Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Giant species layout 10-04-02.pdf
Mekong River Commission
Mekong giant fish species:
on their management and biology
MRC Technical Paper
April 2002
Report prepared by the MRC Fisheries Programme at the request of the
Technical Advisory Body on Fisheries Management in the Lower Mekong Basin
Published in Phnom Penh in April 2002 by
the Mekong River Commission
This document should be cited as: Mattson, Niklas S., Kongpheng Buakhamvongsa, Naruepon Sukumasavin, Nguyen Tuan, and Ouk Vibol. 2002. Cambodia Mekong giant fish species: on their management and biology. MRC Technical Paper No. 3, Mekong River Commission, Phnom Penh. pp. 29. ISSN: 1683-1489
The opinions and interpretations expressed within are those of the authors
and do not necessarily reflect the views of the Mekong River Commission.
Editor: Ann Bishop
Mekong River Commission
P.O. Box 1112, 364 M.V. Preah Monivong Boulevard
Phnom Penh, Cambodia
Telephone: (855-23) 720-979 Fax: (855-23) 720-972
E-mail:
[email protected]
Website: www.mrcmekong.org
Acknowledgements
This report was prepared under the auspices of the Aquaculture of Indigenous Mekong Fish Species (AIMS) project, which is funded by the government of Denmark (through DANIDA). It was prepared at the request of the Technical Advisory Body (TAB) of the MRC Fisheries Programme, which is composed of members from: the Department of Fisheries, Cambodia; the Living Aquatic Resources Research Center (LARReC), Lao PDR; the Department of Fisheries, Thailand; and the Ministry of Fisheries, Viet Nam. The authors are grateful to the Department of Fisheries, Cambodia, the Department of Fisheries, Thailand, the Living Aquatic Resources Research Center in Lao PDR and the Research Institute for Aquaculture Number 2, in Viet Nam for their contribution in compiling this document.
Background of the Working Group on Mekong Giant Fish Species
The Technical Advisory Body on Fisheries Management (TAB) of the Mekong River
Commission (MRC) was established in June 2000. The TAB gives advice to the
MRC Fisheries Programme on technical issues relating to basin-wide fisheries
management. During the first meeting, five main issues were identified. Among these
was the following:
Management and preservation of the giant fish species of the Mekong
The TAB considered under this item, in particular, the giant catfish, C.
siamensis
and Probarbus spp.
The TAB agreed that action should be taken to
conse rve these species, but the strategy for doing this was not entirely clear. More
research may be needed. Considerable knowledge exists among researchers in the
four MRC countries, but this is not readily available for analysis and for development of a conservation strategy. It was agreed that the MRC Fisheries Programme establish a Working Group on Mekong Giant Fish Species, with participants from the four riparian countries. The Working Group will review and compile existing knowledge on the Mekong giant fish species regarding: important habitats, migrations, biology and life cycles, as well as artificial breeding and results of release of artificially-bred fingerlings, etc. The Working Group may analyse management options and will report to the TAB. The Working Group on Mekong Giant Fish Species consists of one advisor from the MRC Fisheries Programme and one officer from each of the four fisheries departments in Cambodia, the Lao People's Democratic Republic, Thailand and Viet Nam.
Table of Contents
Summary. 1
1. Overview of Giant Fish Species . 3
Introduction . 3
Status of Mekong fish. 4
On rarity and size. 4
Population genetics . 5
Management of the giant Mekong fish species . 6
2. Species synopsis – Catlocarpio siamensis. 9
Natural habitats. 9
Natural food .10
Natural spawning season and spawning habitats .10
Age and size at first maturity .10
Natural growth rate and maximum size .10
Growth rate and culture system .11
Constraints and concerns .12
Characteristics of environments supporting self-sustaining populations.12
Other information.12
3. Species synopsis – Pangasianodon gigas. 13
Natural habitats.14
Natural food .14
Natural spawning season and spawning habitats .14
Age and size at first maturity .15
Natural growth rate and maximum size .15
Growth rate and culture system .17
Constraints and concerns .18
Characteristics of environments supporting self-sustaining populations.18
Other information.18
4. Species synopsis – Probarbus jullieni . 19
Natural habitats.19
Natural food .20
Natural spawning season and spawning habitats .20
Age and size at first maturity .21
Natural growth rate and maximum size .21
Growth rate and culture system .24
Constraints and concerns .25
Characteristics of environments supporting self-sustaining populations .25
Other information.25
5. References. 27
Mekong giant fish species – on their management and biology
The Technical Advisory Body on Fisheries Management (TAB) of the Mekong River Commission (MRC) requested the MRC Fisheries Programme to compile existing knowledge on rare giant Mekong fish species, and to recommend further action for their preservation. This report represents part of the response to the request. It includes biological information as well as management options. The species of main interest, as defined by the TAB, are
Catlocarpio siamensis (giant barb),
Pangasianodon (
Pangasi us)
gigas (giant catfish),
Probarbus jullieni (Jullien's Golden Carp or Seven-Striped Barb),
Probarbus labeamajor and
Probarbus labeaminor. These species grow to a large size, generally over 100 cm in length (except
P.
labeaminor), and in the case of the giant catfish, up to three metres. These giant fishes are becoming rare in the Mekong River, which is under increasing pressure from growing human populations and development. Attempts at saving the wild populations have so far largely focused on captive breeding, or spawning of wild broodstock, and subsequent release of hatchery-reared offspring into the wild. The report attempts to clarify why these species are rare. In general, it has been shown that the proportion of rare fish species increases with maximum size. It is assumed that large species, which breed comparatively late in life, are more vulnerable to fishing and changes in the environment, particularly in terms of fragmentation of the normal habitats (often caused by water-related development projects, such as dams). Although general biodiversity concerns are valid, it appears unlikely that the giant species play a significant role in terms of the functionality and stability of the Mekong River ecosystem as a whole. The river is the home of some 1,200 fish species, and the disappearance of a few already very rare species may not make much difference. However, the giant species can and should be promoted as "flagship species", or ecosystem ambassadors. As such, they may be extremely important for the preservation of the ecosystem as a whole. Thus, it is recommended to put a special effort into promoting these species and saving them from extinction. The report summarises a large amount of biological information on the species of interest, which may be used to further refine culture systems, as well as design studies aimed at describing the life histories of wild populations. The preservation of wild populations will depend on several factors, including decreased fishing pressure, but probably, and most importantly, on other ecosystem functions. Although the preservation of the ecosystem as a whole should be the overall goal, this may not be accomplished easily without the support of public opinion, and it is argued that this may be most easily accomplished by promoting the giant fish species as ambassadors of the Mekong River ecosystem. It is difficult to promote something that cannot be easily illustrated (e.g. an ecosystem), but it should be relatively easy to get public response to photogenic species (e.g. the giant panda of the World Wide Fund for Nature). Conservation efforts should involve a deliberate focus on promoting these species and their habitat, the Mekong River. The management and preservation of ecosystem stability and functions, is a highly complex task, which will have to involve multiple sectors. It is suggested that this may be best accomplished through the MRC Basin Development Plan initiative.

Mekong giant fish species – on their management and biology
Overview of 1 Giant Fish Species
The giant fish species of the Mekong, and particularly those identified by the Technical Advisory Body on Fisheries Management (TAB) of the Mekong River Commission (MRC) can be consider ed "flagship species" in the context of conservation. As such, they deserve special attention since they are potential focal points for awareness raising and education on issues relating to the preservation of Mekong biodiversity and fish production. However, the focus on individual species (which is a common feature of conservation projects) is unlikely on its own to ensure preservation of the ecosystem. Even though preservation of biodiversity in a wider sense is necessary for preserving ecosystem functions, it is not sufficient, since many other factors influence the stability of the ecosystem. It is important to note that the focus on these three species by the working group does not imply that other species are not threatened or worthy of preservation efforts (e.g. Table 1). The Mekong basin is one of the World's most biologically diverse inland waters, and is the home of some 1,200 species of fish (Rainboth 1996). In addition, there are little studied areas of the basin, particularly the upper reaches of the tributaries, where it is likely that further studies will reveal new species. Many of the Mekong species are endemic to the basin.
The release of a tagged specimen of Catlocarpio siamensis, Cambodia
Mekong giant fish species – on their management and biology
This report discusses some issues relating to rarity and the development of policies for management, and the final sections contain species synopses, adapted from Leelapatra et al. (2000). The species synopses contain information on general biology, as well as aquaculture.
Status of Mekong Fish
Of the taxa identified by the TAB, Pangasianodon (Pangasius) gigas (giant catfish) and Probarbus
jullieni (Jullien's golden carp or seven-striped barb) are classified as ‘endangered' on the 2000
International Union for the Conservation of Nature (IUCN) Red List, while Probarbus labeamajor and
Probarbus labeaminor are listed, but classified as ‘Data Deficient'(Table 1). Catlocarpio siamensis
(giant barb) is not on the Red List, but is becoming increasingly rare in the Mekong, and Rainboth
(1996) maintains that it is overfished and suggests that the catch should be strictly regulated by size.
Table 1. Mekong finfish listed in the 2000 IUCN Red List of threatened species
Common name(s)
Size (cm)
Aaptosyax grypus
Botia sidthimunki
Chela caeruleostigmata
Chitala blanci
Royal Featherback
Epalzeorhynchos bicolor
Oreoglanis siamensis
Pangasianodon gigas
Pangasius sanitwongsei
Probarbus jullieni
Jullien's Golden Carp
Seven-striped Barb
Probarbus labeamajor
Probarbus labeaminor
Scleropages formosus
Asian Bonytongue (E)
Tenualosa thibaudeaui
Note: CR: Critically Endangered, DD: Data Deficient, EN: Endangered, LR: Lower Risk,
VU: Vulnerable, EW: Extinct in the Wild (for a full description of the classification,
see http://www.redlist.org/categories_criteria.html)
1.3
On rarity and size
It is relevant here to consider the meaning of the term ‘rare' in the context of biodiversity. While it may be rightly assumed that many fish species are threatened due to human activities, such as over-fishing or alterations to the environment (dams, etc.), species may also be rare for other reasons. For example, some taxa are rare because they are evolving, and others may be relics of very old groups. On an evolutionary time scale, new taxa have always evolved and others disappeared. In fact, from this point of view, most of the species that have ever existed on Earth are extinct. The implication is that even in natural environments (with no perceptible influence from human activities) rare species will be found. Therefore, attempting to preserve all species that are rare or appear to be threatened would be counter-productive. However, the rate at which species disappear has accelerated greatly due to human activities in recent years, and evolution will not produce new species at the same rate.
Mekong giant fish species – on their management and biology
Based on the limited information available, it appears that the population sizes of the three giant
species have decreased substantially over the last decades. They have in common that they grow to
large, even colossal, sizes. Froese and Torres (1999) conclude, from data in FishBase, that the
proportion of threatened fishes increases substantially for sizes exceeding 100 cm, and that most fish
species that grow to this size are threatened. In addition, the available evidence indicates that the non-
guarding species (applies to all five taxa) appear to be more at risk of being threatened, than live
bearers and egg guarders (classification by Balon 1990).
On the assumption that large species in general have lower population densities than small-bodied
species, and also that there is a minimum population size that is required to avoid genetic problems
(see below), it may be argued that larger species require larger areas. This is another possible cause
for the decline of the large species, in that increasing disruption of migration corridors as a result of
construction of dams and weirs means fragmentation of existing habitats and isolation of
sub-populations.
Typically, fisheries tend to first deplete the largest species, and subsequently gradually change the
exploitation pattern to take the smaller-sized fish (Pauly et al. 1998). In general, large-bodied fish
tend to be more susceptible to fishing, partly because of their relative mobility, which increases the
likelihood of their encountering fishing gear. Add to this the preference of most fishers for large,
valuable fish, and the fishery itself appears as a plausible cause of their decline. The situation for the
Probarbus spp. is further aggravated because fishers target them at their spawning grounds.
1.4
Population genetics
Reduced genetic variation causes a decrease in the ability of a population to adapt to and withstand normal environmental challenges. Therefore, for a population to avoid extinction in the longer term, it is essential that appropriate and sufficient genetic variation be maintained. This is particularly an issue when breeding fish for release into the wild. Genetic data on Mekong fish are still very limited, although there are initiatives under way to amend the situation. To properly evaluate alternative actions to preserve the Mekong giant fish species, it is crucial that basic genetic data are made available. According to Meffe (1990), genetic data may be used to:
? assess quantity and spatial distribution of genetic variation ? evaluate historical levels of natural isolation and gene flow among (sub-) populations ? identify unique gene pools for special protection ? resolve taxonomic uncertainties ? choose stocks to release in the wild ? monitor hatchery populations
When considering stocking for enhancement or reintroduction of a population, breeding should aim at optimising the genetic variability in the species (FAO 1997):
? by using as large a breeding population as possible (to increase effective population size) ? by avoiding inbreeding ? by avoiding hybridisation (unless sufficient broodstock of both sexes is not available) ? by avoiding "domestication selection"; that is, avoiding producing an organism that is adapted
to the hatchery instead of nature.
Mekong giant fish species – on their management and biology
Management of the giant Mekong fish species
Any management action aimed at improving the situation of threatened species or reintroduction of extinct species, must start by identification of the possible reasons for rarity. Failing this, efforts aimed at improving or re-establishing populations are likely to fall short. Notably, this implies that stocking aimed at enhancement or reintroduction of a threatened or extinct species should only be considered after the factors that cause rarity or extinction have been alleviated. More likely than not, it will not be possible to address the factors that cause rarity of the giant Mekong species in isolation from the rest of the ecosystem, and development of management policies for their preservation will have to be developed together with the other sectors and users that influence the system. It is suggested that the approach most likely to attain the objective is adaptive (or experimental) management, which implies integration of experiences and scientific information from multiple disciplines into models that attempt to make predictions about alternative policies (see for example, Walters 1997). Successful experimental management and application of its results will require a high degree of coordination between those involved, and this may best be achieved through the MRC Basin Development Plan (BDP) initiative. The BDP is a tool for basin-wide planning which MRC is currently designing in order to ensure that the Mekong's resources are developed in a manner which is equitable, sustainable and has as few environmental consequences as possible. The following recommendations are written in terms of outcomes to be achieved. These will have to be translated into agreements and management plans implemented by the riparian countries. It is assumed that the agreed basin-wide management objective is to restore and maintain viable wild populations of the species considered here, and to maintain the rest of the ecosystem (the recommendations would be different for other objectives).
? Studies and workshops have identified the main cause(s) for rarity, and actions have been
taken to reduce these:
Based on available data and knowledge, one or more models have been created (these may range from simple, verbal models to complex, computerised models).
Hypotheses have been formulated and screened to eliminate those that are unlikely to have given rise to the observed data.
Exper iments have been designed and implemented to test the hypotheses (the experiments may range from the small to the large ecosystem scale. The time factor is an issue: large-scale ecosystem experiments may give more reliable results, but take a long time, whereas small-scale experiments usually give quicker results).
The results of the experiment(s) have been analysed and the main reason(s) for rarity identified.
The results of the experiments have been used to further refine the management system(s) to address the factors that cause rarity.
? At all stages in the process, gaps in data and knowledge will be identified and prioritised, and
sufficient resources assigned to fill the high priority gaps.
? The major sub-populations and their breeding grounds are know n, both in terms of ecology
and population genetics.
? A basin-wide monitoring programme is in place, covering ecology, genetics, and life history
? Relevant data and meta data are stored and made available to scientists and the public.
Mekong giant fish species – on their management and biology
? A breeding and stocking programme is in place.
Broodstock is either obtained from the wild, or maintained in captivity in sufficient numbers and with appropriate genetic profiles
Genetically and otherwise appropriate seed fish are stocked if/when considered relevant.
? Aquaculture (which has different objectives) is developed in parallel, recognising that
cultured populations can contribute to understanding the biology of wild populations, either through simple observation or through specifically-designed experiments.
? Participatory management of the breeding grounds is in place, possibly involving
compensation for lost income to fishers.
Conclusions
It seems likely that the giant Mekong fishes considered here are threatened due to human activities. A set of recommended outcomes, including experimental management, are detailed which may help to identify the factors that need to be managed in order to secure the future of these species. Management aimed at preserving self-sustaining natural populations of the giant Mekong fish species populations will most likely be a subset of management of the aquatic resources of the basin. It is unlikely that efforts to save the wild populations of the giant species will be successful unless an ecosystem approach is used. To accomplish this on a basin-wide scale will require collaboration with other sectors, and this may best be carried out through the MRC Basin Development Plan. It is in this context that the special characteristics of the giant species become apparent; they are very obvious and suitable for catching the imagination and interest of non-specialists among the public, as well as policy makers.

Mekong giant fish species – on their management and biology
Species synopsis
Catlocarpio siamensis
Catlocarpio siamensis is an endemic species in the Mekong River and considered one of the world's largest cyprinid fish. C. siamensis is well known to many older people as it used to form an important fishery.
Catlocarpio
siamensis
Common names: English:
Siamese giant carp
Natural habitats
The species is found in Thailand, the Lao People's Democratic Republic (Lao PDR), Cambodia and Viet Nam in the Mekong Basin. Generally, when young, they reside in shallow flooded areas. As they become bigger, they migrate to deeper pools of the river. In Cambodia, several researchers and fishers have reported that in August, adult fish (40-100 kg) migrate out of the floodplain of the Tonle Sap Great Lake through the Tonle Sap River. The fish reach Chaktomuk, Phnom Penh, in October -November and migrate upstream along the Mekong River to Kratie, ìn Steung Treng Province. Adult C. siamensis have a preference for big pools in the Mekong for at least part of the year (MRC 2001). In Thailand, juveniles (2-6 cm long) are found in three places: Chian Saen (Chiang Rai Province), Tad Phanom (Nakhon Phanom Province) and Khemaratah (Ubol Ratchathani Province). In Cambodia, juvenile fish migrate downstream from Stung Treng to the Tonle Sap Great Lake and small tributaries, while juveniles of 10-12 cm are seen in Muk Kampul (Kandal Province) and in August, juveniles of 20-25 cm are seen in Kampong Kleang (Siem Reap Province). In Viet Nam there are juveniles in Can Tho (Can Tho Province) and Cao Lanh (Dong Thap Province) in the Mekong.
Mekong giant fish species – on their management and biology
Natural food
C. siamensis prefers to feed on algae, phytoplankton and the fruits of inundated terrestrial plants. Eung (1995) reported that C. siamensis will not feed if they are disturbed. In pond or cage, this fish also feeds on: dried fish, corn, soy beans, mung beans (e.g. Vigna sesquipedalis) and rice bran.
Natural spawning seas on and spawning habitats
The natural spawning ground of C. siamensis has not been reported clearly yet. However, Touch
Seang Tana (personal communication 2001) claims, on the basis of recent research, that the spawning
ground of C. siamensis is in deep po ols of the Srepok River between Stung Treng, Mondolkiri and
Ratanakiri Province. According to Eung (1995), the spawning season is in July and August.
2.4
Age and size at first maturity
In earthen ponds, C. siamensis will reach maturity at an age of seven years with a body weight of 9 kg (Sukumasavin 1996), while in the wild, the body weight of spawning fish can reach 60 kg. Generally, the female is bigger than the male, and during the spawning season, the females have abdomens that are more bulging than those of the males (Pinit Sihapitukgiant, personal communication 2000).
Natural growth rate and maximum size
In nature, fish can grow from 2 to 4 kg in eight months (Leelapatra et al. 2000). The maximum length is around 3 m, but more commonly about 1-2 m and 70-120 kg. Nadeesha (1994) reported that some people in Cambodia claim to have seen fish weighing more than 200 kg. Today, fish weighing more than 50 kg are rarely caught.
2.6 Breeding
Brood stock care and maintenance
In the past, breeding of C. siamensis was done by collecting mature fish from the wild. Sukumasavin
(1996) reported that C. siamensis have matured in earthen ponds after seven years, at a weight of 9 kg.
In brood stock ponds, fish weighing 5-20 kg can be stocked at a density of one fish/80-160m2
(Sihapitukgiat 2000). Brood fish are fed at a rate of 2 percent of body weight daily, using pellets or
formulated feed with 40 percent protein content (Meewan et al. 1994). Unakornsawat and Upakarat
(1995) reported the use of water-sprinklers in the brood fish pond during the night for two months
before inducing ovulation.
Breeding techniques tried
Leelapatra et al. (2000), reported that spawning of giant barb can be induced using hypophysation or
gonadotropin hormone-releasing hormone analogue (GnRHA) and dopamine antagonist techniques.
Breeding technique that has been successful
A single injection of GnRHA, in combination with Domperidone and pituitary gland seems to be very
effective for induced spawning of giant barb (Unakorsawat et al. 1990).
Assessment of gonadal stage
The external appearances of the female, such as a large, soft abdomen and swollen genital papilla, can be used to judge the stage of maturity. Males will release milt when pressed gently on the abdomen.
Mekong giant fish species – on their management and biology
Induction of spawning
Among the countries in the region, only Thailand has been successful in artificial breeding. To induce
spawning of giant barb, pituitary gland extract (PG) and human chorionic gonadotrophin (HCG) have
been used to inject brood stock at a dosage of 0.8-1.0 PG/fish + 100 IU HGC/kg and 1.8-2.0 PG/fish
+200-500 IU HGC/kg at 8-hr intervals; fish can be stripped 4- 6 hrs after the second injection. Males
are injected with PG once, at the time of the second injection of the female, at the dosage of 0.5-1.0
PG/fish.
Type of eggs
The eggs are light yellow to dark brown and semi-buoyant (Eung 1995), with an initial size of 1 mm.
They swell to 3 mm after water absorption.
Fecundity
Fecundity depends on the size of the female. One female of 61 kg produced 11 million eggs, while
another of 55 kg had about 5 million eggs.
Egg incubation
After fertilisation, the eggs hatch in 11-13 hrs, at a temperature of 28?-29? C (Nukulluk and
Tangtrongpiros 1975), while Unakorsawat and Upakarat (1995) reported that the eg g could hatch in
20-21 hrs at the same temperature. The eggs can be hatched in a jar, a cement tank or in a hapa (net
suspended in the water for rearing eggs and larvae) with high water flow rate or supplemental
oxygenation.
Size of larvae
The size of the larvae at hatching is about 6 mm. After the yolk sac is absorbed, larvae are fed with
milk of hard-boiled egg yolk for about three days and then transferred to the nursery.
Fry nursing techniques
The fry can be nursed in hapas erected in a pond, or nursed directly in the pond. Before stocking the
fry, ponds are drained, dried out and sprinkled with lime at a rate of 0.06 kg/m2. After 2-3 days,
chicken or cattle manure is applied at a rate of 0.25- 0.38/kg/m2 (Leelapatra et al. 2000). The fertilised
pond is then refilled with water to a level of about 0.5 m through a fine nylon net in order to prevent
insects and wild fish from entering the pond. Adding urea and triplesuperphosphate at 3-5 g/m2
enhances natural feed production.
Once the water in the nursery ponds turns green, the 3-5 day-old larvae can be stocked. The stocking
density of larvae depends on the water quality and size at stocking. Leelapatra et al. (2000), claim that
three day-old fry can be stocked at 500-1000 fry/m2, but Pinit Sihapitukgiant (personal
communication 2000) reported stocking fry at only 30 fish/m2.
The larvae are fed with hard- boiled egg yolk for the first 4-5 days following stocking. Thereafter they
are fed with fine rice bran or water fleas and a mixture of dry fish meal and rice bran. After 30 days
the survival rate may be expected to be about 20 percent.
2.7
Growth rate and culture system
At a stocking density of 0.2 fish/m2 in earthen ponds, fish grew from 100 g to 700 g in the first year,
and to 2 kg in the second year (Leelapatra et al. 2000). Nadeesha (1994) reported that C. siamensis
cultured in southeast Cambodia, could grow from 0.4-0.6 kg to 2 kg in eight months. In Bati station,
C. siamensis cultured in earthen ponds, grew from 25 g to 2 kg within one year. The growth rate of C.
siamensis stocked in polyculture was low compared to other species and mortality was also very high
Mekong giant fish species – on their management and biology
(Nadeesha 1994). The food conversion ratio (FCR) of C. siamensis cultured in earthen ponds at a
stocking density of 0.2 fish/m2, ranged between 3.1 and 3.6 (Eung 1995).
2.8
Constraints and concerns
The main constraint in the breeding of giant barb is the source of spawners. In captivity, the fish need
at least seven years to reach maturity, and therefore the artificial breeding is mainly dependant on wild
brood fish. In Cambodia, the giant barb is rarely caught. The total catch of giant barb declined from
200 tonnes in 1964, to 50 fishes in 1980, and 10 fishes in 2000.
Characteristics of environments supporting self-sustaining populations
In Thailand, the giant barb has been introduced into the river, but there are no reports of recapture
(Nadeesha 1994).
2.10 Other information
Artificial breeding of giant barb will be initiated in the near future at Bati station.
Table 2. Mekong giant barb caught in the Cambodian dai fishery, October-December, 2000
Dai Unit
Weight (kg)
Length (m)
Source: Hozan et al . (2000)
Table 3. The number and weight of giant fishes held at research stations, Cambodia
Stations
Weight (kg)
Pangasianodon gigas
Catlocarpio siamensis
Pangasianodon gigas
Catlocarpio siamensis
Catlocarpio siamensis
Probarbus spp
Source: Ngan Heng, Kat Sokhan and Bun Hay Chheng (personal communication 2001)

Mekong giant fish species – on their management and biology
Species synopsis 3 Pangasianodon gigas
The Mekong giant catfish (Pangasianodon (Pangasius) gigas Chevey 1930) is one of largest
freshwater fish in the world, measuring up to 3 m in length and weighing in excess of 300 kg. It is
endemic to the Mekong basin.
The vernacular name "Pla Buk" has been known to the Thai and Lao people since time immemorial.
The word "Buk" may be derived from "Huk", a term meaning big to the Mekong riparian
communities. The English common name, "Mekong giant catfish" also indicates the largeness of its
body size.
Pangasianodon
Mekong giant catfish
Taxonomically, the Mekong giant catfish is Pangasianodon gigas. It was proposed to change its genus from Pangasianodon to Pangasius (Vidthayanon 1993), although Rainboth (1996) maintains Pangasianodon. The major characteristic that distinguishes the Mekong giant catfish from other members of genus Pangasius is the lack of jaw and vomer dentition. Diagnosis: The eye situated below the level of the mouth corner in adults; mouth terminal, with a prominent lower jaw; teeth and gill rakers absent in fish larger than 500 mm standard length (SL). The upper limb of gill arches 1/15- 1/12 is shorter than the lower. The pelvic fin ray count is 8-9. A single -chambered swim bladder runs along the abdominal cavity. Description: The body is robust, the snout truncated or round, and the head equal to 14.3-21 percent SL. The body colour is silvery grey dorsally and pale ventrally. The juvenile body colour is dusky dorsally, and silvery ventrally with two lateral dark stripes.
Mekong giant fish species – on their management and biology
Natural habitats
The Mekong giant catfish is a freshwater fish, and has not been caught in the brackish water estuary
of the Mekong River. The known habitat of this species is the main stream where the water depth is
10 m or more. The fish particularly prefers rocky or gravel substrate, and sometimes underwater
caves.
Natural food
After the yolk sac has been absorbed, the hatchlings are fed zooplankton (Cyclops, Moina, Daphnia)
for two weeks. The fry are cannibalistic (Pholprasith 1983).
When the fish is one year old, it shifts feeding mode and becomes herbivorous (Pookaswan 1969;
Jensen 1997a and 1997b). Adults feed on filamentous algae, but probably also ingest insect larvae and
periphyton. The lack of dentition on the jaws and vomer area has led fishery biologists to believe that
the fish feeds on algae growing on submerged rocky substrates (Pholprasith 1983).
3.3
Natural spawning season and spawning habitats
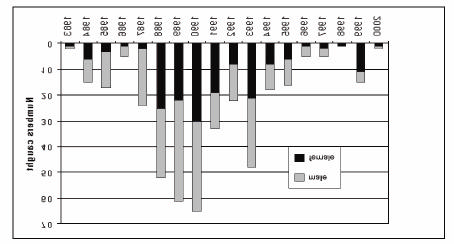
The natural spawning season of Mekong giant catfish is from late April to mid-May. The locations of the spawning grounds of the Mekong giant catfish are poorly known. One well-known spawning site is in the mainstream of the Mekong River northward from Chiang Khong in northern Thailand (Pholprasith and Tavarutmaneegul 1997), where mature specimens have been caught annually during the spawning season (Figure 1). Durand (1940) collected juveniles in the lower Mekong in Cambodia, including a 125 mm specimen from Bac-Lieu; two specimens of 150-185 mm from Kompong Cham; one specimen of 285 mm from Chau-Doc; one specimen of 420 mm from Peam Chikang; and one of 450 mm from the Tonle Sap. The specimens were all caught in 1937-38, and are the smallest naturally- occurring specimens recorded. Thongsaga and Pholprasith (1991) reported that local fishers saw mating behaviour in the spawning grounds at Chiang Khong (above Khone Falls) and the Tonle Sap Great Lake (below Khone Falls).
Figure 1. Number of giant catfish caught at Chiang Khong District,
Chiang Rai Province, Thailand.
Source: Pholprasith and Tavarutmaneegul 1997.
Mekong giant fish species – on their management and biology
Age and size at first maturity
The wild spawners that were caught in the Mekong River near Chiang Rai Province between 1984
and 1990, were estimated to be 6-8 years old, and with a body weight of 150- 250 kg. (Pholprasith and
Tavarutmaneegul 1997). Mature females are larger than the males. Phayao Inland Fisheries Station
(Phayao IFS, 2000), Thailand, reported that P. gigas that had been cultured from the juvenile stage in
earthen ponds finally reached maturity after 15 years, at a body weight of 40-50 kg and a body length
of about 160 cm. The fish were induced to spawn, but the embryos failed to develop beyond the
somite stage.
3.5
Natural growth rate and maximum size
The Mekong giant catfish is one of the fastest growing freshwater fish species in the world. The maximum recorded size of the Mekong giant catfish is 300 kg and 3 m in length (Smith 1945). A Mekong giant catfish (230 cm and 135 kg) was caught at Nong Khai in November 1967. Measurements of annuli in the dorsal spine and the fifth vertebral centra of this fish indicated that it was six years old and that it grew slowly in the first year, faster in the second, and fastest in the third year. Thereafter, it grew more slowly (Pookaswan 1969). In natural habitats, it is reported to grow to 150-200 kg in 3-5 years, or 20-30 kg/year (Vidthayanon 1993). In captivity, fry averaging 13 cm and 17 g grew to an average of 40 cm and 620 g in only four months. This was a 400 percent increase in weight (Roberts and Vidthayanon 1991). Length/weight relationships are described by the following equations (Pholprasith 1995):
W = 1.54217L 1.49797
Female: W = 0.69364L 1.62173 Male and female (sex combined): W = 1.10196L1.54835
The Mekong giant catfish was released into reservoirs in Thailand. Data on recapture indicate that
they could grow up to 100 kg (Pholprasith and Tavarutmaneegul 1997). However, there is no report
on the age of the fish.
3.6
B reeding
Brood stock care and maintenance
At present, induced breeding of the Mekong giant catfish relies mainly on wild spawners caught in the
Mekong River near Chiang Rai in northern Thailand.
Breeding techniques tried
In May 1983, the first successful artificial breeding of wild Mekong giant catfish adults caught in the
Mekong River near Chiang Rai produced some 200,000 fry, but the survival rate was very poor
(Roberts and Vidthayanon 1991).
New ways to propagate the species have been tried in Thailand under the Mekong giant catfish
artificial breeding programme. However, very few individuals are available from the natural
environment each year.
Using cryopreservation, spermatozoa of Mekong giant catfish males were successfully preserved in
liquid nitrogen, retaining fertilising capacity for up to 3-4 months, with a fertilisation rate of around
65 percent (controls: 73 percent) (Mongkonpunya et al. 1995).
Mekong giant fish species – on their management and biology
After 18 months, the fertilisation rate was 67.7 ± 7.1, while controls were 79.0 ± 1.4 percent
(Pholprasith 1995).
Breeding techniques which have been successf ul
The Thai Fisheries Department has had a project for breeding the Mekong giant catfish since 1981.
The first successful artificial breeding of wild spawners caught in the Mekong River was reported in
1983. Since then, successful breeding has been achieve d every year, except in 1986 and 1998.
Initially, hypophysation techniques were used. Since 1992, GnRHa (gonadotropin hormone-releasing
hormone analogue) and Domperidone (dopamine antagonist) have been used successfully. The first
successful breeding using captive broodstock was carried out in 2001, at Phayao Inland Fisheries
Station, Thailand. The broodstock was from the 1984 spawning season. The female and male that
were induced to spawn weighed 54 and 35 kg, respectively (Phayao IFS 2001).
Assessment of the gonadal stage
External appearance cannot be used to judge the gonadal stage. Size and colour of eggs and position
of nucleus (germinal vesicle) obtained by cannulation has been used effectively. Fish that have
uniform size of eggs of yellow-brown colo ur, or that have 40 percent of eggs at the germinal vesicle
migration stage, have been used for induced spawning.
Hypophysation
The Mekong giant catfish can be induced to spawn using the hypophysation method. Pituitary
glands (PG) of common carp, rohu, striped catfish or Chinese carp may be used either fresh or in
acetone-preserved form. Pholprasith (1983) reported that three intraperitoneal injections of PG at
dosages of 0.4, 1.4, and 0.5/fish, at 8-hr intervals, induced spawning in wild broodstock. The second
and third injection was given 6-12 and 5-10 hrs after the first and second injections, respectively. Fish
can be stripped 5-11 hrs after the third injection.
Kuchareonpisarn et al. (1985) used two injections of PG and HCG to induce wild Mekong giant
catfish to spawn. The fish were injected with PG at the dosage of 0.7/fish, and HCG at the
concentration of 1,000 IU/kg for the first injection. After 9-10 hrs, a mixture of PG at 2.4-2.5/fish, and
HCG at the concentration of 3,500-4,000 IU/kg, was injected. The fish was stripped 11-12 hrs after
the second injection.
Gonadotropin-releasing hormone analogues and dopamine antagonist
GnRHa, in combination with dopamine antagonist, has been used to induce the Mekong giant catfish
to spawn since 1988. This method has proven to be very effective and reliable. Ovaprim (20 mg/ml
D-Arg6, Trp7, Leu8, Pro9 Net-salmon GnRHa, in combination with 10 mg/ml Domperidone) and
Buserelin are GnRHas that have been used to induce spawning of the Mekong giant catfish.
Domperidone is the only dopamine antagonist that has been used in combination with GnRHa. The
most effective dosage is 10 mg/kg of Buserelin, in combination with 10 mg/kg Domperidone for the
first injection, and 20 mg/kg Buserelin in combination with 10 mg/kg Domperidone at a 9 hr interval.
Fish can be stripped 12-25 hrs after the second injection (Pholprasith and Tavarutmaneegul 1997).
Type of eggs
Adhesive eggs, yellowish in colour with a diameter of 1.7 mm.
Fecundity
The fecundity of females of 1.5-2.0 m SL, is about 500,000- 2,000,000 eggs (Vidthayanon 1993). A 178-kg female was found to have 13.5 kg of eggs. Each kg contained 800,000 eggs (Pholprasith and Tavarutmaneegul 1997).
Mekong giant fish species – on their management and biology
Egg incubation
The egg of the Mekong giant catfish is of the adhesive type. Two incubation methods have been used: ? Fertilised eggs are washed with water until they start to stick, and then spread onto a nest made of
plastic branches and allowed to stick on the branches.
? Fertilised eggs are treated to remove the sticky layer on the egg surface. Then, eggs are incubated,
as semi-buoyant eggs, in containers such as hapas or Weis jars, which are aerated with airflow or running water.
Size of larvae
At 25º C, hatching occurs 42 hours after fertilisation (Pholprasith and Tavarutmaneegul 1997). The
newly-hatched larvae are 3.8 mm long. At two days, they are 8.45 mm, at seven days 13.4 mm, and at
eleven days, they are 28 mm (Roberts and Vidthayanon 1991).
Fry-nursing techniques
The fry of Mekong giant catfish can be nursed in
Figure 2. P. gigas larvae a: newly hatched larva;
cement tanks or earthen ponds.
b: 2-days old; c: 7-days old; d: 11-days
Cement tanks are suitable for nursing the
hatchlings of the Mekong giant catfish up to day
seven. The tank can be either rectangular or
circular in shape. Stocking density is around
4,000 fry/m2. Running water and aeration should be supplied at all times. To prevent cannibalism,
live feed (Moina) must be given 6-8 times per day
at a rate of 60 g of wet weight per 5,000 fry/day.
However, the expected survival rate for nursing
in cement tanks is only 3- 6 percent.
Earthen nurser y ponds are usually around 800-
1,600 m2. The pond must be limed and dried for
three days. Cow or pig manure is used as fertiliser
at the rate of 250g/m 2. Stocking density is around
18-20 fry/m2 for 5-6 day- old fry. During the first
three days, only Moina is given at 2 kg wet weight/800 m2 pond. On the 4th day, Moina is
reduced to 1 kg/day/pond, and trash fish are fed
every 6 hrs at the rate of 0.6g/m 2 pond. On the
seventh day, trash fish are replaced by 27 percent
protein powder feed and Moina is reduced to 1 kg every three days. The survival rate of fry ranges
from 35-77 percent in earthen ponds.
Growth and culture system
Mekong giant catfish can grew to 3 kg in the first
year, and to 8 kg in the second year when 125
fish were stocked in 1,000 m2 earthen ponds
(Pholprasith and Tavarutmaneegul 1997).
In net pens, with a stocking density of one fish
per 0.5 m2, they can grow up to 1.3 kg within
Drawn by A. Termvichakorn.(Roberts and
seven months (Pholprasith et al. 1992).
Vidthayanon 1991).
Mekong giant fish species – on their management and biology
In culture experiments in 1000 m2 earthen ponds at Chiang Rai Inland Fisheries Station, fish fed on 20, 25 or 30 percent protein diets grew to 3.93 kg within a year. The final body weight of fish cultured for 29.5 months was 9.96-10.61 kg (Pholprasith et al. 1992). The average growth rates (Pholprasith 1995):
? Fed on 20 percent protein diet = 433.50g/month.
? Fed on 25 percent protein diet = 456.83g/month.
? Fed on 30 percent protein diet = 459.66g/month.
3.8
Constraints and concerns
The main constraint in breeding of the Mekong giant catfish is the source of the spawner. At present,
induced spawning of the Mekong giant catfish relies on the availability of wild spawners, and the
number of the spawners that are caught each year is declining rapidly. In 1998, no mature females
were caught. It is very important to determine how to obtain maturity in captivity.
Recently, giant catfish that have been reared in captivity (Phayao IFS, 2000) and introduced into
reservoirs throughout Thailand, have shown signs of maturity. There is an urgent need to study the
technique for induced spawning of these fish in order to replace the wild spawners from the Mekong
River.
3.9
Characteristics of environments supporting self-sustaining populations
Recently, Mekong giant catfish have been released into rivers and reservoirs throughout Thailand and
Cambodia. There are reports of the Mekong giant catfish being caught in reservoirs every year, with
body weights exceeding 100 kg. This indicates that the Mekong giant catfish can grow to their normal
size in reservoirs. However, to date none of the fish caught in the reservoirs had mature gonads.
3.10 Other information
The giant catfish is a flagship species that highlights the need to protect threatened species and limit
habitat loss. The giant catfish is also a potentially valuable aquaculture and commercial aquarium/zoo
species. Even so, the wild populations of Mekong giant catfish are declining rapidly and the species
may go extinct in the near future (Hogan 1998).
In Cambodia, the Mekong giant catfish in an incidental catch of the Tonle Sap River bagnet fishery
(Hogan et al. in press). According to local fishermen, a few giant catfish are caught each year. In
1999, 2000 and 2001 four fish, eleven fish and seven fish respectively, were caught, including two
fish in 2001 that weighed less than 20 kg (Zeb Hogan, personal communication, 18 March 2002).
Beginning in 2000, a buy and release project was established in Cambodia to study and protect wild
Mekong giant catfish (Hogan et al. in press). In the short term, the release of wild fish may decrease
the probability of extinction of the species. In the long term, tagging and genetic studies can be used
to define migratory pathways and population genetic structure, as well as help safeguard wild
populations from unintentional introgression with genetically homogenous cultured stock (Hogan et
al. in press).
Ultimately, the management and protection of this important species must involve a coordinated
conservation effort between all countries of the Mekong River basin, since the giant catfish occurs in
Cambodia, Thailand, Lao PDR, and perhaps China and Viet Nam (Hogan et al. in press).

Mekong giant fish species – on their management and biology
Species synopsis 4 Probarbus jullieni
Probarbus
Common names: English:
Jullien's golden carp, seven-line barb
Pla yi sok
Cá trà sóc
Roberts (1992) reports that there are two other species of Probarbus in the Mekong River i.e., P. labeamajor and P. labeaminor. Both are endemic to the Mekong Basin (Rainboth 1996). In Thailand, there is very little information about P. labeamajor and P. labeaminor.
Natural habitats
P. jullieni is a riverine species found in Thailand, Cambodia and Malaysia . It prefers deep, clear water with sand or gravel substrate and abundant mollusc populations. In Thailand, the fish is reported from the Chao Phraya River, the Pasak River, the Mae Klong River, the Kwai Noi River, the Sei Yok River and the Mekong River. Natural populations appear to have been eliminated from several of the Thai rivers. Dams, weirs and barrages are a particular threat to this migratory species. It is no longer seen in large numbers, and listed as "Endangered" on the IUCN Red List. P. labeamajor and P. labeaminor are listed as Data Deficient" (Table 1). At present, it is not known whether the fish that occur in the Mekong River and the Mae Klong River are genetically the same or not. Although techniques for determining the genetic relationship between fish populations are available, the fact that stocks of P. jullieni have been transferred from the Mekong River to the Mae Klong River and its tributaries, may make it difficult to conclude on this issue.
Mekong giant fish species – on their management and biology
Natural food
The Jullien carp has a superior-oblique and protractile mouth, with pharyngeal teeth. It is a night-time
feeder that consumes aquatic weeds, small molluscs and crabs, aquatic insect larvae and zooplankton.
Amatyakul et al. (1995) reported on an 80 cm fish with the stomach full of bivalves and concluded
that the fish is an omnivore, with a preference for molluscs.
4.3
Natural spawning season and spawning habitat
The spawning season of the fish in the Kwai Noi River is from December to January; while in the
Mekong River, th e fish spawn from December to March (Amatyakul et al. 1995). The spawning
ground has a depth of 0.5-2.0 m, with flowing water and stone or gravel substrate. The spawning
grounds of the fish in the Kwai Noi River were south of the Sei Yok Yai waterfall at Ban Yang Ta,
Ban Wong Pra, Ban Ta Poo, Ban Pra Lom, Ban Kao, Ban Ta Kilen, Ban Loom Suom and Ban Wong
Po in Sei Yok district (Amatyakul et al. 1995). However, these areas have been changed due to the
construction of Kao Laem reservoir.
In the Mekong River, the main spawning ground of the fish is in Nong Khai Province (Figure 3).
However, recently two new spawning grounds were identified in Loei and Mukdahan Provinces.
Details of the spawning grounds, and the number of spawners caught are shown in Table 4. The catch
sites in Loei and Mukdahan have been reported since 2000. In the year 2000 spawning season, the
main spawning ground was in Mukdahan, while in 2001, the main spawning ground was in Chiang
Khan, Loei. It is thought that changes in the direction of the current causes a change in the spawning
habitat, i.e. the current can change the bottom substrate from gravel to sand, which is not suitable for
spawning, so the fish change to a new spawning ground. Also, it is believed that if fishing with
explosives occurs in an area, the fish will not return to spawn there again.
The Thai Department of Fisheries has carried out induced breeding of wild broodstock since 1974.
Fry have been introduced to many reservoirs such as Ubolratana, Lampao, Bhumipon, Sirikit, and
Srinakarin (Amatyakul et al. 1995).
Figure 3. Numbers of P. jullieni caught at spawning grounds in Nong Khai Province
Source: Nong Khai Inland Fisheries Station, personal communication, 2001.
Mekong giant fish species – on their management and biology
Age and size at first maturity
The smallest mature female reported from Kwai Noi River weighed five kg (Plangchawee et al. 1987). Mature males usually weigh 5-20 kg, while females weigh 10-50 kg (Amatyakul et al. 1995). The age of wild mature fish is unknown, but mature broodstock from earthen ponds are more than five years old; males weigh 2-7 kg, while females weigh 5–15 kg (Rodrarung and Jensirisak 1990). The sex ratio (male : female) of the fish is 1:1.59 in the Mekong River (Plangchawee et al. 1987). External sexual characteristics of the fish are clear and can be used to separate males from females longer than 80 cm, as indicated in the following table.
1. Elongated body and smaller than the female.
1. Relatively shorter and rounder than the male.
2. Genital papillae is oblong and small and has a pink
2. Genital papillae is round and big.
papillae plate around the genital.
3. Has more pearl spots on the operculum and the body
3. Has fewer pearl spots than the male.
than the female.
Natural growth and maximum size
Under natural conditions, growth in terms of total length of the Jullien carp has been summarised as follows (Meesawat 1973):
Approximate
Length (cm)
January – February
The maximum size of the Jullien carp is reported to be 86 cm in total length (Smith 1945), 100 cm
(Rainboth 1996) and 126 cm (Amatyakul et al. 1995).
Mekong giant fish species – on their management and biology
Table 4. Catch of P. jullieni at spawning grounds in Thailand.
No. Male Female Wt/L(kg/cm) Wt/L(kg/cm)
7-15/60-80 12-38/75-120
6-15/60-80 15-35/80-115
11-16/60-90 14-42/80-130
Note: (a) No record of total catch; only those that were injected were reported. (b) No record of total catch;
only those that were injected were reported. The total fish landing was about 100.
Source: Loei Inland Fisheries Station, personal communication, 2001
Mekong giant fish species – on their management and biology
B reeding
Breeding techniques tried
Hypophysation and gonadotropin releasing hormone analogue and dopamine antagonist techniques
have been successfully used to induce spawning of the Jullien carp.
Breeding technique that has been successful
Gonadotropin releasing hormone in combination with a dopamine antagonist, Domperidone, seems to
be very effective in inducing spawning in the Jullien carp.
Spawner
In the past, wild broodstock that were collected from the Mekong River during the spawning season
were used for induced spawning. Since 1990, fish cultured in earthen ponds have been used as
broodstock. Males and females can easily be sexed during the spawning season because males release
milt through gentle pressure on the abdomen.
Assessment of the gonadal stage
External appearances, such as a large, soft abdomen and swollen genital papilla, can be used to judge
the maturity of the gonadal stage. Mature females that are suitable for hormonal induction can be
selected by cannulation, look ing for germinal vesicle migration to the periphery of the oocytes
(Amatyakul et al. 1995).
Induction of spawning
Hypophysation
Srithongsuk and Yoovechwattana (1975) injected wild broodstock with pituitary gland extract of
Jullien carp at dosages of 0.7-1.0 and 1.4-2.0, at 6-8 hr intervals. Eggs can be stripped 5-8 hrs after the
second injection. Amatyakul et al. (1995) showed that pituitary gland from stripped catfish
(Pangasianodon hypophthalmus) and HCG can also be used to induce spawning of wild Jullien carp.
The dosages are one dose of PG and 50 IU HGC/kg HCG and two doses of PG and 100 IU HGC/kg
HCG at 6-8 hr intervals. Eggs can be stripped 11 hrs after the second injection.
Gonadotropin-releasing hormone analogues and dopamine antagonist
Buserelin, in combination with Domperidone, can also be used to induce the Jullien carp to spawn
(Rodrarung and Jensirisak 1990). The dosages are Buserelin (10 ? g/kg), in combination with
Domperidone (10 mg/kg) and 6-8 hr later followed by Buserelin (30 ? g/kg) in combination with
Domperidone (10 mg/kg). Eggs can be stripped 4- 8 hrs after the second injection. However,
Suppasansanee et al., (2000) indicated that the most effective dosage for inducing spawning in P.
jullieni is 10mg/kg Buserelin in combination with 5 mg/kg Domperidone for the first injection and
common carp pituitary gland at 1.5 dose1 per fish in combination with 30IU/kg Gonadotroplex, 8 hrs
apart. The fish can be stripped 4-5 hrs after the second injection.
Type of egg
Eggs are of the semi-buoyant type, but slightly heavy and adhesive. The diameter of the egg is about 2 mm, which swells to about 3 mm after water absorption. Hatching time for the fertilised egg is around 72 hrs at 23? C (Amatyakul et al. 1995).
1 dose = body weight of donor fish/body weight of recipient
1 dose = body weight of donor fish/body weight of recipient
Mekong giant fish species – on their management and biology
Fecundity
Suppasansanee and S ukumasavin (in press) report that broodstock with an average weight of 3.82 kg
(3.0-5.5 kg), which are used in induced spawning at Kalasin Inland Fisheries Station, have around
45,000 eggs.
Egg incubation
After fertilisation, the eggs must be washed several times with clean water to remove the sticky
material from the eggshell. Subsequently, the eggs can be incubated in funnel jars with high flow rates
to avoid the eggs sticking to the jar (Amatyakul et al. 1995).
Size of larvae
At hatching, the total length is about 0.8 – 0.9 cm (Amatyakul et al. 1995).
Fry-raising techniques
Two days after hatching, the yolk sac is absorbed, and the larvae start feeding. The larvae can be nursed in hapas suspended in concrete tanks. Fry must be fed 3-5 times/day with hard-boiled egg yolk dissolved in water for three days. Subsequently, fry can be transferred into earthen ponds of 800-1,600 m 2. The pond is prepared by liming it at 38-63g/m2 and drying it for 3-5 days. The pond is filled to about 40-50 cm depth with water filtered through a fine-mesh nylon net in order to prevent other fish from entering the pond. Natural feed is enhanced weekly by adding urea and triplesuperphosphate at 3-6g/m2. The five day-old fry are released into the pond at a density of 31-62/m2. They are fed with rice bran, fishmeal and soybean meal at a ratio of 9:6:5, and supplemented with Moina 2- 3 times daily. After a 30-50 day nursing period, the larvae reach 2-5 cm with a 50-80 percent survival rate (Amatyakul et al. 1995).
Growth and culture system
According to Unakornsawat et al. (1990), the Jullien carp reaches 19-25 cm and 83-190 g after 8 months in earthen ponds, which is similar to the growth in wild fish (Amatyakul et al. 1995). Growth and production of the Jullien carp cultured in 800 m 2 earthen ponds for 5-6 months and fed with 25-30 percent protein at different initial sizes and stocking densities are summarised as follows (Amatyakul et al. 1995):
Initial size
Densities
Final size
Production
The length (TL, cm)-weight (g) relationships are as follows (Amatyakul et al. 1995). Fish with a total length of between 3-24 cm:
log W = -2.1992 + 3.2131 log TL
Fish with a total length of 80 –126 cm:
log W = -1.1615 + 2.6504 log TL
log W = -2.1357 + 3.2024 log TL
Mekong giant fish species – on their management and biology
Constraints and concerns
The main constraint in the breeding of Jullien carp is the source of the spawner. At present, the
broodstock must be at least five years old to reach maturity in captivity.
4.9
Characteristics of environments supporting self-sustaining populations
No information.
4.10 Other information
The Jullien carp has a great potential for aquaculture. The technique for induced spawning has already
been developed, but information for broodstock culture needs to be further developed.
It appears that the information regarding Probarbus in Thailand only refers to P. jullieni. We do not
appear to have any information about P. labeaminor or P. labeamajor. There are unconfirmed reports
that the spawning peak of P. labeamajor is a few months earlier than P. jullieni.
Srithongsuk and Yoovechwattana (1975) reported that if during the spawning season, both male and
female fish develop black stripes from chin to pectoral fin, chin to pelvic fin and chin to abdomen,
then fishers consider the fish to be P. labeaminor.
Mekong giant fish species – on their management and biology
Amatyakul, C., K. Kohanantakul, C. Sansrimahachai, S. Sumanochitraporn, P. Sripatrprasite, R.
Wongsongsarn, C. Wongsongsarn, J. Juntana, D. Rodrarung & N. Yoorong. 1995. Jullien carp Probarbus jullieni Sauvage. Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand, 51 pp.
Balon, E.K. 1990. Epigenesis of an epigeneticist: the development of some alternative concepts on
early ontogeny and evolution of fishes. Guelph Ichthyology Reviews, 1990 (1):1- 48.
Jensen, J.G. 1997a. Report from Chiang Khong: five Giant catfish in 1997. Catch and Culture, May
Jensen, J.G. 1997b. Giant catfish breeding: keep cool and wait for the right female. Catch and
Culture, August 1997 (3) 4.
Chevey, P. 1930. Sur un nouveau Silure geant du bassin du Mekong, Pangasianodon gigas. Bulletin
de la Société zoologique de France, 1930. 55: 536- 542.
Durand, J. 1940. Notes sur quelques poissons d'especes nouvelles ou peu connues des eaux douces
Cambodgiennes. Institute oceanographique de I'Indochine, Nhatrang, 36: 1-40, plates 1-7.
Eung, K. 1995. Evaluation of the suitability of Catlocarpio siamensis in small-scale aquaculture
systems. Fisheries Faculty, The Royal University of Agriculture, Phnom Penh. (BSc graduating paper)
FAO. 1997. FAO technical guidelines for responsible fisheries. Aquaculture development. No. 5,
Froese, R. & A. Torres . 1999. Fishes under threat: an analysis of the fishes in the 1996 IUCN Red
List. In: R.S.V. Pullin, D.M. Bartley & J. Koiman, Editors. Towards policies for conservation and sustainable use of aquatic genetic resources. p. 131-144. ICLARM Conference Proceedings 59, Penang, Malaysia. 277 pp.
Hogan, Z. 1998. The quiet demise of the Mekong giant catfish. Wildlife Conservation, 1998, 101:12.
Hogan Z., N. Pengbun, & N. van Zalinge. In press. Status and conservation of two endangered fish
species, the Mekong giant catfish Pangasianodon gigas and the giant carp Cathlocarpio siamensis, in Cambodia's Tonle Sap River. Natural History Bulletin of the Siam Society 49.
Kuchaleonpisan, N., M. Suphacarat, C. Puhrakkiet, V. Chatchavanchaiyaphun & P. Arayawattanvej.
1985. Artificial breeding of Pla Buk (Pangasianodon gigas Chevey). In: Annual Report 1984-85, p. 40-48. Phayao Fisheries Station, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand.
Leelapatra, W., P. Srisakultiew & N. Sukumasavin. 2000. Biology and breeding of indigenous
Mekong fish species in Thailand. Management of reservoir fisheries in the Mekong Basin II. In: Component Report No. 2, p. 83-86. Mekong River Commission, Phnom Penh.
Meesavadth, V. 1973. Surveying the species of fingerling fishes which were caught by the big lift- net
at Nam Mong, Nong Khai Province. In: Annual rep ort 1973, p. 33-66. Nong Khai Fisheries Station. Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand.
Mekong giant fish species – on their management and biology
Meewan, A., D. Radrawang & S. Phanna. 1994. Progress report on breeding of captive broodfish of
Pla Kaho. In: Annual Report 1992-1994, p. 33-66. Lop Buri Inland Fisheries Station. Ayuthaya Inland Fisheries Department Center, Inland Fisheries Division, Ayuthaya, Thailand.
Meffe, G.K. 1990. Genetic approaches to conservation of rare fishes: examples from North American
desert species. Journal of Fish Biology, 1990, 37A: 105-112.
Mekong River Commission. 2001. Fish Migration and Spawning: Version 1, Mekong Mainstream.
An interactive CD. Mekong River Commission, Phnom Penh.
Mongkonpunya, K., T. Chairak, T. Pupipat & T. R. Tiersch. 1995. Cryopreservation of Mekong Giant
Catfish sperm. Asian Fisheries Science, 1995. (8): 211-221.
Nadeesha, M. C. 1994. Fishes of the Mekong River - conservation and need for aquaculture. Reported
from Naga. ICLARM Quarterly, 1994. 17 (4): 17-18.
Nong Khai Fisheries Station. 1997. Survey and collecting the fry and fingerling of Probarbus jullieni
Sauvage at Nong Khai Province. In: Annual report 1974, p 38-46. Nong Kai Fisheries Station, Aquatic Animal Husbandry Division, Department of Fisheries, Thailand.
Nukulluk L. & M. Tangtrongpiros. 1975. Induced spawning by pituitary injection of Pla Kaho,
Catlocapio siamensis. Thai Fisheries Gazette, 1975. 28(2): 183-194.
Pauly, D., V. Christensen, J. Dalsgaard, R. Froese, & F. Torres Jnr. 1998. Fishing down marine food
webs . Science 279: 860-863.
Phayao IFS. 2000. Result on induced spawning trial of the giant catfish in captivity. In: Annual
Report, p. 70-78. Phayao Inland Fisheries Station, Phrae Inland Fisheries Development Center, Inland F isheries Station, Department of Fisheries, Thailand.
Phayao IFS, 2001. Induced spawning of giant catfish Pangasianodon gigas from broodstock
permanently reared in earthen ponds. Phayao Inland Fisheries Station, Department of Fisheries, Thailand.
Pholprasith. S. 1983. Induced spawning of the Mekong giant catfish. Thai Fisheries Gazette. 36(4):
Pholprasith, S. & P. Tavarutmaneegul. 1997. Biology and culture of the Mekong giant catfish,
Pangasianodon gigas (Chevey 1930). Extension Paper No. 31. National Inland Fisheries Institute, Bangkok. 79 pp.
Pholprasith, S. 1995. Biology and economic studies on the Mekong giant catfish Pangasius gigas
(Chevey, 1930). Department of Fisheries, Bangkok. 27 pp.
Pholprasith, S., M. Benjakarn and R. Ritthaporn. 1992. Development of commercial culture of the
Mekong giant catfish. Technical Paper No. 14, Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives, Bangkok. 51 pp.
Plangchawee, V., V. Toopoocha, O. Nakjinda & K. Sahartsanon. 1987. Spawning ground survey of
the Jullien Carp in Kwai Noi River, Karnjanaburi Province. In: Annual Report 1987, p 16-22. Kanchanaburi Fisheries Station. Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand.
Pookaswan, T. 1969. Pangasianodon gigas Chevey. Inland Fisheries Division, Department of
Fisheries, Bangkok, 12 pp.
Rainboth, W. J. 1996. Fishes of the Cambodian Mekong. FAO species identification field guide for
fisheries purposes. FAO, Rome.
Roberts, T. R. 1992. Revision of the Southeast Asian Cyprinid fish genus Probarbus, with new
species threatened by proposed construction of dams on the Mekong River. Ichthyology Exploration of Freshwaters, 1992. 3 (1): 37-48.
Mekong giant fish species – on their management and biology
Roberts T. R. & C. Vidthayanon, 1991. Systematic revision of the Asian catfish family Pangasiidae,
with biologic observations and descriptions of three new species . In: Proceedings of the Academy of Natural Sciences of Philadelphia, United States of America. 143:97-144.
Rodrarung, D. & S. Janesirisak. 1990. Induced spawning of earthen pond reared Jullien carp. In:
Annual Report 1990, pp. 114-117. Nongkai Inland Fisheries Station. Udonthani Inland Fisheries Development Center, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Nongkai, Thailand.
Smith, H. M. 1945. Freshwater fishes of Siam or Thailand. US Govt. Printing Office, Washington,
Srithongsuk, C. and T. Yoovechwattana. 1975. Breeding of the Jullien carp. Technical Paper No. 14,
Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Bangkok. 25 pp.
Sukumasavin, N. 1996. Induced spawning of giant carp, Catlocarpio siamensis, from the brood stock
permanently reared in earthen pond. Thai Fisheries Gaz ette, 1996. 49 (1): 23-26.
Supasansanee, C., N. Sukumasvin & T. Fathep. 2000. Induced spawning of the Jullien Carp,
Probarbus jullieni, using broodstock permanently reared in earthen ponds. In: Annual Report 2000, p 33-43. Kalasin Inland Fisheries Station. Inland Fisheries Division, Department of Fisheries, Kalasin, Thailand.
Thongsaga, S. & S. Pholprasith. 1991. Some aspects on the biology of the Mekong giant catfish
Pangasianodon gigas (Chevey). In : Proceedings of the 29th Kasetsart University Annual Conference (Fisheries), p 499-511. Kasetsart University, Bangkok, Thailand.
Unakornsawat, Y. & N. Upakarat. 1995. Induced spawning of giant carp, Catlocarpio siamensis
Boulenger. In: Annual report 1992-1995 (in Thai), Lampang Freshwater Fisheries Station. Cha ingmai Freshwater Fisheries Development Center, Freshwater Fisheries Division, Chaingmai, Thailand.
Unakornsawat, Y., S. Janesirisak & D. Rodrarung. 1990. Comparison of growth of Jullien carp fed
with 3 different feeds. In: Annual Report 1990, p 86- 97. Nong Khai Inland Fisheries Station. Udornthani Inland Fisheries Development Center, Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives, Nongkai, Thailand.
Vidthayanon C.1993. Taxonomic revision of the catfish family Pangasiidae. Tokyo University of
Fisheries, Japan. (Ph. D. thesis)
Walters, C. 1997. Challenges in adaptive management of riparian and coastal ecosystems.
Conservation Ecology [online]1(2):1. Website: http://consecol.org/vol1/iss2/art1.
Source: http://www.mrcmekong.org/assets/Publications/technical/tech-No3-mekong-giant-fish-species.pdf
An-Najah National University Faculty of Graduate Studies Fate of Oxytetracycline & Doxycycline in Soil & Underground Water Lama Sameeh Mohammad Awartani Supervised By Dr. Shehdeh Jodeh Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Chemistry, Faculty of Graduate Studies, at An-Najah National University, Nablus, Palestine
4 - Chemoprophylaxis 4.1Principles. 33 Chloroquine plus proguanil Atovaquone plus proguanil 4.3Dosage tables. 4.4Country tables. 4.5Popular destinations. 4.6Emergency Standby 4. Chemoprophylaxis Given the possibility of antimalarials purchased in the tropics being fake24, travellersshould obtain the medication required for their chemoprophylaxis from a reputablesource in the UK before they travel. ACMP also advises against purchasing antimalarialdrugs over the internet.








