expands item 9, but specifically
10 meaning heavy influence), indicating that the participants felt
mentions MRI as a highly valuable imaging method for the
potential follow-up of axSpA. Likewise, non-axial disease mani-festations will influence not only the therapeutic approach but
have to be considered when setting treatment targets. Approval
Since none of the recommendations is based on evidence, the
was voted for by 88% of the members and the SoR was 9.3±0.8.
research agenda has to comprise the search for evidence for allof them. However, beyond mere therapeutic aspects, insightsinto the relationships between individual musculoskeletal mani-
festations, damage and disability are still incomplete especially
10. Quantified measures of disease activity, which reflect the
for the peripheral SpA, including PsA. Table 2 lists a research
individual peripheral musculoskeletal manifestations (arthritis,
agenda as mentioned during the task force's meetings.
dactylitis, enthesitis) should be performed and documented regu-larly in routine clinical practice to guide treatment decisions; the
frequency of the measurements depends on the level of disease
Recommendations to treat axSpA and PsA have been developed
over the recent years.17–19 However, none of these addressed a
This recommendation, while expanding items 5, is here spe-
clear therapeutic target and a strategy to reach this target. This
cifically tailored to peripheral SpA, such as ReA, IBD arthritis or
has now been done in the present set of recommendations, and
former ‘undifferentiated' peripheral SpA. Measures of disease
additional strategic aspects of treatment approaches are pre-
activity are available and have been validated for the arthritis
sented. Thus, the present consensus on treatment targets and
component of ReA9 and there exist measures for dactylitis and
general treatment approaches complements the published man-
enthesitis which have not been primarily developed for periph-
agement recommendations,17–20 but a notable difference is the
eral SpA, but rather for PsA or AS.85–87 While they will have to
absence of suggestions or recommendations regarding a particu-
be validated in peripheral SpA, they can be assumed useful for
lar drug in any of the overarching principles or individual
clinical practice until proven otherwise. Also this recommenda-
tion calls for documentation of the measured results. Of the par-
Treatment recommendations should usually be based on evi-
ticipants, 100% approved this item and the SoR score achieved
dence. However, where evidence is missing, expert opinion has
was 9.3±0.9.
to come into play. The recommendations presented here are not
11. Other factors such as spinal and extra-articular manifesta-
based on hard evidence, because strategic therapeutic trials, in
tions, imaging results, changes in function/quality of life, as well
which therapy was consistently adapted to reach a prespecified
as comorbidities may also be considered for decision.
treatment target and compared with a non-steered approach, as
This item reiterates and expands recommendation 9 and
performed in RA,88 89 are currently not available for axSpA,
achieved 100% approval; the SoR was 9.4+0.8.
peripheral SpA or PsA, and other pertinent literature is scarce.
While a SLR has provided indirect evidence from clinical trials
Psoriatic arthritis
which targeted specific endpoints37 and thus supplied some
10. Validated measures of musculoskeletal disease activity (arth-
information towards the work of the task force, the individual
ritis, dactylitis, enthesitis, axial disease) should be performed
recommendations can only be regarded as expert opinion (con-
and documented regularly in routine clinical practice to guide
sensus) and therefore call for more research in the field.
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
behind other disease areas which have already defined their
targets years to decades ago, when at least indirect evidence for
Specific questions
the benefit of attaining certain treatment targets is available; and(2) the tremendous therapeutic advances of the past decade
Composite activity measures
Validation where needed, definition of
have greatly improved the chances of achieving good outcomes
(mainly PsA and peripheral SpA)
disease activity states and responsecategories
and, therefore, setting stringent treatment milestones has
Remission definition
Is it important that all clinical domains of
become a reality which should not be concealed. Moreover, in
axial SpA, peripheral SpA or PsA are in
the course of its initial discussions on this issue, the task force
remission or is it sufficient to define some of
felt that with these advances and the concomitant formulation
of a research agenda, investigations towards providing respective
Is there a difference in long-term outcome
evidence could be fostered and accelerated. Importantly, this
when comparing remission with low disease
view was shared among all task force members, which com-
prised patients and an international group of physicians with
Activity and damage
What is the progression of joint damage indifferent disease activity states in PsA?
expertise in SpA.
Are there differences in responsiveness and
At all three steps of this activity, which included initial discus-
thus differences in attaining certain targets
sions by the steering committee, formulation of recommenda-
with different disease duration in PsA?
tions by an expanded working group and development of
Treatment to target
There is a need to design therapeutic trials
treatment recommendations for all three entities, axSpA, periph-
that compare steered therapy aiming at
eral SpA and PsA, unanimous agreement was attained.
remission or low disease activity withnon-steered treatment (like TICORA)88
Moreover, all items achieved strong consensus in an anonymous
Axial involvement in PsA
Do spinal and peripheral involvements
voting process, with the lowest result being a mean of 9.0 on a
respond similarly or differently?
scale of 0–10, indicating that the task force stood quite united
Enthesitis, dactylitis
More data need to be attained on the
behind the recommendations.
response of dactylitis or enthesitis to different
The complexity of the current endeavour resulted from the
heterogeneity of the diseases covered. After long discussions and
Care by rheumatologist
Is care of axial SpA, peripheral SpA or PsA by
the intermediate development of more than one document, it
a rheumatologist advantageous for outcomes
was decided to produce a single set of recommendations for
when compared with care bynon-rheumatologists?
axSpA, peripheral SpA and PsA, in line with recent criteria for
Maintenance of response
How can response be maintained? Can the
classification of SpA.15 Five overarching principles and nine
dose of the therapy employed be reduced or
recommendations were developed in common for all forms of
the interval of applications be expanded and
SpA, including PsA. Only two recommendations were separately
outcome maintained?
produced for axSpA, peripheral SpA and PsA, although their
Is outcome different when patients are
general scope was still very similar and differences only very
informed in a structured way when comparedwith more general means of information?
subtle. The overall activity was partly influenced by the treat-to-target recommendations for RA.32
PsA, psoriatic arthritis; SpA, Spondyloarthritis.
Several of the recommendations stand out in their import-
ance, while others can be seen as supportive or operational.
So why then were the recommendations developed now
A call for remission or inactive disease became item 1, because
rather than waiting for more evidence? Because the definition of
this was regarded the foremost treatment target. Indeed, we can
a treatment target and strategy is timely at present in light of
anticipate that reducing inflammation and disease activity to the
two major aspects: (1) a field like that of SpA should not stay
minimum is optimal for the patients, at least for their quality of
Algorithm to treat
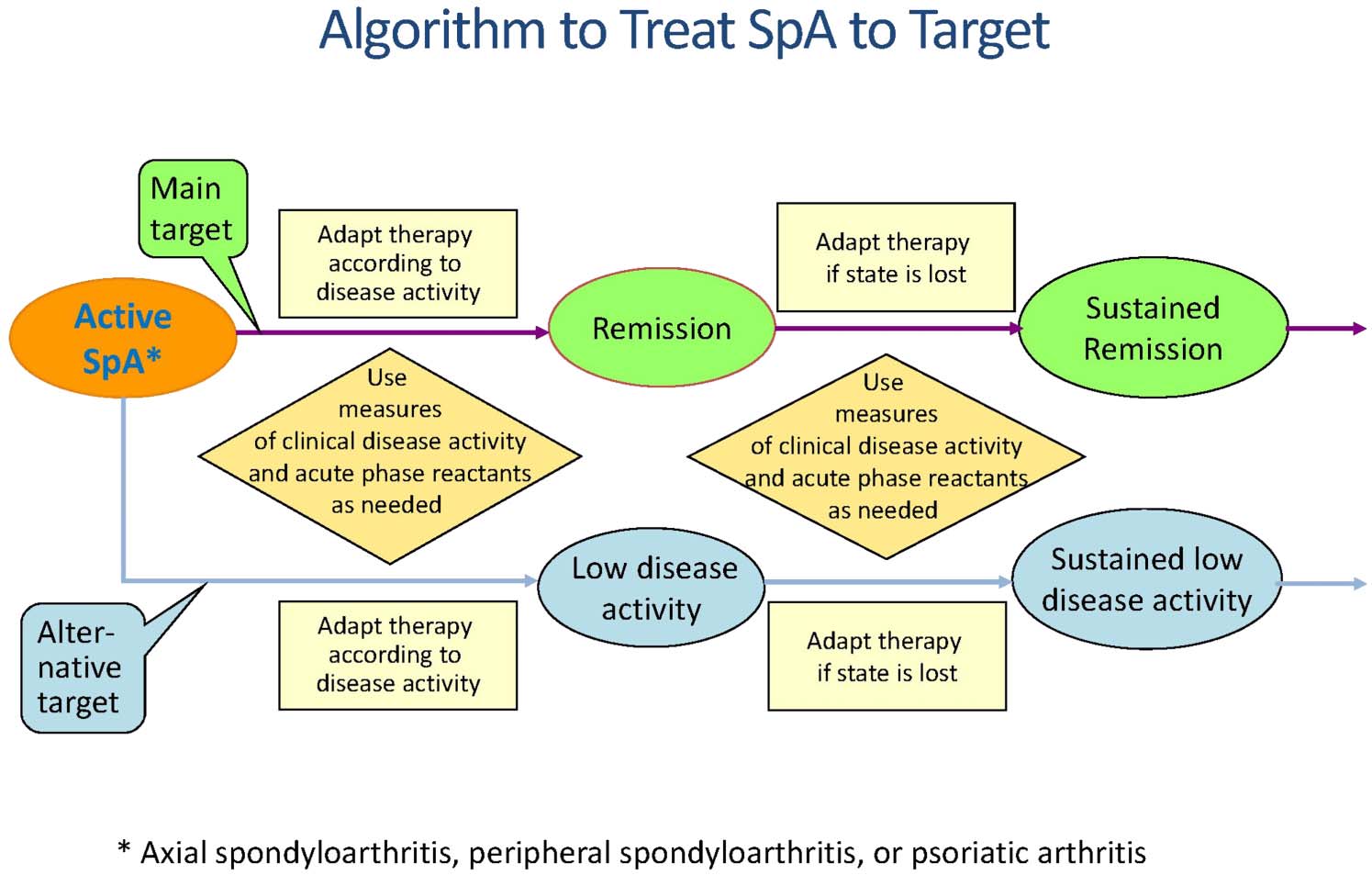
spondyloarthritis (SpA) to target. Thealgorithm depicts the main (remission)and the alternative target (low diseaseactivity), the need to adapt therapy ifthe target is not reached, therequirement to use measures thatreflect clinical activity and acute phasereactants and the sustainment ofremission (developed in considerationof the figure for the rheumatoidarthritis algorithm).32
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
life. However, the members were aware that remission may not
22Laboratory for Molecular Immunology and Inflammation, Department of
be achievable in all patients and, therefore, formulated an alter-
Rheumatology, Ghent University Hospital, Ghent, Belgium
native treatment target, especially for patients with long-standing
Department of Rheumatology, Pitié Salpêtrière Hospital, Pierre et Marie Curie
University, Paris, France
disease, namely low disease activity (recommendation 4).
24Division of Allergy, Immunology and Rheumatology, Institute of Medicine, Chung
Importantly, this acknowledgement indicates that disease activity
Shan Medical University Hospital, Taichung, Taiwan
states other than remission or low disease activity constitute
25Department of Public Health and Preventive Medicine, Oregon Health & Science
unacceptable clinical states, unless justified because of other
University, Portland, Oregon, USA
26Department of Rheumatology, Leiden University Medical Center, Leiden,
reasons, such as comorbidity (items 6, 9 and 11). Importantly,
while validated measures of disease activity are available for PsA
Contributors All authors participated in the development of the consensus
and ReA, disease activity states have not yet been sufficiently
statement and the voting process and provided inputs into the statements presented.
defined, contrasting the situation in AS. Another complexity
Funding This study was supported by an unrestricted grant from Abbott/AbbVie to
relates to the necessity to use measures that reflect the individual
the Medical University of Vienna.
manifestations of a patient which in some instances may involve
Competing interests JSS received grant support from and/or provided expert
assessment of peripheral joint disease, axial involvement, dacty-
advice or speaking engagements for Abbott, Celgene, Janssen, MSD, Pfizer and
litis and enthesitis. To identify an individual treatment goal can
UCB. JB received grant support from and/or provided expert advice to AbbVie,
in itself be seen as an important part of a treatment strategy
Celltrion, Janssen, MSD, Novartis, Pfizer, UCB. MD has participated in advisory
when an intervention is initiated and should be accompanied by
boards and symposia organised by Pfizer, AbbVie, UCB and Roche; his departmenthas received research grants from Pfizer, AbbVie, UCB, Roche, Lilly, MSD and Sanofi.
a monitoring programme. It is also important that the agreed
PE has undertaken clinical trials and provided expert advice to MSD, Pfizer, Abbott
goal is documented in the records of the patient.
and Novartis. OF received grants from AbbVie, Pfizer, BMS, Roche and MSD and
Patient involvement in defining the treatment target and selec-
served as speaker at meetings for AbbVie, Pfizer, MSD, UCB and Celgene.
tion of therapies based on their risks and benefits was deemed
PH received honoraria from Abbott, Celgene, BMS, Pfizer, Merck, J&J and UCB.
so important, that it is stated in the first overarching principle
AK conducted clinical research sponsored by AbbVie, Amgen, UCB and Jannsen,and provided expert advice for AbbVie. TKK received honoraria for speaking and/or
and additionally in one of the recommendations.
consulting engagements from AbbVie, AstraZeneka, BMS, Hospira, MSD/
As indicated above, given the small evidence base, the research
Schering-Plough, Nicox, Pfizer, Roche and UCB and Diakonhjemmet Hospital
agenda is of utmost importance. Research activities should focus
received research funds from AbbVie, BMS, MSD, Pfizer, Roche and UCB. RL has
on strategic therapeutic trials, and on addressing missing infor-
received research grants from Abbott, Amgen, Centocor, Novartis, Pfizer, Roche,UCB; speaker fees from Abbott, Amgen, BMS, Janssen, MSD, Pfizer, Roche, UCB
mation, such as the definition of disease activity states in PsA.
and is director of Rheumatology Consultancy BV, a registered company under Dutch
The recommendations are summarised in a simplified form in
law. TL has no conflict to declare. PM has received research grants, consultation
an algorithm presented in figure 1. Like most types of recom-
fees and/or speaker honoraria from AbbVie, Amgen, BiogenIdec, BMS, Celgene,
mendations, it will be necessary to revise the current document
Genentech, Glaxo SmithKline, Lilly, MSD, Novartis, Pfizer, UCB, Vertex. IO has
in due course, presumably in about 4 years or 5 years or earlier,
received consulting fees, research or institutional support and educational grantsfrom AbbVie, MSD and Pfizer. JR has consulted for AbbVie and UCB and has
when significant evidence accumulates regarding the individual
participated in clinical trials of AbbVie and UCB on axial SpA. CR received research
points of the recommendations. The task force hopes for an
support from Amgen, Janssen, UCB and has consulted for AbbVie, Amgen, Janssen,
expansion of high quality research activities that either allow
UCB, Boehringer Ingleheim, Regeneron and Lilly. MR has received honoraria from or
confirmation or modifications of its conclusions.
has served as consultant for AbbVie, BMS, MSD, Pfizer, Roche/Chugai, UCB. MShas received honoraria from AbbVie. JS has received grant support from AbbVie,MSD, Pfizer, Janssen and Honoraria for consultancies and/or lecturing from AbbVie,
Author affiliations
Merck, Pfizer, Janssen, UCB, Novartis, Lilly, Sanofi, Roche. MdW received honoraria
1Division of Rheumatology, Department of Medicine 3, Medical University of Vienna,
from Abbott. XB has received research grants and consultant's, advisory boards'
and/or speaker's honoraria from AbbVie, Celgene, Janssen, Chugai, MSD, Novartis,
22nd Department of Medicine, Hietzing Hospital Vienna, Vienna, Austria
Pfizer, UCB. NB has received consultancy fees from Roche, BMS and Pfizer.
3Rheumazentrum Ruhrgebiet, Herne, Germany
RB-V has received honoraria for speaking and/or advisory board engagements from
4Department of Rheumatology B, Cochin Hospital, René Descartes University, Paris,
AbbVie, BMS, Pfizer, Roche and UCB. EC-E declares no conflicts. AD has
participated in advisory board activities for UCB, Pfizer, AbbVie and has received
5Division of Rheumatic and Musculoskeletal Disease, Leeds Institute of Molecular
research grants from AbbVie, Amgen, Janssen, Novartis, Pfizer and UCB. DE received
Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, UK
grant support from Merck and Abbott. LG has received honoraria from AbbVie,
6Department of Rheumatology, St. Vincents University Hospital, Dublin, UK
Chugai, Pfizer, Roche and UCB. MJ declares no conflicts. MM declares no conflicts.
7Division of Rheumatology, Allergy, Immunology, University of California, San Diego,
KR has received grant support and/or provided expert advice and/or presentations
for AbbVie, BMS, Glaxo, MSD, Novartis-Sandoz, Pfizer, Roche and UCB. FvdB
8Department of Rheumatology, Diakonhjemmet Hospital, Oslo, Norway
provided advice for Abbott. JC-CW declares no conflicts. K-W has received research
9Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
grants from Pfizer and consulting fees from Pfizer, UCB, Genentech and AbbVie.
10Atrium Medical Center, Heerlen, The Netherlands
DvdH has received consulting fees and/or research grants from AbbVie, Amgen,
11Clinic and Polyclinic of Dermatology, University of Münster, Münster, Germany
AstraZeneca, Augurex, BMS, Centocor, Chugai, Daiichi, Lilly, Glaxo-Smith-Kline,
12Swedish Medical Center and University of Washington, Seattle, Washington, USA
Janssen Biologics, MSD, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche,
13Rheumatology Department of Lucania, San Carlo Hospital of Potenza and
Sanofi-Aventis, UCB and Vertex, and is Director of Imaging Rheumatology bv.
Madonna delle Grazie Hospital of Matera, Potenza, Italy
14Division of Rheumatology, University of Texas Health Science Center at Houston,
Provenance and peer review Not commissioned; externally peer reviewed.
Houston, Texas, USA
Open Access This is an Open Access article distributed in accordance with the
15Allergy, Immunology and Rheumatology Division, The Center for Musculoskeletal
Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits
Medicine, University of Rochester Medical Center, Rochester, New York, USA
others to distribute, remix, adapt, build upon this work non-commercially, and license
16Endokrinologikum Berlin, Berlin, Germany
their derivative works on different terms, provided the original work is properly cited and
17Medical Department I, Rheumatology, Charité Campus Benjamin Franklin, Berlin,
the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
18EULAR standing committee of People with Arthritis/Rheumatism in Europe (PARE),Zurich, Switzerland
19Rheumatology Department, Faculty of Medicine, Hospital General de MéxicoUniversidad Nacional Autónoma de México, Mexico City, Mexico
20Department of Rheumatology, Reina Sofia University Hospital of Córdoba/IMBIC,
Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment
of SpondyloArthritis international Society classification criteria for axial
21Division of Arthritis and Rheumatic Diseases, Oregon Health & Sciences University,
spondyloarthritis ( part II): validation and final selection. Ann Rheum Dis
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
Kaltwasser JP, Nash P, Gladman D, et al. Efficacy and safety of leflunomide in the
Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal
treatment of psoriatic arthritis and psoriasis: a multinational, double-blind,
cardiovascular disease in Europe: the SCORE project. Eur Heart J
randomized, placebo-controlled clinical trial. Arthritis Rheum 2004;50:1939–50.
Antoni C, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy
Egan BM, Lackland DT, Cutler NE. Awareness, knowledge, and attitudes of older
for dermatologic and articular manifestations of psoriatic arthritis: results from the
americans about high blood pressure: implications for health care policy, education,
infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum
and research. Arch Intern Med 2003;163:681–7.
Rachmani R, Slavacheski I, Berla M, et al. Treatment of high-risk patients with
Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis
diabetes: motivation and teaching intervention: a randomized, prospective 8-year
with infliximab: a randomised controlled multicentre trial. Lancet
follow-up study. J Am Soc Nephrol 2005;16(Suppl 1):S22–6.
Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects
Mease P. Update on treatment of psoriatic arthritis. Bull NYU Hosp Jt Dis
of treatment on morbidity in hypertension. II. Results in patients with diastolic blood
pressure averaging 90 through 114 mm Hg. JAMA 1970;213:1143–52.
Sieper J. Developments in therapies for spondyloarthritis. Nat Rev Rheumatol
The Diabetes Control and Complications Trial Research Group. The effect of
intensive treatment of diabetes on the development and progression of long-term
Song IH, Hermann KG, Haibel H, et al. Relationship between active inflammatory
complications in insulin-dependent diabetes mellitus. N Engl J Med
lesions in the spine and sacroiliac joints and new development of chronic lesions on
whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week
Pincus T, Gibofsky A, Weinblatt ME. Urgent care and tight control of rheumatoid
48. Ann Rheum Dis 2011;70:1257–63.
arthritis as in diabetes and hypertension: better treatments but a shortage of
van der Heijde D, Machado P, Braun J, et al. MRI inflammation at the vertebral unit
rheumatologists. Arthritis Rheum 2002;46:851–4.
only marginally predicts new syndesmophyte formation: a multilevel analysis in
Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target:
patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:369–73.
recommendations of an international task force. Ann Rheum Dis 2010;69:631–7.
Eberl G, Studnicka-Benke A, Hitzelhammer J, et al. Development of a disease
Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to
activity index for the assessment of reactive arthritis (DAREA). Rheumatology
target: results of a systematic literature search. Ann Rheum Dis 2010;69:638–43.
Haraoui B, Smolen JS, Aletaha D, et al. Treating Rheumatoid Arthritis to Target:
Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for
multinational recommendations assessment questionnaire. Ann Rheum Dis
assessment of disease activity in psoriatic arthritis. Ann Rheum Dis
Dougados M, Betteridge N, Burmester GR, et al. EULAR standardised operating
Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease
procedures for the elaboration, evaluation, dissemination, and implementation of
activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis
recommendations endorsed by the EULAR standing committees. Ann Rheum Dis
Machado P, Landewe R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score
Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline
(ASDAS): defining cut-off values for disease activity states and improvement scores.
development, reporting and evaluation in health care. CMAJ 2010;182:E839–42.
Ann Rheum Dis 2011;70:47–53.
Schoels M, Braun J, Dougados M, et al. Treating axial and peripheral spondyloarthritis,
Helliwell PS, Fitzgerald O, Fransen J, et al. The development of candidate composite
including psoriatic arthritis, to target: Results of a systematic literature search to support
disease activity and responder indices for psoriatic arthritis (GRACE project). Ann
a consensus statement. Ann Rheum Dis 2013. Published Online First 5 Jun 2013.
Rheum Dis 2013;72:986–91.
Mease PJ. Measures of psoriatic arthritis: tender and swollen joint assessment,
Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in
Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI),
patients with non-radiographic axial spondyloarthritis: results of a randomised
Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index
placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22.
(MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada
Teutsch C. Patient-doctor communication. Med Clin North Am 2003;87:1115–45.
(SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds
Ortendahl M. Shared decision-making based on different features of risk in the
Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality
context of diabetes mellitus and rheumatoid arthritis. Ther Clin Risk Manag
Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of
Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria
Staiger TO, Jarvik JG, Deyo RA, et al. BRIEF REPORT: patient-physician agreement
(PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic
as a predictor of outcomes in patients with back pain. J Gen Intern Med
Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis
Care Res (Hoboken) 2011;63(Suppl 11):S64–85.
Schmieder A, Schaarschmidt ML, Umar N, et al. Comorbidities significantly impact
Rudwaleit M, van der Heijde D, Landewe R, et al. The Assessment of SpondyloArthritis
patients' preferences for psoriasis treatments. J Am Acad Dermatol
International Society classification criteria for peripheral spondyloarthritis and for
spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31.
Schaarschmidt ML, Schmieder A, Umar N, et al. Patient preferences for psoriasis
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis:
treatments: process characteristics can outweigh outcome attributes. Arch Dermatol
development of new criteria from a large international study. Arthritis Rheum
Fajri DW, Brand CA, Dharmage SC, et al. What factors determine patients'
Ritchlin CT, Kavanaugh A, Gladman DD, et al. Treatment recommendations for
preference for tumour necrosis factor inhibitors in ankylosing spondylitis? Clin
psoriatic arthritis. Ann Rheum Dis 2009;68:1387–94.
Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism
Umar N, Schaarschmidt M, Schmieder A, et al. Matching physicians' treatment
recommendations for the management of psoriatic arthritis with pharmacological
recommendations to patients' treatment preferences is associated with improvement in
therapies. Ann Rheum Dis 2012;71:4–12.
treatment satisfaction. J Eur Acad Dermatol Venereol 2013;27:763–70.
Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR
Criswell LA, Such CL, Yelin EH. Differences in the use of second-line agents and
recommendations for the management of ankylosing spondylitis. Ann Rheum Dis
prednisone for treatment of rheumatoid arthritis by rheumatologists and
non-rheumatologists. J Rheumatol 1997;2:2283–90.
van den Berg R, Baraliakos X, Braun J, et al. First update of the current evidence
Gladman D, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical
for the management of ankylosing spondylitis with non-pharmacological treatment
features, course, and outcome. Ann Rheum Dis 2005;64(suppl 2):ii14–17.
and non-biologic drugs: a systematic literature review for the ASAS/EULAR
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol
management recommendations in ankylosing spondylitis. Rheumatology (Oxford)
Stamm TA, Nell V, Mathis M, et al. Concepts important to patients with psoriatic
Khan MA. Ankylosing spondylitis: a dual perspective of current issues and
arthritis are not adequately covered by standard measures of functioning. Arthritis
challenges. J Rheumatol Suppl 2006;78:1–3.
Louie GH, Reveille JD, Ward MM. Challenges comparing functional limitations in
Ward MM, Reveille JD, Learch TJ, et al. Impact of ankylosing spondylitis on work
rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol
and family life: comparisons with the US population. Arthritis Rheum
2009;27(4 Suppl 55):S83–91.
Weisman M, Learch TJ, Baraliakos X, et al. Current controversies in
Taylor WJ, Mease PJ, Adebajo A, et al. Effect of psoriatic arthritis according to the
spondyloarthritis: SPARTAN. J Rheumatol 2010;37:2617–23.
affected categories of the international classification of functioning, disability and
Ritchlin CT. Therapeutic considerations in spondyloarthritis patients who fail tumour
health. J Rheumatol 2010;37:1885–91.
necrosis factor antagonists. Best Pract Res Clin Rheumatol 2010;24:683–92.
Verstappen SM, Watson KD, Lunt M, et al. Working status in patients with
Warram JH, Manson JE, Krolewski AS. Glycosylated hemoglobin and the risk of
rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: results from the
retinopathy in insulin-dependent diabetes mellitus. N Engl J Med
British Society for Rheumatology Biologics Register. Rheumatology (Oxford)
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Clinical and epidemiological research
Averns HL, Oxtoby J, Taylor HG, et al. Radiological outcome in ankylosing
van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in
spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J
patients with ankylosing spondylitis: results of a multicenter, randomized,
double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46.
Kennedy LG, Jenkinson TR, Mallorie PA, et al. Ankylosing spondylitis: the correlation
Song IH, Hermann K, Haibel H, et al. Effects of etanercept versus sulfasalazine in
between a new metrology score and radiology. Br J Rheumatol 1995;34:767–70.
early axial spondyloarthritis on active inflammatory lesions as detected by whole-body
Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other
MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis
comorbidities in patients with psoriatic arthritis: a comparison with patients with
psoriasis. Arthritis Care Res (Hoboken) 2011;63:1729–35.
Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy
Jamnitski A, Symmons D, Peters MJ, et al. Cardiovascular comorbidities in patients
for dermatologic and articular manifestations of psoriatic arthritis: results from the
with psoriatic arthritis: a systematic review. Ann Rheum Dis 2013;72:211–6.
infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum
Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing
spondylitis: a nationwide population-based study. Ann Rheum Dis
Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of
patients with moderately to severely active psoriatic arthritis: results of a
Cresswell L, Chandran V, Farewell VT, et al. Inflammation in an individual joint
double-blind, randomized, placebo-controlled trial. Arthritis Rheum
predicts damage to that joint in psoriatic arthritis. Ann Rheum Dis 2011;70:305–8.
Bond SJ, Farewell VT, Schentag CT, et al. Predictors for radiological damage in
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic
psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6.
arthritis: a proposed objective target for treatment. Ann Rheum Dis
Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic
sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann
Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic
Rheum Dis 2011;70:1369–74.
arthritis using interventional trial data. Arthritis Care Res (Hoboken)
Gladman DD, Mease PJ, Healy P, et al. Outcome measures in psoriatic arthritis.
J Rheumatol 2007;34:1159–66.
Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ
Wanders A, van der Heijde D, Landewe R, et al. Nonsteroidal Antiinflammatory
hybridization techniques in the examination of sacroiliac joint biopsy specimens from
Drugs Reduce Radiographic Progression in Patients With Ankylosing Spondylitis: a
patients with ankylosing spondylitis. Arthritis Rheum 1995;38:499–505.
Randomized Clinical Trial. Arthritis Rheum 2005;52:1756–65.
Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by
Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal
acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med
anti-inflammatory drugs on radiographic spinal progression in patients with axial
spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann
Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial
Rheum Dis 2012;71:1616–22.
spondylarthritis: results from the German Spondyloarthritis Inception Cohort.
van der Heijde D, Landewe R, Einstein S, et al. Radiographic progression of
Arthritis Rheum 2009;60:717–27.
ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis
Kroon F, Landewe R, Dougados M, et al. Continuous NSAID use reverts the effects
of inflammation on radiographic progression in patients with ankylosing spondylitis.
van der Heijde D, Landewe R, Baraliakos X, et al. Radiographic findings following
Ann Rheum Dis 2012;71:1623–9.
two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis
Baraliakos X, Listing J, Brandt J, et al. Clinical response to discontinuation of
anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous
Caperon A, Helliwell PS. Remission in psoriatic arthritis. J Rheumatol Suppl
treatment with infliximab. Arthritis Res Ther 2005;7:R439–44.
Covelli M, Scioscia C, Iannone F, et al. Repeated infusions of low-dose infliximab
Anderson JJ, Baron G, van der Heijde D, et al. Ankylosing spondylitis assessment
plus methotrexate in psoriatic arthritis: immediate benefits are not maintained after
group preliminary definition of short-term improvement in ankylosing spondylitis.
discontinuation of infliximab. Clin Exp Rheumatol 2005;23:145–51.
Arthritis Rheum 2001;44:1876–86.
Heuft-Dorenbosch L, Spoorenberg A, van TA, et al. Assessment of enthesitis in
Brockbank JE, Stein M, Schentag CT, et al. Dactylitis in psoriatic arthritis: a marker
ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32.
for disease severity? Ann Rheum Dis 2005;64:188–90.
Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment
van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic
of existing measures and development of an instrument specific to psoriatic arthritis.
progression in the spines of patients with ankylosing spondylitis treated with
Arthritis Rheum 2008;59:686–91.
adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127.
Helliwell PS, Firth J, Ibrahim GH, et al. Development of an assessment tool for
Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated
dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:
acute-phase reactant levels, and cigarette smoking status predict spinal radiographic
progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388–98.
Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control
Machado P, Landewe R, Braun J, et al. A stratified model for health outcomes in
for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled
ankylosing spondylitis. Ann Rheum Dis 2011;70:1758–64.
trial. Lancet 2004;364:263–9.
Haibel H, Rudwaleit M, Listing J, et al. Efficacy of adalimumab in the treatment of
Verstappen SMM, Jacobs JWG, van der Venn MJ, et al. Intensive treatment with
axial spondylarthritis without radiographically defined sacroiliitis: results of a
methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted
twelve-week randomized, double-blind, placebo-controlled trial followed by an
Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial).
open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91.
Ann Rheum Dis 2007;66:1443–9.
Smolen JS, et al. Ann Rheum Dis 2013;0:1–11. doi:10.1136/annrheumdis-2013-203419
Source: http://www.reumatologia-es.med.br/website/template/uploads/recomenda%E7oes%20alvo%20de%20tto%20espondiloartrites%20jun%202013%20Ann%20Rheu%20Dis.pdf
Mekong River Commission Mekong giant fish species: on their management and biology MRC Technical Paper April 2002 Report prepared by the MRC Fisheries Programme at the request of the Technical Advisory Body on Fisheries Management in the Lower Mekong Basin Published in Phnom Penh in April 2002 by
Brain Research 1010 (2004) 151 – 155 Stimulation of the superior cerebellar peduncle during the development of amygdaloid kindling in rats Carmen Rubioa, Vero´nica Custodioa, Francisco Jua´rezb, Carlos Paza,* a Departamento de Neurofisiologı´a, Instituto Nacional de Neurologı´a y Neurocirugı´a M.V.S., Insurgentes Sur 3877, Mexico 14269 D.F., Mexico b Instituto Nacional de Psiquiatrı´a R.F., Mexico




