Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Doi:10.1016/j.brainres.2004.03.015
Brain Research 1010 (2004) 151 – 155
Stimulation of the superior cerebellar peduncle during the development
of amygdaloid kindling in rats
Carmen Rubioa, Vero´nica Custodioa, Francisco Jua´rezb, Carlos Paza,*
a Departamento de Neurofisiologı´a, Instituto Nacional de Neurologı´a y Neurocirugı´a M.V.S., Insurgentes Sur 3877, Mexico 14269 D.F., Mexico
b Instituto Nacional de Psiquiatrı´a R.F., Mexico
Accepted 2 March 2004
Cerebellar manipulations have been used successfully in some intractable epileptic patients, however, their intrinsic mechanisms are not
fully understood. To further clarify the cerebellar participation in epilepsy, we stimulated 10 rats with 100 Hz, 20 AA at the superior cerebellarpeduncle (SCP) during amygdaloid kindling. Results were compared to 10 rats with an electrode placed at the SCP without stimulation and10 rats without electrodes at the SCP used as control. We found that SCP stimulation increased the theta and alpha rhythms at thecontralateral motor cortex. Such a stimulation produced hypertonicity of the forelimbs and tremor of the head. In this condition, we found thateach of the behavioral stages during amygdaloid kindling in the SCP stimulated rats was reached earlier, while the amygdaloid electrographicafterdischarges (ADs) were longer during the first and shorter in the final trials as compared to controls. Moreover, amygdaloid ADs recordedexclusively during the behavioral stage-5 were significantly shorter than those recorded in the control conditions. We suggest that SCPstimulation could change the customary electrographic and convulsive expression of amygdala kindling in such a manner as to initiallyfacilitate the limbic seizures and impede the secondary generalized seizures.
D 2004 Elsevier B.V. All rights reserved.
Theme: Disorders of the nervous systemTopic: Epilepsy, basic mechanisms
Keywords: Epilepsy; Kindling; Cerebellum; Power spectra; Amygdaloid afterdischarge
scharges (ADs) and the secondary generalized seizuresprovoked by kindling stimulation
Some clinical improvement in epileptic patients submit-
Kindling has been referred to as a progressive develop-
ted to cerebellar electrical stimulation suggests that the
ment of electroencephalographic and behavioral seizures
cerebellum participates in epileptic activity Exper-
produced by repeated application of low-intensity electrical
imentally, cerebellar cortical stimulation in cats results in a
stimulation to discrete forebrain structures resulting in
significant decrease on epileptic like activity produced by
secondary generalized seizures However, it is not clear
systemic or cortical penicillin applications Electri-
how the lengthening of the amygdaloid ADs could be
cal stimulation of the cerebellar dentate nucleus decreases
related to the development of kindling. Nevertheless, the
the epileptic activity produced by systemic penicillin appli-
fact that repetition of the stimulation that initially produced
cation in rats and by cortical penicillin application in
local ADs results in the eventual development of general-
anesthetized cats On the contrary, total cerebellectomy
ized convulsion suggests that progressive recruitment of
magnifies the epileptic activity produced by cortical peni-
related cerebral structures underlies this phenomenon.
cillin stimulation in anesthetized rats and increases the
Moreover, It has also been reported that lesions of the
duration of both the amygdaloid electrographic afterdi-
cerebellum shortened the mean duration of the amygdaloidADs using the kindling stimulation In this view,other sites within the brain would not only contribute in
* Corresponding author. Tel.: +55-56063822x2021; fax: +55-
propagation of seizure activity but would also participate in
E-mail address:
[email protected] (C. Paz).
a reverberatory network that helps to maintain the AD
0006-8993/$ - see front matter D 2004 Elsevier B.V. All rights reserved.
doi:10.1016/j.brainres.2004.03.015
C. Rubio et al. / Brain Research 1010 (2004) 151–155
duration at the site of stimulation. Thus, to further under-
amygdaloid AD was attained. On each subsequent trials, the
stand the cerebellar participation in epilepsy, we assessed
intensity required to generate amygdaloid ADs was applied
the amygdaloid AD during the kindling in rats with changes
daily until 10 stage-5 seizures were obtained. During the
in the EEG power spectra produced by the electrical
search of the amygdaloid response the SCP was not stim-
stimulation of the superior cerebellar peduncle (SCP).
ulated, though once the kindling study began the SCPstimulation was applied from 10 s before amygdaloidstimulation until 10 s after termination of the AD.
Once the best SCP and amygdaloid stimulation param-
eters were determined, amygdaloid kindling was studied in
Thirty male Wistar rats weighing 280 – 310 g were
three different groups: (a) 10 rats with an electrode placed
handled daily and kept under controlled environmental
over the left SCP with no stimulation; (b) 10 rats with an
conditions (20 – 23 jC and 12/12-h light/dark cycle) for at
electrode in the left SCP and electrical stimulation (100 Hz,
least 2 weeks before surgery. Seven days later, the rats were
20 AA); and (c) 10 rats without electrodes at the SCP used as
anesthetized (Ketamine, 100 mg/kg, i.p.) and placed on a
the control group. During kindling, the following parame-
stereotaxic apparatus in order to conduct bipolar electrodes
ters were measured: (a) duration of the amygdaloid AD
for stimulation and recording in the left basolateral amyg-
regardless of the behavioral stage progression; (b) duration
daloid nucleus (coordinates: anterior 6.2 mm, lateral 5.0
of the amygdaloid AD recorded during 10 consecutive
mm, height 1.5 mm using the interaural line as a reference
stage-5 seizures; and (c) the number of trials required to
point), and in the right sensory-motor cortex to record the
reach each of the behavioral stages following Racine's
secondary EEG activity. Twenty rats were chosen for further
criteria Briefly: Stage-1 mouth and facial movements,
electrode implantation over the left SCP (coordinates: ante-
Stage-2 head nodding, Stage-3 forelimb clonus, Stage-4
1.0 mm, lateral 2.0 mm, height 1.5 mm). Electrodes
rearing, Stage-5 rearing and falling.
were led to their loci using the Paxinos' and Watson's
We applied a Student's t-test in those conditions where
stereotaxic atlas Each electrode consisted of two
the absolute power analysis was used with an accepted
twisted insulated wires (0.005-in. diameter) made of stain-
nominal level of significance of p<0.02. The number of
less steel and coated with teflon except for the tips. A screw
trials to reach each of the behavioral stages and the duration
implanted in the skull served as an indifferent source of
of amygdaloid AD during kindling and during 10 stage-5
reference. Electrodes were arranged and soldered to a mini-
seizures were compared between groups using a one-way
connector and secured to the skull with dental acrylic. Skin
analysis of variance (ANOVA) followed by a Tukey test
cuts were sutured while exposing the mini-connector.
with an accepted nominal level of significance of p<0.01.
After 10 days of post-operative recovery, the rats were
The amygdaloid AD duration was analyzed until the 18th
placed in a soundproof chamber while their mini-connectors
trial because some groups were incomplete since some rats
were connected to a 78D Grass polygraph via flexible
reach 10 stage-5 seizures earlier. Once electrographic
cables. Electrographic activity records were stored and
recordings were concluded, rats were deeply anesthetized
digitized in a 486 PC computer provided with software
and intracardiacally perfused with isotonic saline solution
from Stellate Systems that could discriminate between
followed by 10% formalin. After keeping the brains for 30
different bands using fast Fourier transform language.
Electrographic activity was assessed using an absolutepower analysis extracted in three frequency bands as sug-gested by Kubicki et al. (theta, 3.5 – 7.5 Hz; alpha 7.5 –12.0 Hz; beta 12.0 – 30.0 Hz).
Once ordinary electrographic activity was determined,
the stimulation parameters to obtain cerebellar or amygda-loid responses were selected. For this purpose, all rats withelectrodes over the SCP were stimulated at 1-h intervalsusing 0.5-ms rectangular pulses at a frequency of 1, 10 or100 Hz each with 10, 15 or 20 AA until a behavioral orelectrographic changes in the absolute power analysis wasproduced. Amygdala stimulation parameters were deter-mined when an electrographical response was produced(amygdaloid AD). Initially, 0.5-ms rectangular pulses at
Fig. 1. Absolute power bands comparing EEG activity before (white bars)
60 Hz for 1 s with an intensity of 5 V were employed using
and after SCP stimulation (gray bars) extracted in three different frequency
an electronic circuit breaker device which permitted us to
bands: (theta 3.5 – 7.5 Hz, alpha 7.5 – 12.0 Hz, and beta 12.0 – 30.0 Hz).
record and stimulate through the same electrode If a
Values are expressed as means F S.E.M. in AV2 during SCP stimulation
response was not elicited, the intensity of the stimulation
with 100 Hz 20 AA. Statistical differences were analyzed using Student's t-
was increased in 1 V on subsequent stimulation until one
test, *p<0.02.
C. Rubio et al. / Brain Research 1010 (2004) 151–155
Fig. 2. Number of trials required to reach each kindling stage according to
Fig. 4. Ten consecutive amygdaloid ADs recorded during stage-5 seizures
Racine et al. Bars indicate means F S.E.M. for the control group
for the control group (black circles), rats with an electrode over the SCP
(black bars), with an electrode over the SCP without stimulation (gray bars)
without stimulation (gray circles) and rats stimulated with 20 AV at 100 Hz
and with left SCP stimulation (white bars). Statistical differences were
over the left SCP (white circles). Significant differences were found using
evaluated for each stage using a one-way ANOVA with a significance of
the ANOVA test ( p<0.001), though only the SCP stimulated rats showed
p<0.01, followed by a Tukey test within groups *p<0.01.
significant differences within groups using the Tukey test (ap<0.01).
days in 10% formalin, the mesencephalon and pyriform lobe
ioral or electrocorticographic changes with light SCP stim-
were coronally or sagittally sliced in 8-Am serial sections
ulation, though on the whole they showed the clearest
and stained using the Klu¨ver – Barrera technique in order to
response with the highest frequency and intensity of stim-
verify the correct position of the electrodes.
ulation. Therefore, the highest parameters were chosen totest the possible effects of SCP stimulation during thedevelopment of amygdaloid kindling.
We found significant differences between groups in the
number of trials required to reach each of the behavioral
We stimulated the SCP with the highest frequency and
stages. When comparison was done within groups, rats
intensity (100 Hz and 20 AA) and found a significant
stimulated at the SCP required fewer trials to reach each
increased in theta and alpha rhythms In this
of the behavioral stages
conditions, we found that the SCP stimulation elicited alight hypertonicity of the forelimbs in addition to a slightand quick tremor of the head. When the animals moved, gaitwas characterized by ataxia. Some animals showed behav-
Fig. 3. Temporal evolution of the amygdaloid AD duration for the controlgroup (black circles), rats with an electrode over the left SCP withoutstimulation (gray circles) and rats stimulated at the left SCP (white circles).
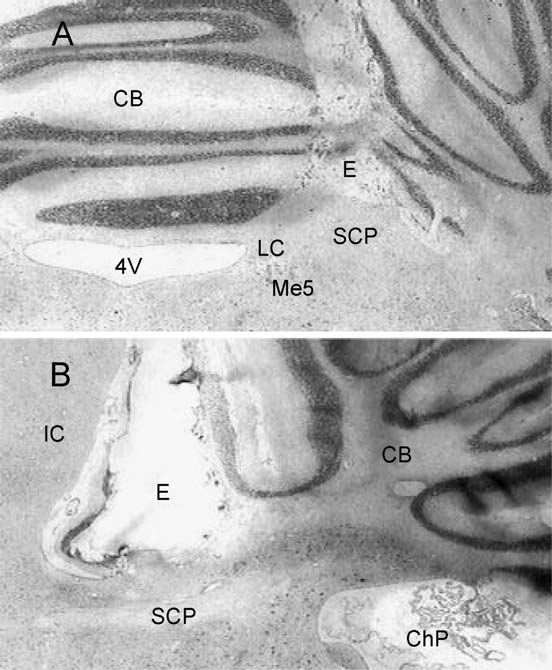
Fig. 5. Low-power amplification of coronal (A) and sagittal (B) sections at
Data are expressed as means F S.E.M. After a comparison between groups
the mesencephalum and cerebellum (CB) showing the track of the bipolar
using the ANOVA test ( p<0.01), rats stimulated at the SCP showed either
electrodes (E) placed on the superior cerebellar peduncle (SCP). Closely
the longest or shortest AD duration when compared within groups using the
situated structures like the locus coeruleus (LC), mesencephalic trigeminal
Tukey test, ap<0.01. Non-stimulated rats also showed shorter AD duration
nucleus (Me5), fourth ventricle (4V), inferior colliculus (IC) and choroids
as compared to control, bp<0.01.
plexus (ChP) are indicated.
C. Rubio et al. / Brain Research 1010 (2004) 151–155
Significant differences were found from the first to the
structures and fibers other than the dentate and interpositus
7th but not the 4th trials ( F2,27=4.94, 4.85, 6.14, 10.54,
forebrain pathways. However, we excluded the possibility
4.75, 5.26; p<0.01) and from the 14th to the 18th trials
that the stimulation of the SCP could reach the midbrain
( F2,27=8.73, 40.01, 64.62, 49.22, 46.48; p<0.01) when
reticular formation, because we found an increase in slow
compared to the amygdaloid AD duration between groups.
wave bands instead of the classical arousal response medi-
Comparisons within groups (Tukey) indicate that the group
ated via reticulo- thalamo-cortical pathway The SCP
of rats stimulated at the SCP showed the longest AD
also includes fibers from the locus ceruleus though we
duration at the 3rd, 5th, 6th and 7th trials, though this group
discarded its possible participation in our results because the
also showed the shortest from the 14th to the 18th trials
stimulation of this structure significantly delays the appear-
when compared to control and non-stimulated groups.
ance of stage-5 seizures, contrary to our findings The
Additionally, rats with electrodes at the SCP without stim-
parabrachial nuclei show a remarkable c-fos expression
ulation during kindling also showed shorter ADs duration at
associated with seizures that occur in genetically epilepsy-
the 15th, 16th and 17th trials when compared to the control
prone rats or produced by thalamic microinjections of
carbachol However, c-fos expression has also been
We found significant differences between groups ( F2,27=
found throughout the brain using these experimental models
37.80, 49.10, 42.07, 70.72, 63.53, 43.79, 9.43, 29.82, 18.01,
of epilepsy and a direct participation of the parabrachial
47.14; p<0.001) when compared to 10 consecutive stage-5
nuclei has not been related to amygdaloid kindling.
seizures. However, only the SCP stimulated rats showed
We found that each of the behavioral stages in SCP
significant differences within groups The EEG
stimulated rats was reached earlier, while the amygdaloid
changes induced in the motor cortex by SCP stimulation
ADs were longer during the first seizures and shorter during
indicate the accuracy of electrode placement, which was
the last as compared to the control group. However, sec-
further confirmed histologically
tioning of the left SCP done 1 week before the beginning ofthe kindling stimulation did not interfere with the typicalstage progression, though the amygdaloid AD durations
recorded in kindled rats were significantly shorter We do not know how the cortical EEG activity could be
Most experimental models of epilepsy show considerable
after the SCP lesions. Nevertheless, we suppose that its
controversy regarding the role of the cerebellum in seizures.
effects on the EEG activity could vary with time and cannot
On account of such differences, we only compared results
be compared to those SCP stimulations applied simulta-
where the kindling model was used. We found that the
neously with the amygdaloid stimulation. Decreases in
lengthening of the amygdaloid ADs and the development of
glutamate, aspartate and GABA were detected on the 7th
the behavioral seizures in rats without electrodes at the SCP
day after cerebellectomy in the thalamus and on the 15th
occurred as have been previously described by others
day in the cerebral cortex and both areas showed a total
However, we found that the mere presence
recovery at the 30th day The participation of the
of an electrode at the SCP without electrical stimulation
cerebellum directly over the cerebral cortex has also been
restricts expression of secondarily generalized seizures. We
studied using the kindling paradigm. Lesions in the lateral
believe that such restriction could be due to a potential
thalamus or the red nucleus and SCP abolished the tonic
damage produced by the electrode as has been previously
component of the seizures produced by neocortical kindling
found in rats with SCP lesions Nevertheless, the most
However, kindling from neocortical areas differed
outstanding effect was found when SCP electrical stimula-
substantially from amygdaloid kindling, specifically the
tion, besides the motor upset, initially facilitated the devel-
ADs remained relatively steady along subsequent stimula-
opment of kindling but later prevented the secondary
tions while behavioral convulsions occurred according to
generalization of epileptic seizures. Similar motor impair-
the stimulated neocortical area
ments have also been reported by electrical stimulation of
The cerebellar dentate and interpositus nuclei innervate
the dentate nucleus or by deep cerebellar lesions
thalamic neurons, which in turn project to the amygdaloid
Then, we hypothesize that the SCP stimulation
complex. It is known that the dentate and interpositus nuclei
could exert a blocking effect over the SCP in such a way
send efferents to the parafascicular and ventromedial tha-
that the dentate and interpositus efferents remain temporally
lamic nuclei Such thalamic nuclei are anatomically
related to the ipsilateral amygdala Thus, SCP stimula-
Several behavioral and electrographic manifestations
tion inducing cortical electrographic changes through their
observed in cerebellar lesions are the result of damage to
thalamic relays could participate in increasing the AD
extra-nuclear structures rather than to the nuclei themselves.
duration and the stage of seizure progression as have been
Lesions of inhibitory pathways, such as those exerted by
observed in our results. It has been suggested that the
Purkinje cells, could lose their inhibitory influence over the
circuits of the peri-amygdaloid cortex might act as a critical
deep cerebellar nuclei and increase their excitatory output.
conduit for limbic seizures which in turn provide access to
The stimulation of the SCP could include closely related
the frontal motor cortex that can drive a convulsive response
C. Rubio et al. / Brain Research 1010 (2004) 151–155
Moreover, the basal ganglia could also redistribute
[11] J.J. Hablitz, Intramuscular penicillin epilepsy in the cat: effects of
epileptic activity from the amygdala to the motor cortex
chronic cerebellar stimulation, Exp. Neurol. 50 (1976) 505 – 514.
[12] J.T. Hutton, J.D. Frost, J. Foster, The influence of the cerebellum in
Then, once epileptic activity reaches the motor cortex,
penicillin epilepsy, Epilepsia 13 (1972) 401 – 408.
the cortical electrographic changes produced by SCP stim-
[13] J. Jansen, On cerebellar evolution and organization from the point of
ulation could modify the AD and the convulsive expression
view of a morphologist, in: R. Llinas (Ed.), Neurobiology of Cere-
of amygdala-kindled seizures. Moreover, the contralateral
bellar Evolution and Development, American Medical Association,
cerebral cortex has been found necessary to reach kindling.
Chicago, 1969, pp. 881 – 893.
[14] M.E. Kelly, D.C. McIntyre, Perirhinal cortex involvement in limbic
Cerebral infarcts produced by ligation of the middle cerebral
kindled seizures, Epilepsy Res. 26 (1996) 233 – 243.
artery increase the number of days required to develop
[15] S. Kubicki, W.M. Herrmann, K. Fichte, G. Freund, Reflections on the
kindled seizures in rats stimulated in the contalateral amyg-
topics: EEG frequency bands and regulation of vigilance, Pharmakop-
dala, but not in the ipsilateral amygdala Therefore, we
sychiatr. Neuro-Psychopharmakol. 12 (1979) 237 – 245.
suppose that SCP stimulation could change the customary
[16] F.J. Madryga, G.V. Goddard, The effects of disconnection of two
phases of kindled frontal-cingulate motor seizures, Exp. Neurol. 86
electrographic and convulsive expression of the amygdala
(1984) 240 – 260.
kindling in such a manner as to initially facilitate the limbic
[17] J.K. Min, P.A. Valentine, G.C. Teskey, Effect of complete and partial
seizures and subsequently impede the secondary generalized
bilateral lesions of the deep cerebellar nuclei on amygdaloid kindling
in rats, Epilepsia 39 (1998) 692 – 699.
[18] S. Mraovitch, Y. Calando, Interactions between limbic, thalamo-
striatal-cortical, and central autonomic pathways during epilepticseizure progression, J. Comp. Neurol. 411 (1999) 145 – 161.
[19] O.L. Ottersen, The afferent connections of the amygdala of the rat as
studied with retrograde transport of horseradish peroxidase, in: Y.
We thank Francisco Gutierrez Baeza for his technical
Ben-Ari (Ed.), The Amygdala Complex, Elsevier, North-Holland,
assistance and Carlos Melendez for reviewing the
1981, pp. 91 – 104.
[20] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates,
Academic Press, Sydney, 1986.
[21] C. Paz, E. Reygadas, A. Ferna´ndez-Guardiola, Amygdala kindling in
totally cerebellectomized cats, Exp. Neurol. 88 (1985) 418 – 424.
[22] C. Paz, F. Gutierrez-Baeza, B. Baza´n-Perkins, Transection of the
cerebellar peduncle interferes with the onset and duration of genera-
[1] F. Cicirata, P. Angaut, M.R. Panto, M.F. Serapide, Neocerebellar
lized seizures induced by amygdaloid kindling, Brain Res. 558 (1991)
control of the motor activity: experimental analysis in the rat. Com-
parative aspects, Brain Res. Rev. 14 (1989) 117 – 141.
[23] R.J. Racine, Modification of seizure activity by electrical stimulation:
[2] F. Cicirata, C. Meli, C. Castorina, M.F. Serapide, V. Sorrenti, C. Di
II. Motor seizure, Electroencephalogr. Clin. Neurophysiol. 32 (1972)
Giacomo, G. Gambera, A. Vanella, Neurotransmitter amino acid lev-
281 – 294.
els in rat thalamus and cerebral cortex after cerebellectomy, Int. J.
[24] R.J. Racine, Modification of seizure activity by electrical stimula-
Dev. Neurosci. 9 (1991) 365 – 369.
tion: cortical areas, Electroencephalogr. Clin. Neurophysiol. 38 (1975)
[3] I.S. Cooper, I. Amin, M. Riklan, J.M. Waltz, T.P. Poon, Chronic
cerebellar stimulation in epilepsy, Arch. Neurol. 33 (1976) 559 – 570.
[25] J.M. Schwartz, C.L. Ehlers, W.M. Detmer, F.E. Bloom, Amygdala
[4] M. Culic, J. Saponjic, B. Jankovic, J. Rakicaponjic, B. Jankovic, L.
kindling after ligation of the middle cerebral artery in the rat, Exp.
Rakic, Effect of cerebellar stimulation on EEG power spectra in the
Neurol. 80 (1983) 484 – 490.
acute model of epilepsy, Indian J. Med. Res. 100 (1994) 135 – 139.
[26] A.A. Shandra, L.S. Goldlevsky, Antiepileptic effects of cerebellar
[5] R. Davis, Cerebellar stimulation for cerebral palsy spasticity, function,
nucleus dentatus electrical stimulation under different conditions of
and seizures, Arch. Med. Res. 31 (2000) 290 – 299.
brain epileptisation, Indian J. Exp. Biol. 28 (1990) 158 – 161.
[6] R. Davis, S.E. Emmonds, Cerebellar stimulation for seizure control:
[27] S.J. Slaght, T. Paz, S. Mahon, N. Maurice, S. Charpier, J.M. Deniau,
17-year study, Stereotact. Funct. Neurosurg. 58 (1992) 200 – 208.
Functional organization of the circuits connecting the cerebral cortex
[7] J.B. Eells, R.W. Clough, J.W. Miller, P.C. Jobe, R.A. Browning, Fos
and the basal ganglia: implications for the role of the basal ganglia in
expression and 2-deoxyglucose uptake following seizures in deve-
epilepsy, Epileptic Disord. 4 (2002) 9 – 22.
loping genetically epilepsy-prone rats, Brain Res. Bull. 52 (2000)
[28] R.S. Snider, A cerebellar-ceruleus pathway, Brain Res. 88 (1975)
379 – 389.
[8] A.Z. Ferrer, A. Fernandez-Guardiola, H. Solis, An electronic circuit
[29] N. Tsuru, H. Kawasaki, S. Genda, K. Hara, H. Hashiguchi, Y. Ueda,
breaker from recording and stimulation from same electrode, Electro-
Effect of unilateral dentate nucleus lesions on amygdaloid kindling in
encephalogr. Clin. Neurophysiol. 45 (1978) 299 – 301.
rats, Epilepsia 33 (1992) 213 – 221.
[9] I.B. Gartside, The effects of cerebellectomy on a penicillin epilepto-
[30] C.H. Vanderwolf, The electrocorticogram in relation to physiology
genic focus in cerebral cortex of the rat, Electroencephalogr. Clin.
and behavior: a new analysis, Electroencephalogr. Clin. Neurophy-
Neurophysiol. 44 (1978) 373 – 379.
siol. 82 (1992) 165 – 175.
[10] G.V. Goddard, D.C. McIntyre, C.K. Leech, A permanent change in
[31] G.K. Weiss, J. Lewis, C. Jimenez-Rivera, Antikindling effects of
brain function resulting from daily electrical stimulation, Exp. Neurol.
locus coeruleus stimulation: mediation by ascending noradrenergic
25 (1969) 295 – 330.
projections, Exp. Neurol. 108 (1990) 136 – 140.
Source: http://rincondepaco.com.mx/rincon/Inicio/Curri/Articulos/2004A_2.pdf
COMMON QUESTIONS ABOUT BIPS A MANUAL FOR NEW AND EXPERIENCED "BIPERS" TABLE OF CONTENTS ♦ What are BIPS? ♦ What are the indications for performing a BIPS study? ♦ What are the contraindications & limitations of BIPS? ♦ How do BIPS compare with other diagnostic methods? ♦ Common questions about the administration of BIPS
DECEMBER 12, 2012 Core Values: If there is content you would like to see covered in future Integrity, Passion for newsletters, please email [email protected] People, Excellence, Strategic Goal Update Vision: My life has meaning My home provides comfort I have friends I make a difference in the lives of others.




