Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Untitled
Laser Treatment of Cutaneous
Vascular Lesions
Mitchel P. Goldman
Laser treatment of cutaneous vascular lesions began with
keep the laser energy within the thermal relaxation time
Dr Leon Goldman in 1963 at the Children's Hospital
of the blood vessel.
Research Foundation in Cincinnati, Ohio, with the treat-
The latest advance was the development of the intense
ment of port-wine stain (PWS) and cavernous hemangioma
pulsed light (IPL) devise in 1993. This therapeutic modal-
using ruby, neodymium : yttrium-aluminum-garnet ity did not use a single wavelength of light, but a broad(Nd:YAG), and argon lasers. His initial report of 45 patients
spectrum of visible light where the lower portion was
treated with these three lasers appeared in 1968 and stim-
cut off to limit the wavelength to various band-widths
ulated great interest in this new treatment modality.1
between 515 nm and 1000 nm. The output was pulsed in
Beginning in 1970, early pioneering work in argon laser
single, double or triple pulses of 1.5–20 ms with delays
surgery was accomplished at the Palo Alto Medical Clinic
between the pulses to allow for epidermal cooling. IPL
by Apfelberg, Maser, and Lash2 for treatment of cutaneous
technology, which at first was stated by the ‘experts' as
vascular lesions. By 1984 the argon laser was generally
being both dangerous and ineffective is now adopted by
accepted as the treatment of choice for PWS.3 Indeed,
every laser manufacturer and laser surgeon and is the single
before the development of the argon laser, no effective
most popular technology for the treatment of vascular
form of treatment could be recommended to patients.
lesions word-wide. This chapter reviews current laser and
The next major advance was the development of the
IPL technologies in the treatment of vascular lesions. Other
flashlamp pumped pulse dye laser (PDL) in the mid-1980s
chapters specifically discuss the treatment of leg veins, pig-
to treat PWS. This laser was developed to encompass the
mented lesions, photorejuvenation and light activation of
theory of selective photothermolysis. This theory states
photosensitizers: photodynamic therapy.
that a specific laser wavelength and energy be delivered to vaporize a specific target. The target was oxygenatedhemoglobin in the red blood cell in the blood vessel of the
PWS. The most important factor was to keep the thermaldamage within the blood vessel's 20–50-mm diameter
Importance of Pulse Duration
through the use of a pulsed laser output of 350 msec, whichhas been expanded to 450 msec then 1500 msec and now up
To limit thermal damage to the intended target, the pulse
to 40 ms. The original wavelength chosen was 577 nm,
duration must be shorter than the thermal relaxation time
which soon changed to 585 nm and then 595 nm to allow
of the target tissue (Tables 2.1 and 2.2). The thermal relax-
both deeper penetration as well as more efficient use of the
ation time of tissue is defined as the time necessary for
excitation dye between the flashlamps of the laser. Finally,
target tissue to cool down by 50% through transfer of its
techniques providing epidermal cooling and copper vapor
heat to surrounding tissue through thermal diffusion. If a
lasers emitting a wavelength of 577 nm were also devel-
targeted tissue can be heated sufficiently to affect it
oped to target oxygenated hemoglobin. The use of a scan-
irreversibly before its surrounding tissue is damaged by
ning hand-piece for use with non-pulsed lasers was used to
thermal diffusion, selective photocoagulation occurs.4,5
Cutaneous and Cosmetic Laser Surgery
Classification of Vascular Malformations
Capillary Malformation (CM)
Port-wine stain
Sturge–Weber syndrome
Lymphatic Malformation (LM)
Venous Malformation (VM)
Arterial Malformation (AM)
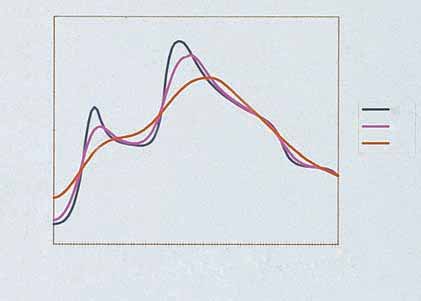
Figure 2.1
Temperature distribution achieved during treatment of
four different-size vessels with PDL. Two 0.05-mm-diameter vessels
are located at 0.4-mm and 0.5-mm depths. A large 1-mm-diametervessel is located at a depth of 1.1 mm; a medium-sized 0.4-mm-
diameter vessel is located at a depth of 0.6 mm. Treatment
Klippel–Trenaunay syndrome (CLM, VM)
conditions: 585-nm wavelength, 6-mm-diameter spot size, 10-J/cm2
Parkes–Weber syndrome (CLM, VM, AM)
fluence. (Reprinted with permission from Goldman MP, Eckhouse S
et al: Dermatologic Surgery: Official Publication for American Society
Maffucci syndrome (LM, VM, enchondromas)
for Dermatologic Surgery 22:323, 1996. With permission from
Solomon syndrome (CM, VM, intracranial AM,
Blackwell Publishing Ltd.)
epidermal nevi, osseous defects, tumors)
Riley–Smith syndrome (LM, VM, macrocephaly,
Table 2.2
Thermal Relaxation Time (Tr) of Laser Targets
Bannayan syndrome (AM, LM, VM, macrocephaly,
Diameter (m
m, approx)
Proteus syndrome (CM, VM, macrodactyly,
hemihypertrophy, lipomas, pigmented nevi, scoliosis)
Modified from Mulliken JB The classification of vascular
birthmarks. In: Tan OT, ed. Management and treatment of
benign cutaneous lesions. Philadelphia: Lea & Febiger; 1992.
than 20 ms result in vessel rupture and hemorrhage sec-
Table 2.1
Approximate Thermal Relaxation Time (Tr)
ondary to RBC explosion.6 This will lead to hemosiderin
for Vessels of Different Diameters
pigmentation. Therefore, with single laser pulses, the ther-
apeutic window is small. This argues for the developmentof a wider single pulse or a multipulsed laser that is able
to transfer absorbed heat to the endothelium without
causing its rupture (Figs 2.1 and 2.2).
Extension of the pulse duration of the PDL from
0.45 ms to over 1.5 ms limits purpura without decreasing
efficacy. The immediate purpuric threshold increases from
6.2 to 8, 10.4, and 13.8 J/cm2 with an increase in the pulseduration from 0.5 to 2.0, 20 and 40 ms.7 Even though a
decrease in purpura can be obtained by extending the pulseduration to 10 ms, patients are still erythematous for 3–4
Data from Anderson RR, Parrish JA. Lasers Surg Med 1981;
days and posttreatment edema is present to a similar extent.7
Importance of Wavelength
For vascular lesions, the exposure time should be long
enough to conduct heat from the red blood cell (RBC)-
The theory of selective photothermolysis is the basis for
filled lumen to the entire blood vessel wall. The thermal
the development of the PDL with a wavelength of 577,
relaxation time of vessels 10 to 50 mm in diameter is 0.1 to
585 or 595 nm to increase penetration into the dermis
10 ms, averaging 1.2 ms.4,5 However, pulse durations less
without loss of vascular specificity.8 Although blood
Laser Treatment of Cutaneous Vascular Lesions
surface usually cools to about -30°C, whereas the tempera-ture of the epidermal basal layer will not drop below 0°C.13This allows for higher fluences to be given without adverseepidermal effects. The result of epidermal cooling and
higher laser fluences increases efficacy, allows deeper laser
penetration, and minimizes treatment pain.14,15
One potential problem is the effect on the cryogen spray
on laser light. One study on 594 and 785-nm light demon-
strated a 3% decrease in light transmission after a 30-ms
pulse and a 30-ms delay before the laser pulse.16
Other methods for cooling the epidermis are air cooling,
contact cooling through a quartz or sapphire crystal or
topical cold gel or ice. The cold air cooling devise com-
monly used, SmartCool or Cryo 5 (Zimmer Elektromedi-
zin, Ulm, Germany) generates a continual air current of
500–1000 L/min at a minimal temperature of -30°C. Thelevel of airflow can be regulated. At a cooling level of 6
Figure 2.2
Temperature distribution achieved during treatment of
(1–6 scale), a temperature of -15°C has been measured at
four different-size vessels with IPL. Two 0.05-mm-diameter vessels are
the skin surface after 8 seconds of cooling.17 In addition,
located at 0.4- and 0.5-mm depths. A large 1-mm-diameter vessel is
the air transport system can be modified with an adapter
located at a depth of 1.1 mm; a medium-sized 0.4-mm-diameter
so that the stream of cold air hits the skin exactly at the
vessel is located at a depth of 0.6 mm. Treatment conditions: 590-nmcutoff filter, double pulse of 5 and 10 ms, 150-ms delay between
laser impact site. A study on 166 patients treated with a
pulses, irradiance of 55 J/cm2. (Reprinted with permission from
variety of lasers for hair removal, tattoo removal and the
Goldman MP, Eckhouse S et al: Dermatologic Surgery: Official
treatment of vascular abnormalities were treated with air
Publication for American Society for Dermatologic Surgery 22:323,
cooling or ice pack cooling or chilled tip cooling; 86% of
1996. Blackwell Publishing Ltd.)
patients felt that the cold air cooling was better than othercooling methods.18 The analgesic effect was better in 37%
absorbs 585-nm light about one-half as efficiently as it
of patients than ice gel. There was also a reduction in
absorbs 577-nm light,9 585-nm light will coagulate larger
erythema and purpura of 70% and 83% respectively.
vessels better than 577-nm light at a given depth because
However, contact cooling has certain advantages in
of deeper penetration of laser energy. In addition, deeper
comparison to air cooling. As a physician you can use the
vessels absorb laser energy at longer wavelengths.10
contact cooling handpiece to compress the skin up to a
The tissue depth to which a given fluence will coagulate
certain degree. By doing this, the amount of blood in the
the target vessel depends largely on the blood volume of
vein to be coagulated can be adjusted and optimized. This
vessels above the target vessels. Superficial vessels contain-
procedure can reduce pain and result in good coagulation.
ing blood will absorb laser light before it reaches deeper
In this connection it is important to have a handpiece
target vessels. This explains why multiple treatments are
that provides parallel cooling through a cooled sapphire
necessary for complete PWS resolution.
window as well as long post cooling through an integrated
Because blood also has an absorption peak at 532 nm,
cooled metal piece. The handpiece of the long-pulsed
the frequency doubled Nd : YAG laser is also effective in
Nd : YAG laser MYDON from WaveLight is the only device
treating superficial vascular lesions. However, to obtain a
that features not only these options but also has a remov-
degree of selectivity over the high melanin absorption
able cooled sapphire window. The advantage of this possi-
at 532 nm and to penetrate to clinically useful depths,
bility is remarkable when it comes to treating fine vessels
cooling the overlying epidermis is important.
where you need optimal vision at the treatment area. Addi-tionally, it also avoids the blood disappearing completely
Effect of Epidermal Cooling
by the pressure of the contact cooling when treating thesevery small veins.
Cooling the epidermis has been shown to increase the
The effect of epidermal cooling to enhance clinical effi-
depth of penetration of effective thermocoagulation.11,12
cacy has been shared by other therapeutic modalities, par-
Addition of the pretreatment epidermal cooling allows
ticularly the IPL and long-pulsed Nd : YAG systems described
treatment to occur at higher energy fluences (9–10 J/cm2
later in this chapter and hair removal lasers and IPLs.
versus 6–7 J/cm2 without cooling) without increasing theincidence of scarring or pigmentary changes.
The Beckman Laser Institute developed the concept of
Lasers Commonly Used to
delivering a cryogen spurt of tetrafluorethane (boiling point
Treat Vascular Lesions
-26.2°C) just before laser impact. They term this ‘dynamiccooling'. The Candela Laser Corporation and CoolTouch
Flashlamp-pumped Pulsed Dye Lasers
Corporation have proprietary rights to this technology. Skinsurface temperature is reduced by as much as 40°C with a
Tan et al's successful treatment of children with PWSs
20- to 80-ms cryogen spurt after PDL exposure. The skin
established the safety and efficacy of the PDL.19 Garden
Cutaneous and Cosmetic Laser Surgery
Figure 2.3
Beam profile of Candela SPTL-1 from laser head. It
exhibits Gaussian-like distribution of energy with some irregularities.
Figure 2.4
Cynosure PhotoGenica V laser from laser head. Like the
(Reprinted from Jackson BA, Arndt KA, Dover JS. Journal of the
Candela SPTL-1 (see Fig. 2.3), it exhibits Gaussian energy distribution
American Academy of Dermatology 34:1000, 1996, with permission
with irregularities. (Reprinted from Jackson BA, Arndt KA, Dover JS.
from the American Academy of Dermatology.)
Journal of the American Academy of Dermatology 34:1000, 1996,with permission from the American Academy of Dermatology.)
and others20–22 further refined the treatment parametersand expanded the use to lesions in adults. This clinical effi-
spot size of the two lasers were tested, the Candela laser
cacy has been confirmed by other authors,23–29 and the use
spot size was up to 35% larger than 5 mm while the Cyno-
has been expanded to include the early treatment of cap-
sure laser was up to 8% smaller. Therefore it is prudent to
illary hemangiomas as well as many different cutaneous
check the diameter of the spot with burn paper before
lesions that have a vascular component.30–32
switching from one PDL machine to another.
Three manufacturers produce this type of laser. Candela
Corporation (Wayland, MA) manufactures the SPTL line of machines, which originally emitted a wavelength of
585 nm and now can emit wavelengths of 590, 595, and
600 nm. The original pulse duration was 450 ms and nowcan be increased to 40,000 ms. The beam profile can be
Doubling the frequency and halving the wavelength to
circular at 3, 5, 7, 10 and 12 mm in diameter or elliptic
532 nm has made the 1064 nm Nd : YAG laser more poten-
at 2 ¥ 7 mm. Maximal energy fluences of 10 or 20 J/cm2
tially useful in the management of vascular lesions. This
are available. The Candela machine uses cryogen spray
laser is produced by Continuum (CB Diode/532), and other
cooling. Another PDL is the PhotoGenica V with a 585 nm
laser companies. The 532-nm wavelength is at one of the
wavelength and the Photogenica V-Star with a 595 nm
hemoglobin peaks for 50-mm superficial blood vessels.
wavelength and similar spot sizes and power as the Candela
This allows some selectivity in treating vascular lesions.
machine manufactured by Cynosure, Inc. (Chelmsford,
However, its shorter wavelength does not allow deep
MA). Currently produced Candela systems, according to
penetration. A preliminary study using the Con-Bio
their website, include the Vbeam (6 J output, 595-nm, 0.45-
Laser (Continuum) at fluences ranging from 1 to 6 J/cm2
to 40-ms, with 5-, 7-, 10-, 3 ¥ 10-mm handpieces) and
(maximum 10 ns, 150 mJ) in mice and rabbit ear veins
Cbeam (6 J output, 585-nm, 0.45-ms, 5-, 7-, 10-mm hand-
showed a depth of coagulation of 0.73 ± 0.44 mm and hem-
pieces). Cynosure currently produces the PhotoGenica V-
orrhage of 0.68 ± 0.41 mm. In addition, edema (blistering)
Star (8 J output, 585- or 595-nm, 0.5- to 40-ms, with 7-, 10-,
was observed at all fluences tested with mild subcutaneous
12-mm and elliptical handpieces and PhotoGenica V (4 J
fibrosis and epidermal hypertrophy. Therefore this laser
output, 585-nm, 0.45-ms, with 7- and 10-mm handpieces).
can produce vascular injury, but because of its interaction
Currently, no manufacturer produces a multi-wavelength
with epidermal melanin, it is relatively nonselective.
dye laser. Both the Candela Sclerolaser and the CynosureVLS multi-wavelength systems have been discontinued,
Potassium Titanyl Phosphate (KTP)
although they may be available on the used market. The
Cynosure PDL uses cold air cooling. The third company isDEKA (Florence, Italy), which produces the Dermobeam at
LaserScope (San Jose, CA) has developed a modulated KTP
595 nm with similar spot sizes and pulse durations as the
laser that uses an arc lamp running at a constant current
other PDL. DEKA uses an integrated cooling system.
pulsed to a much higher current than the lamp can toler-
The PDL beam profiles may be different between laser
ate under current operation. This produces an average
companies. With the Candela PDL a 10% to 20% over-
power up to 160 W packaged into a single pulse that can
lapping spot provides for an even distribution of energy
be adjusted to pulse durations of 1 to 100 ms at pulse rates
fluence. This is because of the Gaussian distribution of
that are adjustable from one to ten pulses per second. Ten
beam output. An 18% overlap has been found to cover the
watts of power are available, with peak power of up to
largest surface area with the least overlap.33 In contrast, the
60 W for pulse widths of 1 to 50 ms. The fluence is deliv-
Cynosure PDL has a ‘top hat' distribution of energy fluence
ered through a bare fiber 1–5 mm in diameter, which can
(Figs 2.3 and 2.4).34 In addition, when the 5-mm-diameter
be connected to various scanning delivery systems.

Laser Treatment of Cutaneous Vascular Lesions
The KTP lasers are used in three modes when treating
directly correlated with the energy fluence, so that if
vascular lesions. In one mode the laser is used with the
a higher fluence is needed, the pulse duration is longer.
Dermastat (LaserScope), which applies a spot size to the
The Palomar Palmalite/Prolite features advanced pulse-
skin varying in size from 0.1 to 2.0 mm in diameter.
forming. The target tissue experiences the pulse burst as
The maximum average laser power is 5 W, and the pulse
continuous. The first third of the pulse train with higher
duration can be varied in the range of 0.1 to 1.0 s. The 1-
power is said to heat up the target tissue with the lower
mm spot size at 5 W with a pulse duration of 0.2 s gener-
power last third of the pulse train maintaining the target
ates a fluence of 127 J/cm2 on the skin. Higher fluences can
tissue temperature.
be selected by using longer pulse durations or smaller spot
The fluence is delivered through a quartz or sapphire
sizes. These high energies may not be appropriate for treat-
light guide with a spot size/surface area of 8 ¥ 15 to 15 ¥
ing PWS in children or pink superficial lesions.
35 mm (Figs 2.6–2.8). This spot size can be further mini-mized by covering the surface area with any opaque covering, such as white paper. All settings are computer
Intense Pulsed Noncoherent
controlled to deliver the desired energy to a flashlamp. Theexact wavelength spectral output is proprietary.
The ability to pulse the intense light rapidly within the
thermal relaxation time of the target vessel allows an accu-
The PhotoDerm VL is the original intense pulsed light
mulation of heat to occur within the target vessel with dis-
source emitting a continuous light spectrum with most of
sipation of heat within the epidermis (Figs 2.9 and 2.10).
its energy fluence between 515 and 1000 nm. Xenon-filled
The use of a cool gel on the skin surface and/or a cooled
flashlamps are the primary light source with the lamps
crystal that touches the epidermis provides epidermal pro-
powered by a capacitor bank. The lamps are cooled by
tection to heat generated by the light output (Fig. 2.11).
water which surrounds the lamps and helps in cutting
The ability of the epidermis to cool more quickly than the
down longer infra-red emissions. The intense pulse light
target vessel is a function of the vessel size. When one com-
source produces incoherent light whose spectrum can be
bines the longer wavelength, longer pulse duration, larger
cut off through the use of colored filters. Filters in standard
spot size, and ability to deliver multiple pulses within the
use are 515, 550, 560, 570, 595, 610, 645, 695 and 755 nm.
thermal relaxation time of the target vessel, treatment effi-
These filters block out the shorter wavelengths, allowing
cacy is enhanced. Multiple sequential pulsing is a propri-
the energy fluence to be concentrated up to 1000 nm with
etary technology of Lumenis. All other IPL devises use
resulting deeper penetration of the high-intensity pulsed
variations of increasing fluence with pulse duration and
light (Fig. 2.5). Different manufacturers use different
various methods of epidermal cooling to selectively heat
absorbing filters to cut-off the lower wavelengths. Palomar
blood vessels.
uses a fluorescent filter to shift the wavelengths to the right
The BBLTM intense pulsed light is available as a module
preserving the 800–950-nm band (this is said by the
for the ScitonProfile platform or as a stand-alone system
company to help with dermal heating).
(Sciton, Sunnyvale, CA). BBL has the widest single pulse
The pulse durations of the Lumenis IPLs can be adjusted
width of presently available IPLs and can also deliver
from 2 to 25 ms/pulse given as a single, double, or triple
double or triple pulses. An integrated thermo-electric
pulse with delays between pulses of 2 to 100 ms. The total
cooled sapphire crystal cools the treatment area and can
energy fluence emitted can range from 3 to 90 J/cm2. Other
control skin temperature to within 1°C during an entire
IPL devises do not have this degree of variability. Mostsystems have only one or two pulse durations that are
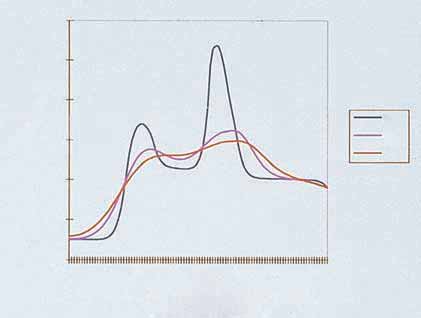
Effective penetration (cm)
Figure 2.6
Application of intense pulsed light (IPL) treatment head
Figure 2.5
Light penetration into tissue. (Courtesy ESC Medical,
to the skin. Pulsed light passes through quartz crystal light guide and
layer of clear coupling gel before going into skin.
Cutaneous and Cosmetic Laser Surgery
Figure 2.8
Footprint of elliptic pulsed dye laser (PDL) compared
with intense pulsed light (IPL) footprint. (Courtesy ESC Medical, Inc.)
External light on the skin
Figure 2.7
(A) Footprint of the intense pulsed light. (B) Footprint of
5-mm-diameter pulsed dye laser (PDL). (C) Footprint of typical CVL.
(Courtesy ESC Medical, Inc.)
heat penetrationduring the time delaysbetween pulses
Figure 2.9
Diagram of effect of repetitive pulses of intense pulsed
procedure. The contact cooling system uses a high-power
light on 2-mm vessel, 1 mm below epidermis. (Courtesy ESC Medical,
quad thermoelectric temperature regulator that can be set
from 0°C to 30°C allowing the physician to control thelevel of epidermal cooling.
The vascular network leading to facial flushing, redness
and fine telangiectasia is very near the surface as is dys-
BBL uses an advanced dual-lamp configuration. The life-
pigmentation. Surface cooling systems may affect the
time of lamps decreases rapidly as they are driven to higher
response of the superficial vascular targets and surface pig-
energies. Using dual lamps results in each lamp supplying
mentation to the IPL. With the proper temperature control
half of the energy for a lifetime that is an order of magni-
BBL is able to treat vascular conditions and pigmented
tude greater than that of a typical single-lamp system.
lesions with only 50% of the fluence of many other sys-
As a result, BBL comes with a standard 300,000-shot
tems leading to greater comfort, safety, and consistency. For
warranty. BBL has the following filters: 420 nm, 515 nm,
deeper targets the high power of the quad-thermoelectric
560 nm, 590 nm, 640 nm, 695 nm, and 755 nm.
system can provide deep regulated cooling for maximum
Table 2.3 details the present variety of vascular-specific
patient comfort.
lasers available.
Laser Treatment of Cutaneous Vascular Lesions
Temperature (°C)
Temperature (°C)
Figure 2.10
(A) Vessel size is 0.2 mm in diameter. Double pulse with 550-nm cut-off filter is used with energy of 35 J/cm2 given in 2.4-ms
pulses. (B) Vessel size is 1 mm in diameter. Double pulse is given with 590-nm cutoff filter and energy of 40 J/cm2 given in 2.4- and 4.0-mspulses. (Courtesy ESC Medical, Inc.)
Figure 2.12
A 48-year-old woman 20 years after radiation therapy
for thyroid tumor with development of telangiectasia. Clinicalappearance 6 months after treatment with pulsed dye laser at
Figure 2.11
Application of cool gel to skin minimizes thermal
7 J/cm2. Hypopigmented macules took approximately 18 months
damage and allows thermocoagulation to occur in vessel. Without
for complete resolution.
gel, skin is thermally damaged before the underlying vessel isthermocoagulated.
Adverse Effects of Vascular Lasers
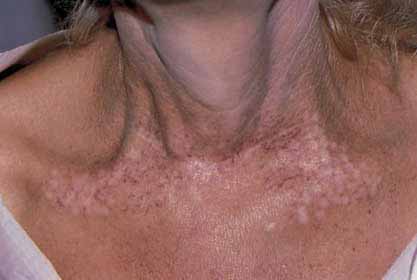
Hypopigmentation can occur in treated areas in dark-skinned patients and was found in 3.2% of patients in onestudy, who were Hispanic or Middle Eastern treated withthe PDL.35 Persistent hypopigmentation is more commonon the neck, legs, and chest (Fig. 2.12). Persistent hyper-pigmentation may also occur with premature sun exposureon facial areas and after treatment of vascular lesions onthe leg. This is especially common and appears as ‘skipped'areas of normal skin on a background of sun-damaged skin.
(Fig. 2.13) Fortunately, most hypopigmented areas resolvespontaneously within 6–12 months. Treating these areaswith an Eximer laser or Relume narrow band UVB
Figure 2.13
Patient treated with the intense pulsed light. Each
light source (Relume, Lumenis, Santa Clara, CA) can speed
impulse is spaced too far apart and areas of ‘skipped' treated skin are
Table 2.3
Vascular Specific Lasers and Intense Pulsed Light
Product name
Device type
V-StarSmartEpill II
46 ¥ 18; None46 ¥ 10
adjustable crystal
adjustable crystal
adjustable crystal
adjustable crystal
Cooled sapphirecrystal
Cooled sapphirecrystal
15 ¥ 35, Cooled
10 ¥ 20, None20 ¥ 25
pulsed lightNd : YAG
30 ¥ 30,13 ¥ 15
10–30/2–25 RF
Contact or aircooling
Laser Treatment of Cutaneous Vascular Lesions
Although rare, hypertrophic scarring has been reported
(rings)43 (Figs 2.15 and 2.16). Patients with type 1 vascu-
with high laser fluences and when treating lesions on the
larity have a better response to PDL treatment because type
neck, arms, or shoulder with any vascular specific laser.35–37
2 PWS lesions are more deeply situated and consist of freely
Another report described ‘isolated, superficial, depressed
anastomosing dilated vessels of the superficial horizontal
scars' in 2 of 35 children treated, reportedly in areas trau-
vascular plexus. Videomicroscopy may allow the physician
matized within 24 hours after PDL treatment.19 Two of 92
to choose the most appropriate laser or pulsed light source
adults with facial telangiectasia developed dermal atrophy
for treatment of PWS.
with normal skin texture on the nose, nasolabial folds, andmalar regions that lasted at least 6 months. These areaswere treated with a 1-mm spot diameter PDL at a fluence
Adverse Medical Effects
of 7 J/cm2.38 Laser fluence, lesion location, and posttreat-ment care are important factors that may contribute to the
In addition to their abnormal cosmetic appearance, PWSs
risk of scarring.
may be associated with medical problems, the mostcommon and serious being glaucoma and less commonand serious being inflammation. Glaucoma occurs inapproximately 45% of patients with a PWS involving
Port-wine Stain
both the ophthalmic (V1) and maxillary (V2) divisions of
Port-wine stains (PWSs) occur in 0.3% to 0.5% of new-
borns39,40 and represent a congenital malformation of the superficial dermal capillaries. They should not be con-fused with the common pink patches known as ‘nevusflammeus neonatorum', ‘angel's kiss', ‘stork bite', or‘salmon patch'. These ‘stains' fade within the first year oflife in 50% of patients. A midline PWS-appearing lesionmay represent a capillary malformation that clears quicklywith one or two treatments and thus is not typical of themore ectatic and venular PWS (Fig. 2.14). These midlinelesions may represent a maturation delay in autonomicinnervation because up to 60% of lesions resolve spontaneously.41
Most PWSs are superficial, with a mean vessel depth of
0.46 mm.42 The lesion is first present as a relatively sharplymarginated pink patch, most often involving the head andneck in 90% of patients, especially in the areas of the firstand second trigeminal nerves.39,40
Videomicroscopy has demonstrated two patterns of vas-
cular abnormality: type 1 consists of tortuous, superficial,dilated capillary loops (blobs); type 2 consists of dilatedectatic vessels in the superficial horizontal vascular plexus
Figure 2.15
(A) Results of transcutaneous videomicroscopy of
patient with port-wine stain (PWS) showing type 1 blob abnormalities(¥200). (B) Results of transcutaneous videomicroscopy of patient with
Figure 2.14
(A) Four-month-old girl with urticaria pigmentosa and
PWS showing type 2 ring abnormalities. Note the small, fine capillary
midline port-wine stain before treatment. (B) 6 1/ years after single
dots that are capillary loops in normal dermal papillae. Dilated vessels
treatment with pulsed dye laser at 5.5 J/cm2 using 5-mm-diameter
of horizontal plexus lie in a deeper plane (¥200). (Reprinted from
spot size, lesions show 100% clearance without adverse sequelae and
Arch Dermatol 133:921, 1997. Copyright 1997 American Medical
no evidence of recurrence.
Association. All rights reserved.)
Cutaneous and Cosmetic Laser Surgery
existence of these associated lesions should not cause confusion in diagnosis.
Various support groups are available for children with
congenital vascular abnormalities and their families. TheSturge–Weber Foundation (PO Box 460931, Aurora, CO80046, USA) publishes an excellent booklet for childrenthat clearly explains the syndrome as well as multiple treatment options. The Klippel–Trenaunay Support Group(4610 Wooddale Ave, Edina, MN 55424, USA) publishes auseful quarterly newsletter and holds support group andeducational meetings for the public. The National Con-genital Port-Wine Stain Foundation (125 E. 63rd St, NewYork, NY 10021, USA) also provides information andsupport to patients and families of children with PWSs;
Figure 2.16
Diagrammatic representations of vascular abnormalities
www.birthmarks.com is an excellent website for patient
found with videomicroscopy. (Left) Tortuous, dilated papillary tipvessels. (Right) Dilated vessels of superficial horizontal vascular plexus.
information. Patients and parents should be encouraged to
Note that type 1 abnormality (left) presents a superficial target with
use these resources.
limited blood supply. Type 2 abnormality (right) is more deeply
PWSs can also present with an inflammatory compo-
situated, and vessels anastomose freely. (Reprinted from Arch
nent consisting of scaling, excoriations, oozing, and crust-
Dermatol 133:921, 1997. Copyright 1997 American Medical
ing, resembling a dermatitis.55 PWS with this secondary
Association. All rights reserved.)
inflammation has been reported in lesions on the nuchaland occipital areas. Treatment with topical steroids helps
the trigeminal nerve. The most well-known condition is
decrease the inflammation, but the PDL is curative after
Sturge–Weber syndrome, which consists of a PWS involv-
one treatment.
ing the first branch of the trigeminal nerve, a high inci-
The natural history of a PWS is that the vessels become
dence of glaucoma of the ipsilateral eye (especially if the
progressively ectatic over time.42,56 This results in gradual
upper lid is involved), angioma of the lids, choroidal
darkening, thickening, and development of nodularity
hemangiomas (in up to 40% of patients),44 calcification
(Fig. 2.17). One study found that two-thirds of patients
and vascular anomalies of the brain with associated seizure
develop hypertrophy and nodularity by age 46, with a
disorders, and, in some cases, mental retardation.45–47 In
mean age of 37 years for hypertrophy.57 Rarely, sponta-
70% to 80% of patients with glaucoma, Sturge–Weber
neous improvement may occur,58 possibly during the first
syndrome presents as buphthalmos, a grossly enlarged
3 years of life.
eye soon after birth. The remaining patients develop glau-
Giant proliferative hemangiomas may also arise in PWSs
coma in childhood, with 44% diagnosed after 4 years of
and can develop without any prior history of trauma.59
age.48 Therefore repeated intraocular pressures should betaken every 3 to 4 months if glaucoma is not present initially.
The extent of the PWS does not usually correlate with
neurologic disease.49 However patients with bilateral PWS
In addition to lesion characteristics that may cause
have a greater likelihood of neurologic involvement with
bleeding and produce physical deformity, a PWS carries a
an earlier onset of seizures.46,50 Epileptic seizures occur in
definite risk for lasting detrimental effects on a child's
72% of Sturge–Weber patients with unilateral lesions and
psychologic, social, interpersonal, and cognitive develop-
93% of patients with bihemispheric involvement.44,46
ment.60–62 The exact age when psychosocial development
Mental retardation occurs in up to 30% of Sturge–Weber
is affected is speculative. A psychiatric study of 19 children
patients, with a 92% incidence of retardation in patients
3 to 5 years old with face, head, or neck hemangiomas
with bilateral lesions.46 Recommended neurologic tests
found no association with major problems in psychosocial
include electroencephalography and functional testing
development.63,64 However, early treatment improves the
with positron emission tomography (PET), single-photon
responsiveness, decreases the number of treatments, and
emission computed tomography (SPECT), computed
reduces the likelihood of permanent adverse seque-
tomography (CT) of cranium, or magnetic resonance
lae.19,26–28,64 Therefore we recommend that treatment be
started at the earliest possible age.
Other congenital syndromes include the Klippel–
A common misperception regarding PWS in adults is
Trenaunay and Klippel–Trenaunay–Weber syndromes
that if one has reached adulthood without psychologic
(PWS with associated varicose vein and hypertrophy of
damage from a cosmetic deformity, one does not require
skeletal tissue52 with or without arteriovenous malforma-
treatment. Former Soviet president Mikail Gorbachev is an
tions [AVMs], respectively) and Cobb syndrome (PWS with
example of successful ‘coping' with a cosmetic handicap.
underlying AVM of the spinal cord).53 A PWS may be
(Interestingly, the Soviet news agency, Tass, airbrushed out
associated with an underlying venous malformation or
Gorbachev's PWS from published photographs until pere-
occasionally an arterial malformation or AVM,54 so the
stroika.) However, such presumptions are often incorrect.
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.17
Progressive nodularity of port-wine stain (PWS) is
Figure 2.18
(A) Twelve-year-old girl with congenital port-wine stain
noted with aging. (A) Patient, age 15, has light-pink PWS on right
on her right cheek. (B) After third treatment with pulsed dye laser.
cheek. (B) Patient, age 35, has marked nodularity and darkening of
First treatment used a fluence of 7.25 J/cm2 with 213 5-mm pulses,
PWS. (C) Clinical appearance after 12 separate treatments with pulsed
second treatment 7.5 J/cm2 with 109 5-mm pulses, and third
dye laser at average fluence of 7.0 to 7.5 J/cm2. Each treatment
treatment 7.5 J/cm2 with 40 5-mm pulses. Patient and parents noted
averaged 600 5-mm impacts. (Courtesy of Gerald Goldberg, MD.)
90% resolution of entire lesion. (From Goldman MP, Fitzpatrick RE,Ruiz-Esparza J. J Pediatr 1993; 122:71.)
More often, the misperception of treatment complicationsalong with cost considerations are the primary reasons foravoiding treatment. These misconceptions are often used
1989 annual meeting of the American Academy of Der-
by insurance companies to deny coverage and save money.
matology dramatically demonstrates this point. A model
Unfortunately, cosmetic considerations are not the only
had a PWS painted on her face and then feigned an illness
reasons PWS should be treated in adults. Hypertrophy,
that led to unconsciousness on a public bus. Not one pas-
hemorrhage, and infection are the medical reasons for
senger came to her aid. When the same model feigned the
same illness on a bus without the facial PWS, all those
Adult PWS can also have an adverse impact on social
present eagerly came to her aid. Pena Clementina Mas-
relationships. A questionnaire given to 186 patients who
clarelli,66 a senior occupational therapist who also has an
sought treatment for their PWS found that 29% thought
extensive PWS, wrote a poignant chapter about her inter-
the PWS was disadvantageous in forming interpersonal
actions with others that should be required reading for all
relationships with members of the opposite sex.65 Half
rated their PWS as unattractive, although only 33%
Multiple studies have demonstrated an improvement
thought that other people perceived their PWS to be mod-
in psychological health after successful treatment of
erately to very unattractive. The true incidence of psycho-
PWS.63,67,68 We have noted a change in personal percep-
logic problems from PWS may be higher or lower because
tions dramatically in our treated patients. A 12-year-old girl
this study was obviously skewed to patients who were
first sought treatment in our practice for a PWS on the
actively seeking treatment. Nevertheless, the survey does
right cheek. Initially, although of above-average intelli-
show that a significant number of adults with PWS would
gence, she was introverted and interacted sparingly with
benefit psychologically from treatment.
her classmates. After three treatment sessions, resulting in
Psychologic difficulties in interactions with others occur
75% clearance, she began dating, joined the school band,
as often in adults as in children. An experiment during the
and excelled academically (Fig. 2.18). These are the obser-
Cutaneous and Cosmetic Laser Surgery
Table 2.4
Childhood Port-wine Stains: Treatment Response by Age
No. lesions
Age 0–4 years
21
Age 4.5–14 years
22
Total 0–14 years
43
From Goldman MP, Fitzpatrick RE, Ruiz-Esparza J. J Pediatr 1993; 122:71.
vations so gratifying to the physician and medical staff
children less than 14 years old (mean age 7 years 2 months)
involved in laser treatment.
with an average of 6.5 treatments.19 Subsequent clinical
Despite the psychologic and medical complications of
studies demonstrated notable efficacy and defined more
PWS, insurance coverage in the US for laser treatment
reasonable expectations. Reyes and Geronemus26 success-
of PWS varies from state to state. A study by McClean
fully treated 73 patients between age 3 months and 14
and Hanke69 of insurance reimbursement in 18 States
years. The overall average lightening after one treatment
found that determination for approval of treatment
was 53%, and the percentage of lightening increased
was made on a case-by-case basis, with the majority
with subsequent treatments. More than 75% lightening
requiring preauthorization. The percentage of requests
was achieved with an average of 2.5 treatments in 33
approved for coverage varied from 50% to 100% without
apparent reason. Some insurance carriers would only
Morelli and Weston88 advocate beginning treatment as
approve treatment if functional impairment existed and
early as 7 to 14 days of age so that three treatments can be
some only if the patient was less than 1 year of age.
done before the infant reaches 6 months of age. They
Only Minnesota has a law requiring all health insurance
noted a 50% resolution with this protocol by the third
to cover the elimination or maximum feasible treatment
treatment. In their population of 132 patients, complete
clearance was obtained in 25% of PWSs when treatmentwas begun before 18 months of age (average 7.8 treatmentsessions) versus 7% to 10% having total clearance when
treatment was begun between ages 11/2 and 18 years(average 7.0 treatment sessions). A follow-up evaluation
Many therapeutic methods have been attempted to treat
of this patient population confirmed the authors' initial
PWSs. These include surgery (excision, grafts, flaps, observations with 83 children: 32% of children who began
dermabrasion),45,70,71 radium implants,72 X-ray therapy,45
treatment before 1 year of age had complete clearing of
their PWS compared with 18% of children treated after 1
ing,74,75 and cosmetic camouflage.76 These methods all have
year of age.89 In this later study, 32% of patients with PWS
limited and unpredictable results as well as potentially
less than 20 cm2 in size completely cleared compared with
serious complications.64 In addition to currently recom-
an 8% complete clearance rate in patients with larger
mended laser treatments, the CO2 laser,77–80 Nd : YAG laser,81
copper vapor laser (CVL),82,83 and argon laser56,84–87 have
Our studies on the treatment of 43 children between
previously been used to treat PWS in children. As previously
ages 2 weeks and 14 years with 49 lesions of capillary mal-
discussed, cosmetic results with these lasers have been
formation confirm these results.28 Lesions treated in chil-
poor in children, with the risk of scarring unacceptably
dren under age 4 had greater overall improvement with
less treatment sessions compared with those in childrenover age 41/2 years (Table 2.4). In general, improvement and
Childhood Port-wine Stains
clearance were gradual and required 5 to 10 treatments.
The PDL was specifically designed to treat the small vessels
However, very superficial lesions cleared more quickly,
found in childhood PWSs.19,21 The first published reports
with four lesions reaching a level of 95% clearing in one
noted complete clearing of pink-to-red macular PWSs in 35
or two treatments (Table 2.5).
Laser Treatment of Cutaneous Vascular Lesions
Table 2.5
Childhood Port-wine Stains: Response Per Number of Treatments
No. lesions
Improvement of nonclear lesion
Median (%)
No. lesions
Median (%)
energy (J)
From Goldman MP, Fitzpatrick RE, Ruiz-Esparza J. J Pediatr 1993; 122:71.
An additional study of 12 children 6 to 30 weeks of age
with the argon laser. Third, treatment is usually painful to
confirmed that treatment of infants only a few weeks old
can be undertaken safely and with an accelerated response:
In our experience, treatment is considerably eased with
45% demonstrated 75% or more lightening of their lesions
topical anesthetic creams, cooling the epidermis with
after a mean of 3.8 treatments.64 Alster and Wilson90
cryogen spray or ice, or conscious sedation administered
reported an 87% clearance rate in patients less than 2 years
by a pediatric anesthesiologist, but necessary only in chil-
of age, 78% clearance in patients ages 3 to 8, and 73%
dren 8 years old or younger. Fortunately, earliest childhood
clearance rate in patients 16 years and older. All these
memories usually occur after 2 or 3 years of age.93 Thus
studies demonstrate a better treatment outcome with
treatments given before this time should not have long-
younger patients.
term psychologic effects. We have been treating infants
Only one study of 23 facial PWS lesions in patients up
with the PDL since 1985 and have yet to observe adverse
to age 17 showed no difference among different ages in the
psychologic effects in our patients with continuing long-
average number of treatments to obtain maximum lesion
term follow-up. Some of our initial patients, treated in the
lightening.91 However, this study only evaluated four
first few months of life with continued treatment at 4-
lesions in children less than 1 year of age and eight lesions
to 6-month intervals, are now 8 to 10 years old and con-
in those 1 to 7 years of age.
tinue to receive treatment without apparent psychologic
Although treatment is efficacious and most laser sur-
geons recommend treatment at the earliest sign of a lesion,
For children less than age 12 years, we recommend laser
photothermolysis of blood vessels does result in the release
fluences that generate slight purpura. We like to use a large
of free Hb into the circulation. Hemoglobinemia may
spot size (10–12 mm in diameter for PDL or the large foot-
theoretically lead to renal impairment in a young patient.
print of the IPL) to minimize skipped areas. The initial
Therefore a study of 15 patients under age 5 treated with
treatment session usually results in an average improve-
the PDL tested serum haptoglobin and urine hemosiderin
ment of approximately 50%. Each subsequent treatment
postoperatively.92 Even though patients had a treatment
provides an additional increment of approximately 10%
region more than 3% of total body surface area and
improvement. After six treatments, 40% of our patients
received more than 1500 5-mm-diameter pulses in some
completely clear, and those who are not clear have an
cases, the authors found no evidence of urine hemoglobin
average improvement of approximately 80% (Fig. 2.19).
and reported normal levels of serum haptoglobin We have not had patients with scarring or persistent pig-levels.
mentary changes despite rare episodes of vesiculation and
Therefore the advantages of early treatment are: (1)
crusting after treatment. Skin type (I–III) also does not
quicker resolution requiring fewer treatments; (2) fewer
appear to influence the ultimate treatment outcome, but
laser pulses because of smaller size (children triple in size
darker skin requires more treatment sessions to achieve the
from birth to age 2 and further double in size from ages 2
same degree of clearing as in PWSs of fair-skinned patients
to 8); and (3) less need for anesthesia.
as we recommend lower fluences and a higher degree of
Unfortunately, pediatricians and family practitioners are
epidermal cooling. Lesions on distal limbs respond with
reluctant to refer their patients for treatment. This is most
less fading than lesions elsewhere, such as on the neck and
likely a result of too few reports in all but the most recent
torso (Table 2.6). The diminished response of PWS on the
pediatric literature and thus lack of knowledge. Second,
limbs has been reported by others.94,95
older physicians may remember the failures of the argon
Fortunately, treatment of PWS in childhood and infancy
laser in treating these lesions and equate all laser treatment
not only has been very efficacious with the PDL, but also
Cutaneous and Cosmetic Laser Surgery
has proved to be safe. Swelling and erythema are fre-quently present immediately after treatment, especiallyaround the eyes, but resolve within 24 to 48 hours. Hyper-pigmentation of the treated site occurs in 25% to 30% ofpatients but is temporary and resolves over 2 to 3 months.
Hypopigmentation occurs infrequently and resolves spon-taneously over 3 to 6 months. Cutaneous depressions oratrophic scars have occurred in isolated laser impact sitesand have been associated with excessive delivery of energy,excessive spot overlap, or posttreatment trauma to the site.
Almost all reported cases have resolved spontaneouslywithin 1 year.21,26,28
Adult Port-wine Stains
The treatment of PWS in adults has been as equally grati-
fying as our experience in children (Figs 2.20 and 2.21).
The PDL is used in the same manner as with children
except that fluences are usually increased depending on
the lesion's color and thickness. A 7- to 12-mm-diameterspot size is preferred because of its deeper penetration. Werecommend beginning with a fluence of 5.0 to 5.5 J/cm2with a 7-mm-diameter spot size and increasing by 0.5 J/cm2with each subsequent treatment at 3- to 4-month intervals.
A mathematical model as well as clinical experience
predict a 10% clearance of the PWS with each of the firstfive or six treatments. Additional treatments result in adecreased therapeutic response so that 20 treatments arerequired to produce a 90% clearing.96
The IPL can also be used in a number of different set-
tings to effect vascular-specific thermocoagulation of PWS.
Multiple studies have demonstrated an enhanced efficacyof clearing in comparison to the PDL. This holds true evenfor Asian patients.97–101
Different settings are used with each treatment, which
Figure 2.19
(A) Initial appearance of extensive port-wine stain
can be given at monthly intervals. We usually increase the
(PWS) on a 10-week-old girl. Evaluation by pediatric neurologist was
fluence with each subsequent treatment as well as the pulse
entirely within normal limits. (B) The same patient 4 months after her
duration, cutoff filter, and number of simultaneous pulses
fourth treatment with the pulsed dye laser. First and second
(Fig. 2.22). The following settings are effective for initial
treatments were performed at an energy of 6 J/cm2 and third and
and subsequent treatments (these settings apply for the
fourth treatments at 6.25 J/cm2. Total of 400 pulses were given to theentire PWS during each treatment visit. Parents and physician noted
Lumenis vasculite system; parameters will vary with other
almost 90% resolution of PWS. (From Goldman MP, Fitzpatrick RE,
Ruiz-Esparza J. J Pediatr 1993; 122:71.)
Table 2.6
Childhood Port-wine Stains: Treatment Response by Location
No. lesions
Average no.
No. lesions
Average no.
From Goldman MP, Fitzpatrick RE, Ruiz-Esparza J. J Pediatr 1993; 122:71.
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.20
Port-wine stain on left anterior chest of 44-year-old woman. (A) Before treatment. (B) Near 100% resolution after six treatments.
Each treatment used a fluence of 7.25 J/cm2 with PDL. First treatment used 136 5-mm impacts, second treatment 175 5-mm impacts, thirdtreatment 117 5-mm impacts, fourth treatment 87 5-mm impacts, fifth treatment 77 5-mm impacts, and sixth treatment 37 5-mm impacts. Fig.
2.20A and B: Reprinted from Fitzpatrick RE: American Journal of Cosmetic Surgery 9:107, 1992. With permission from American Academy ofCosmetic Surgery. (C) Continued clearance 12 years after original treatment clearance.
in these patients. Ice-cold coupling gel and/or cold contactcrystals are used.
One advantage when using the IPL is the minimization
of purpura after treatment (Fig. 2.23). Other advantages aredescribed later.
Complications and Adverse Sequelae
Complications and adverse sequelae when treating vascu-lar lesions with the PDL, IPL or any vascular specific laseras described previously are rare. Adverse sequelae areusually temporary and limited to purpura and epidermalcrusting. Purpura is more common with the PDL.
The most common long-term adverse sequelae are
pigmentary changes. Because melanin competes as anabsorber with Hb in patients with Fitzpatrick type III skinor greater, hypopigmentation (especially in patients withtanned skin) is not uncommon. Alternatively, postinflam-
Figure 2.21
This 34-year-old patient had a port-wine stain of her
matory melanocytic hyperpigmentation may occur. At
chin (A). She underwent a series of five pulsed dye laser treatments
times this may appear as a ‘checkerboard' pigmentation
with approximately 2 months between sessions. Treatmentparameters were the following: 585-nm wavelength; 7- to 10-mm
(Fig. 2.24). When this occurs, additional treatments are
spot size; 8–12 J/cm2; cryogen spray cooling spurt duration of 30–
usually necessary to even out the skin color. Oftentimes,
50 ms with a 30–50-ms delay. The patient achieved an excellent result
switching to the IPL, which with its larger spot size evens
(B). (Courtesy Kristen Kelley, MD.)
out the dyspigmentation in addition to the nontreatedareas (discussed later). In addition, the use of depigment-ing agents such as hydroxyquinone, alpha-hydroxy acids,azelaic acid, and kojic acid alone or in combination with
515-nm or 550-nm cutoff filter, single pulse at 2 to 5 ms
retinoic acid both before and after treatment is helpful.
with 20 to 25 J/cm2
Fortunately, permanent scars or pigmentary changes are
550-nm cutoff filter, double-pulsed at 2.4 ms with 10-ms
delay, 4.0 ms with 35 to 42 J/cm2
More serious potential adverse events include atrophic
570-nm cutoff filter, double-pulsed at 3.0 ms with 20-ms
and hypertrophic scarring and keloid formation. The for-
delay, 6 ms with 40 to 45 J/cm2
mation of keloids may be enhanced when the patient
590-nm cutoff filter, triple-pulsed at 3 ms with 30-ms
is also taking isotretinoin. Multiple case reports of the
delay, 4.5 ms with 30-ms delay, 7 ms with 30-ms delay
development of keloid formation in patients receiving
at 50 to 60 J/cm2
isotretinoin have been reported with argon laser and
As with other lesions, patients with more darkly pigmented
dermabrasion treatment.102,103 A single case report of this
skin are treated with higher cutoff filters to circumvent
occurrence with PDL treatment of a neck PWS has
melanin absorption and longer delay times between
appeared in the literature.104 Although the mechanism for
double and triple pulses because epidermal heat is higher
enhancing scarring is speculative and the length of time
Cutaneous and Cosmetic Laser Surgery
Figure 2.22
(A) Port-wine stain on 24-year-old male before treatment. (B) Immediately after treatment with PhotoDerm VL. Note purpuric
response to treated areas (C) One month after treatment. Note resolution at various treatment parameters, all given in single pulses. (Top tobottom) 590-nm cutoff filter at 50 J/cm2, 570-nm cutoff filter at 40 J/cm2, 550-nm cutoff filter at 30 J/cm2.
from cessation of treatment to ‘safety' is unknown, it
scarring were treated in the early stages of the PDL
would seem prudent to avoid laser treatment within 1 to
development, when problems with the dosage meter were
2 years of isotretinoin use.
Numerous retrospective studies have detailed adverse
A study of 701 patients who received 3,877 full treat-
sequelae. A study of 133 patients (89 females, 44 males)
ments with the PDL using a 5- or 7-mm-diameter spot and
with PWS who had been treated with the PDL over a 2-
fluences of 5.5 to 9.5 J/cm2 with the Candela and the Cyno-
year period showed good or excellent results in 84% of the
sure systems reported severe blistering in 1.08%, severe
PWSs.27 The average number of treatments increased from
crusting in 0.13%, hypopigmentation in 0.26%, hyperpig-
1.7 to 2.3 to 3.5 as the clinical results improved from slight
mentation in 1.7%, atrophic scarring in 0.7%, and hyper-
to good to excellent, accordingly. After a treatment session,
trophic scarring in 0.13% of treatments.105 Seven patients
discoloration and purpura were seen in all patients, crust-
developed atrophic scarring despite uneventful test area
ing in 51.9%, and scaling or peeling in 19.6%. Three
treatments. Thirty percent of patients with scarring had
patients reported swelling, and two reported blisters. Many
clinical resolution over 6 to 12 months. Hypertrophic scar-
patients reported crusting only after their first treatment
ring also occurred after an uneventful test and four or five
and not with subsequent treatments. The average duration
uneventful treatments. Hypertrophic scarring showed no
of these immediate skin changes was reported to be 7
resolution in these patients.
to 14 days. Long-term skin changes with PDL therapy
Complaints of discomfort from treatment were rated as
included hyperpigmentation in six patients, hypopigmen-
moderate in 49.1% of patients, which is higher than pre-
tation in five, and isolated punctate depressions in two.
viously reported.21 However, pain in adults was not a lim-
Pigmentation changes in patients who had completed
iting factor in treatment. Although patients, in retrospect,
therapy lasted an average of 6 months. Atrophic surface
have rated treatment pain as moderate, treatment was not
changes noted in two patients involved small areas of exco-
discontinued because of pain in any patient.
riation. No hypertrophic scarring was noted. This apparentscarring was transient in nature, and both episodesresolved completely within 12 months. No significant
Variable Treatment Response by Lesion
differences in adverse sequelae were apparent between
Location and Size
original lesions that were flat or raised.
An additional study of 500 patients treated with the PDL
In addition to an obvious decrease in responsiveness to
found an incidence of atrophic scarring of less than 0.1%.
treatment on the extremities compared with the face (see
Hyperpigmentation was seen in 1% of patients and tran-
the next section), PWSs responded differently even within
sient hypopigmentation in 2.6%. Patients with atrophic
the same anatomic location. The centrofacial regions
Laser Treatment of Cutaneous Vascular Lesions
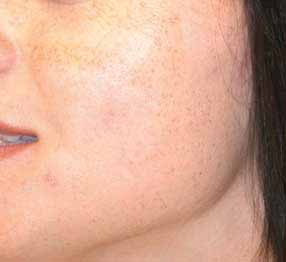
Figure 2.24
(A) Facial telangiectasia in 32-year-old woman 3 years
after one treatment with the PDL at 7.0 J/cm2 with 5-mm-diameterspot. Note hypopigmented circles and persistent telangiectasia.
(B) Ten months after three treatments with PhotoDerm VL. Firsttreatment was with 570-nm cutoff filter at 37 J/cm2 given as a doublepulse of 2.4 and 2.4 ms with a delay time of 10 ms. Second and thirdtreatments given 4 weeks apart, 4 weeks later through a 550-nmcutoff filter at 40 J/cm2 given as a 2.4- and 4.0-ms double pulse witha 10-ms delay. Note complete resolution of the telangiectasia andhypopigmented circles.
(medial aspect of the cheek, upper cutaneous lip, nose)respond less favorably than the periorbital, forehead,temple, lateral cheek, neck, and chin areas.91,106 Evaluationby dermatomal distribution revealed that V2 lightened onaverage 74%, whereas combined dermatomes V1 and V3
Figure 2.23
(A) Port-wine stain (PWS) on the cheek of 4-year-old
lightened on average 82% when treated with the PDL at
girl immediately before treatment. (B) Purpuric response immediately
585 nm, 5-mm-diameter spot size, and 5.75 to 8.5 J/cm2
after treatment with PDL at 7.0 J/cm2 delivered through 7-mm-
in an average of four treatments. V
diameter spot size. (C) Opposite cheek with identical PWS
2 lesions also require
immediately after treatment with PhotoDerm VL with 570-nm cutoff
more treatments to reach maximal clearance than V1 or V3
filter at 40 J/cm2 given as a single 8-ms-duration pulse. Note
lesions (Fig. 2.25). Lesions in the V3 dermatome have been
diminished purpuric response.
found to have more superficially ecstatic blood vessels,whereas lesions in V2 dermatome and on distal extremi-ties have more deeply placed vessels.107
Lesion size may be an independent factor determining
lesion response. A study of 74 adult PWSs on various loca-
Cutaneous and Cosmetic Laser Surgery
Mean lightening, 70.7%;
Mean lightening V2, 73.8%;
Mean lightening, 82.3%;
Mean lightening V1, V3, C2/C3;
excellent response
82.3%; excellent response
Figure 2.25
(A) Anatomic subdivision of therapeutic response of port-wine stain (PWS) to pulsed dye laser (PDL) treatment. (B) Dermatomal
distribution of therapeutic response of PWS to LPDL treatment. (Reprinted from Arch Dermatol 129:182, 1993. Copyright 1993 AmericanMedical Association. All rights reserved.)
PDL Treatment of Extremity Lesions
The poor response of lesions located on the distal extrem-ities has been seen in our practice and reported by others.
In one review, 7 of 10 patients with PWS on the extremi-
ties responded only slightly or poorly.94 This finding mightreflect an artifact of fewer treatments because the average
Rate of clearing
number of treatments was 2.6 (Fig. 2.27). In contrast, thoselesions on the face and neck that responded slightly or
poorly had an average of only 1.1 treatments. Twenty-seven patients with lower limb PWS treated with the PDL
10.1-20 20.1-40 40.1-60 60.1-80 80.1-100
at fluences up to a maximum of 8.5 J/cm2 also had a
Lesion size (cm2)
poor response.95 In this population, only one patient had greater than 95% clearance, despite these patients
Figure 2.26
Facial PWS lesion size affects rate of clearing after PDL
treatment in adults. Data are represented as mean ± standard error of
having an average of 9.4 treatments. Overall, 26% of these
mean for each lesion-size category. Asterisk (*) designates significant
patients had no response, 22% had a poor response, 33%
difference (p < 0.05) in rate of clearing versus size category of greater
had less than 50% lightening, 15% had 50% to 75% light-
than 100 cm2. (From Yohn JJ, Huff JC, Aeling JL et al. Reprinted with
ening, and only one had greater than 95% clearance after
permission from Cutis. 1997; 59:267–270. 1997, Quadrant
seven treatments. In addition, 18% of patients developed
hyperpigmentation, which lasted for an undisclosedperiod.
The effect of decreased response to treatment with
lesions located in extremities was also noted by Orten
tions found that all lesions responded, with 25% to 90%
et al,91 who found only 33% lightening despite a mean of
lightening.108 However, only 36.5% achieved 50% clearing
approximately six treatments, compared with 80% to 90%
despite 4 to 19 treatments, depending on the size of the
lightening of PWS lesions in fewer or similar number of
lesion. No lesions had greater than 75% clearance despite
treatments in other locations. Lanigan109 also reported
6 to 15 treatments when their size was greater than 60 cm2,
poor response to treatment in 23 patients with lower
and no lesions had greater than 50% clearance despite a
limb PWS. Ten patients had no discernible lightening
mean of 17 treatments when their size was larger than
after one treatment at 7.75 J/cm2. Seventeen patients with
100 cm2 (Fig. 2.26).
a median of seven treatments at a median fluence of
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.27
Port-wine stain on entire right hand, arm, and chest of
38-year-old woman. Photograph was taken 2 years after treatment ofhand and arm. Right dorsal hand has been treated three times withthe pulsed dye laser at fluences ranging between 7 and 7.5 J/cm2.
Area from wrist to elbow has been treated once at an energy of 7 J/cm2. Area of demarcation from midforearm to lateral aspect of
photograph was treated a second time with a fluence of 7.5 J/cm2.
Note significant improvement in proximal forearm without noticeableimprovement (by comparison) of distal forearm, dorsal hand, orfingers.
7.25 J/cm2 showed median lightening of 40%. Of thoseresponding, only three patients had lightening of 70% orgreater after six, ten, and five treatments. However, someauthors have reported a similar treatment efficacy despitelesion location with a similar average number of treatments.90
The reason PWSs on the distal limbs respond slowly is
Figure 2.28
Extensive port-wine stain (PWS) of left hand, forearm,
unknown but may be related to gravitational or deoxy-
and chest of 18-month-old boy. (A) Before treatment. (B) Six months
genation effects on circulation. Distal vessels have a thicker
after two treatments to dorsal hand with pulsed dye laser using a
wall, which may require increased thermal effects for irre-
fluence of 6.75 J/cm2. Total of 80 5mm impacts were given duringeach treatment session. Note significant resolution of PWS.
versible damage (see p. 50). Because distal areas respondmore poorly than more proximal areas, early treatment ofextremity lesions, before ambulation, may be beneficial.
We have found this to be true in the small number ofpatients we have treated at an early age with distal limb
density may have been chosen. Generally, three or four
PWS (Figs 2.28 and 2.29).
energy fluences are tested and evaluated at approximately
As previously mentioned, even treating extremity
6 weeks (Fig. 2.29). The most effective one is then chosen
lesions with high-energy fluences has been unsuccessful.
for treatment. If none of the test sites shows improvement,
Near-complete resolution in only two treatments has
a second series of test energies is chosen. With experience,
been reported when fluences of 9 J/cm2 are used. However,
the test period may be eliminated and the treatment
this degree of laser energy poses a risk of pigmentary and
energy chosen by evaluation of the laser's immediate
nonspecific epidermal changes.88 Epidermal cooling may
allow these necessary higher fluences to be used.
Treatment of the entire lesion or a portion of the lesion
may be accomplished by covering the treatment area withlaser or IPL impacts that overlap 10%. Overlapping can
minimize a mottled ‘egg crate' or ‘foot-print' appearance.
However, as previously described, the degree of overlap-
The treatment protocol involves first performing a test of
ping is determined by the type of PDL used, with the Cyno-
several different energy densities to determine efficacy.
sure PDL better used without any overlapping, except with
Immediately after impact, a dark purple discoloration
a ‘fuzzy spot'. Overlapping may also be achieved with the
occurs. Conversely, if the site does not discolor, too low an
Candela PDL by use of the so-called fuzzy spot.110 With this
energy fluence may have been chosen. If edema, blistering,
treatment modification, the laser handpiece is moved away
or blackening of the impact site occurs, too high an energy
from the skin beyond the laser's focal point, resulting in a
Cutaneous and Cosmetic Laser Surgery
Figure 2.29
(A) Immediately after application of test doses with
pulsed dye laser at laser energies specified. (B) Four months after testdoses. Note significant clearing in all areas, with maximum clearing atfluences of 7.0 and 7.25 J/cm2. Patch treated at 7.5 J/cm2 shows light-brown hyperpigmentation. Treatment will therefore proceed at anenergy of 7.0 J/cm2.
larger, defocused impact spot. This has a lower energydensity than that indicated for the focused spot and alsoa more indistinct or ‘fuzzy' border that blurs the edges ofclearing from a single treatment. Re-treatments are gener-ally done at 11/2- to 4-month intervals.
The use of overlapping pulses should be undertaken
with caution, however, because biopsies of PWSs treated
Figure 2.30
Congenital port-wine stain on thigh of 66-year-old
with single impact and consecutive double-impact therapy
man. (A) Before treatment. (B) Immediately after treatment with
reveal an additive thermal effect resulting in nonspecific
pulsed dye laser at 7 J/cm2. S, area treated with single pulse; D, area
thermal damage to the superficial dermis and epidermis
treated with double pulse. Double-pulse area has darker
(Figs 2.30 and 2.31). This double-pulsing technique does
promote greater resolution of nodular, thicker, and darkerlesions but results in loss of specificity.
The reason for multiple treatments is the layered nature
effective wavelength related to their depth.) However,
of ectatic vessels of a PWS. Modeling studies using the
purple lesions are not completely or always unresponsive
histologically correct layered vessel demonstrate that
to treatment. We found that although purple lesions are
the more superficial vessels receive most of the delivered
graded most often as excellent responders (52%), they also
energy. The deeper vessels receive less energy and are there-
are the leading group of poorly responsive lesions (21%).27
fore not thermocoagulated because of mutual shadowing
This paradox may occur because two distinct populations
of the superficial vessels111 (Fig. 2.32).
of purple lesions exist: macular and nodular. Exophyticnodular lesions respond well, whereas purple and macularlesions respond poorly because of deeper and larger dermal
Other Factors in Treatment Response
Paradoxically, lesions that respond poorly may appear
In addition to size and location of the PWS (lesions on
clinically similar to good responders. In these lesions,
extremities), other factors may be important in predicting
biopsy demonstrates vessel walls that are thicker despite
responsiveness to treatment. PWSs that are dark red or
a vessel diameter that is smaller than 0.056 to
purple may be less responsive to laser treatment. The rela-
0.102 mm.112,113 Such lesions are usually on the trunk or
tive difficulty in treating these lesions results from the
extremities. Alternatively, lesions with deeper vessels
presence of deeper, larger vessels that are beyond the laser's
respond poorly, because the PDL at 585 nm and 6 to 8 J/cm2
penetration depth19,21 or that are too large to be photo-
has been found to coagulate the entire vessel wall only to
coagulated completely within treatment parameters of the
a maximum depth of 0.65 mm (mean 0.37 mm), even in
PDL.10 (As discussed previously, the thermal relaxation
vessels not shielded by more superficial vessels.114 Superfi-
time of target vessels is related to their diameter, with the
cial PWS vessels up to 0.15 mm in diameter were found
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.33
Biopsy of port-wine stain on the face of a 43-year-old
female. Lesion is violaceous and thick immediately after treatmentwith pulsed dye laser at 6.5 J/cm2. There is complete coagulation of
Figure 2.31
(A) Biopsy specimen immediately after single pulse as
red blood cells (RBCs) and vessel wall in a 150-mm vessel (right) and
described in Figure 2.47A. Note coagulation of superficial dermal
no damage to the lower third of RBCs and vessel wall in a larger
ectatic blood vessels without any change in overlying epidermis or
vessel. Note the ‘steam bubble' formation in upper half of vessels and
perivascular tissue (hematoxylin–eosin; ¥40). (B) Biopsy specimen
perivascular dermal coagulation zone. (Reprinted from Hohenleutner
immediately after double-pulse technique as described in Figure
U, Hilbert M, Wlotzke U et al: Journal of Investigative Dermatology
2–47B. Note nonspecific thermal damage to overlying epidermis and
104:798, 1995. With permission from Blackwell Publishing Ltd.)
perivascular tissue. Ectatic blood vessels in superficial papillary dermisare thrombosed with evidence of perivascular collagenhomogenization (hematoxylin–eosin ¥40).
histologically to coagulate completely without nonspecificdamage to epidermal or perivascular tissues (Table 2.7 and
Therefore both vessel size and vessel depth in addition
to vascular wall thickness are important determinants in
predicting treatment efficacy. Vessel size has an important
effect because the entire vessel (not just the superficialportion) must be thermocoagulated. This assumes that forvessel coagulation, heating the center of the vessel is necessary for thermal radiation to the entire vessel wall.
This requires both an adequate wavelength (for depth of
penetration) and an adequate pulse duration (thermal
Finally, Waner115 has proposed that autonomic inner-
Deposited energy (
vation is an important determinant of treatment efficacy.
In a study of 118 PWSs in 102 patients, recurrence of the
PWS depended on the time lapsed since the completion oftreatment. Although only 3% of patients showed evidence
of recurrence at 1 year, 20% and 40% showed evidence forrecurrence at 1 to 2 and 2 to 3 years after treatment, respec-
tively. Cutaneous venules of the PWS vasculature areinnervated by sympathetic postganglionic neurons as wellas sensory neurons.116–118 The apparent underlying cause
Deposited energy (
of a venular malformation is an absolute or relative defi-
ciency of autonomic innervation of the cutaneous vascu-
Figure 2.32
(A) Geometry with 17 multiple straight vessels in three
lar plexus. Smaller and Rosen116 demonstrated a deficit in
layers at different depths z of 300, 435, and 570 mm, and lateral
the number of perivascular nerves in PWS. Kane et al119
spacing between vessels' centers of 270 mm. Energy deposition in
also found a decrease in autonomic innervation in six
multi-blood-vessel geometry for (B) wavelength 577 nm, and (C)
patients with poorly response PWS despite 5 to 21 treat-
wavelength 585 nm. Laser beam diameter is 1 mm. Upper vessels
ments with the PDL at fluences up to 10 J/cm2.
receive most of the energy. Deeper vessels receive less energy by
Therefore Waner115 postulates and we concur that even
decreasing light fluence with depth and also by mutual shadowing ofvessels. (Fig. 2.32A, B and C. Lucassen GW, Verkruysse W, Keijzer M
though effective treatment decreases the number of ectatic
et al: Lasers in Surgery and Medicine 18:345, 1996. Reprinted with
vessels significantly, the remaining milieu allows for a con-
permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.)
tinuing course of progressive ectasia. Recurrence of com-
Cutaneous and Cosmetic Laser Surgery
Table 2.7
Treatment of Port-wine Stains with Flashlamp-pumped Pulsed Dye Laser (PDL, 6.5 J/cm2): Lightening Per Vessel
Size and Depth
Measurement (mm; mean ± SD)
Poor lightening (n = 8)
Moderate lightening (n = 12)
Good lightening (n = 22)
From Fiskerstrand EJ, Svaasand LO, Kopstad et al. J Invest Dermatol 1996; 107:671.
pletely resolved capillary malformations, however, has not
IPL as previously discussed. To enhance the therapeutic
been observed by the authors.
effects of the PDL one can use cryogen spray with higherfluences, increase the spot size of the PDL, change from a595 to a 585 dye, increase the pulse duration from 0.45 ms
to 1.5–20 ms, and perform multiple passes at the samesession with variable pulse durations.
Improvement and clearance are gradual and usually
The primary purpose of cryogen cooling is to allow
require 5 to 10 treatments, although some lesions may not
higher fluences to be used without damage to the epider-
fully clear despite more than 20 separate treatments. It is
mis. Higher fluences will produce higher temperatures
sometimes difficult to determine when a lesion has reached
within the dermal ecstatic vessels. Studies have found that
a point of maximum improvement, but treatments should
the use of cooling with higher fluences enhances the
be continued as long as each results in an increment of
resolution of PWS.121–123
improvement. We have found the point of diminishing
Performing multiple passes with 0.45-ms and 1.5-ms
return to occur about the seventh treatment. Finally,
pulse durations has been reported to accelerate resolution
areas within a PWS that fail to respond to treatment
of PWS.124 However, this has not been uniformly con-
should be closely examined because we and others have
firmed by our group and others.125
reported the development of basal cell carcinoma within a
Increasing the pulse duration from 0.45 ms to 1.5 or
even 2.0 ms has been reported to result in further clearing
A retrospective photographic analysis was performed on
of ‘resistant' PWS.126,127
69 patients who failed to achieve greater than 75% lesional
Changing from a 585-nm dye to a 595-nm dye has
lightening in nine treatment sessions with the PDL at
resulting in enhanced clearing in some PWS. It appears
typical treatment parameters.120 Retreatment at similar
that pink or red PWS do best with 585 nm whereas blue or
parameters with a 5-mm-diameter spot size and fluences of
dark red PWS do better with 595-nm dyes.127–131 This is
5.75 to 8.0 J/cm2 resulted in continued improvement in
due to the shift of absorption to higher wavelengths with
these patients. An additional 25% to 100% lightening was
production of methemoglobin, which is formed by a
achieved in these patients treated up to 25 separate times.
photoinduced oxidation of hemoglobin with laser
Extensive surface area involvement and limb lesions were
the slowest to respond.
Finally, other maneuvers, such as increasing the diame-
As mentioned previously, the dominant vessel size in
ter of the blood vessels by using a proximal tourniquet has
childhood PWS is 10 to 50 mm in diameter. The average
also enhanced absorption with a 585-nm, 1.5-ms PDL.133
thermal relaxation time of PWS vessels is estimated at 1.2 ms.4 As the child grows to adulthood, progressiveectasia results in larger and deeper vessels up to 300 mm indiameter with a thermal relaxation time of approximately
Recurrence of Port-wine Stains
10 ms. It is reasonable to assume that these larger vesselswould be more common in the darker, thicker lesions of
The use of the PDL in treating PWS has been widely prac-
adults. Although the PDL has been shown to be very effec-
ticed for almost 20 years. Recently, we and others have
tive in the treatment of adult PWS,22,23,25,27 larger vessels
begun to evaluate our patients treated in the early years for
in these lesions are not ideally suited for the specific
signs of persistent or recurrent lesions. Although we have
parameters of this laser. Therefore other laser or pulsed
not seen a significant number of patients returning to our
light sources or photodynamic therapy may be useful.
practice with progressing lesions, other physicians havereported recurrence after completion of treatment (see Fig. 2.17). Orten et al91 have evaluated a small number of
Treatment of ‘Resistant' Lesions
patients who were 2, 3, and 4 years posttreatment. Theyhave reported recurrence (darkening of the lesion) in 5 of
Multiple methods are available for treating PWS lesions
24 patients at 2 years, 4 of 10 patients at 3 years, and 2 of
that fail to respond to the PDL. The first is to switch to the
4 patients at 4 years posttreatment. The most obvious
Laser Treatment of Cutaneous Vascular Lesions
patient, an adult female whose PWS was on the midlateral
pulses at 15 or 20 J/cm2. Posttreatment purpura developed
cheek, had near-complete clearance of the PWS only to
at all effective treatment sites. No scarring or ulceration
have almost total recurrence after 42 months. Whether
occurred. Histology showed that selective vessel closure
these recurrent lesions would respond more favorably to
was achieved without hemorrhage. Perivascular collagen
alternative treatment modalities (e.g. IPL) or respond
damage was always present at the effective treatment sites.
equally as well to additional treatment is unknown. Recur-
These findings show that a 532-nm laser pulsed in the 1-
rence of PWS in a subset of patients may support a
to 30-ms domain is capable of inducing selective vessel
‘neuronal' theory of PWS evolution.
Raulin et al134 reported complete resolution of a facial
PWS in a 35-year-old male that did not respond to a single
532 nm Nd:YAG Long-pulse Laser
treatment with the PDL at 6.5 J/cm2. They used a 550-nm
By extending the pulse duration to within the thermal
cutoff filter with single and double pulses of 25 J/cm2 3 ms
relaxation time of the vessel treated, purpura is avoided
in duration. Treatments were given every 8 weeks until
without loss of efficacy (see earlier). This laser has been
clearance occurred after the fourth treatment.
effective in treating PWSs that have sometimes proved tobe recalcitrant to treatment with other laser modalities136
Treatment with Other Lasers
(Fig. 2.35). Our experience in using this modality, bothwith and without epidermal cooling, has been variable but
Carbon Dioxide Laser
positive. The 532 nm laser is less effective in Asian patients
In our opinion, nodular lesions are best treated with the
with a higher risk of complications.137 A dual pulsed 532
UltraPulse CO2 (UPCO2) laser (see Chapter 6) or the IPL.
and 1064 nm laser (Dualis KTP+, Fotana d.d., Ljubljana,
With the UPCO2 laser the lesion can be sculpted to reestab-
Slovenia) has been found in a pilot study on 10 patients
lish a normal facial contour in addition to thermocoagu-
to be effective in treating PWS. Cryogen spray cooling as
lating the ectatic blood vessels. The advantages of the
well as topical anesthesia is necessary but atrophic scars
UPCO2 laser are its precise hemostasis and avoidance of
still occurred.138
nonspecific thermal damage (Fig. 2.34). After normal con-tours are obtained, the PDL or IPL can be used to lighten
1064 nm Nd:YAG Long-pulse Laser
the remaining erythema.
Most recently, this longer wavelength laser has been usedin the treatment of PWS. It is thought that the deeper pen-
Potassium Titanyl Phosphate Laser
etration of the 1064 nm wavelength would allow further
The 532-nm Starpulse KTP laser (LaserScope) has been used
improvement especially in blue PWS. Although effective,
in conjunction with a scanner to treat adults with PWS.
this laser has a relatively high incidence of hypertrophic
Best clearing of the test sites was obtained with 3- or 5-ms
and atrophic scarring due to the high energies needed
Figure 2.34
(A) Congenital port-wine stain with large hypertrophic
Figure 2.35
Port-wine stain on left upper cheek of female patient
mass on right lateral distal nose and nodules on right inner canthus
treated under general anesthesia with copper bromide laser (CBL);
present in 47-year-old Hispanic man. Over last 20 years, lesion has
actual treatment required about 15 minutes. (A) Before treatment.
grown progressively darker in appearance as well as developing
(B) After treatment with exposure time of 7 ms, 2.5 W, and spot size
nodular hypertrophies. (B) Three weeks after treatment with Coherent
of 0.7 to 1.0 mm (4.5 J/cm2), with 10 ms (6.5 J/cm2) for darker areas.
Ultra Pulse CO2 Laser (Coherent Laser Corp., Palo Alto, CA) at 250 mJ,
(Courtesy Sue McCoy, MD.)
pulse width of 794 ms at 20 W, 80 pulses per second, in continuous-wave mode with 2-mm spot.
Cutaneous and Cosmetic Laser Surgery
gioma. Other times it is used to (mis)label a noninvolut-ing ‘cavernous hemangioma', which in reality is a venousmalformation with spongy architecture.141 A venous mal-formation may be difficult to differentiate clinically froma deep hemangioma. Failure to evacuate blood with com-pression occurs in hemangiomas, not in venous malfor-mations, and this is a useful maneuver to differentiate thetwo conditions.
Hemangiomas are the most common tumor of infancy;60% occur on the head and neck, 25% on the trunk, and15% on the extremities.142 Eighty percent of hemangiomasare present as a single, well-circumscribed lesion 0.5 to 5.0 cm in diameter, and the remainder occur as multiplecutaneous and visceral lesions.141 The lesions are generally
Figure 2.36
Long-standing PWS before and 1 month after 4-
absent at birth, but a localized area of pallor or macular
monthly treatments with a long-pulse Nd : YAG laser using a 7-mm-diameter handpiece with a fluence of 60–65 J/cm2 at 10–15 ms pulse
erythema or telangiectasia may be present. The majority of
duration. (Courtesy of Don Groot, MD.)
hemangiomas (70%–90%) appear during the first monthof life, and by age 12 months the incidence is reported tobe in the 10% to 12% range.39,143,144
Hemangiomas present at birth result from in utero
growth. They are difficult to diagnose with routine ultrasonic evaluation, even when associated with
to thermocoagulate red blood cells at this wavelength.
Kasabach–Merritt syndrome. They tend not to grow after
Although patients prefer the lack of significant purpura
birth and usually regress by 14 months, leaving atrophic,
with the 1064 nm laser, scarring has been noted to occur
redundant, or scarred skin.145
even with epidermal cooling after establishing a minimal
When lesions have distinct borders, homogeneous
purpuric laser fluence.139 This may be related to the differ-
color, no overlying telangiectasia they are usually a capil-
ent absorption characteristics of the vessels of a PWS in dif-
lary malformation. When the borders are indistinct and
ferent locations within the same lesion. At this time, this
the lesions are variably colored with visible overlying
laser modality is only recommended for thick, blue lesions
telangiectasia they are true hemangiomas.
recalcitrant to other treatment modalities (see Fig. 2.36).
Congenital hemangiomas of eccrine glands have also
been reported.146 These rare lesions usually appear at birthas bluish, slightly elevated tumors. None shows evidenceof hyperhidrosis, and all resolve spontaneously. Diagnosis
is made on histologic examination of dilated capillary-likevessels located around sweat glands.
The treatment of hemangiomas remains a challenge. Part
The female/male ratio for hemangiomas is estimated to
of the problem in evaluating published reports and in
be 3 : 1.142,147 Caucasian infants have been reported to have
designing rational therapeutic plans resides in the confu-
an increased incidence over other racial groups.119,143,144
sion in terminology that still exists.
The clinical appearance is related to the depth of the pro-
Hemangiomas are benign vascular tumors composed of
liferating lesion. Superficial lesions are raised and bright
proliferative, plump, endothelial cells. They can occur in
red (Fig. 2.37). Deeper dermal lesions appear as bluish
skin, mucous membranes, and other soft tissues. A heman-
subcutaneous nodules. The overlying skin may have a fine
gioma often begins as a field transformation, frequently in
network of telangiectasia (Fig. 2.38). With regression,
multiple sites simultaneously or over a large area of skin.140
superficial lesions leave a flaccid, pedunculated, waxy,
The tumor may begin in subcutaneous tissue or muscle or
yellow-colored skin (Fig. 2.39). Deep lesions usually leave
may infiltrate the skin densely without elevating it, giving
a smooth skin surface with overlying telangiectasia. A
an appearance similar to that of a PWS.141 However,
mottled grayish mantle spreads towards the periphery of
whether superficial, deep, or mixed, the lesion is thought
the lesion and is less tense to palpation. Parents note that
to have the same histologic and biologic behavior pattern
deep lesions do not swell as much as superficial lesions
throughout.140 Hemangioma typically present with both
when the child cries.
a superficial component and a deep cutaneous compo-
The hallmark of hemangiomas is a rapid proliferative
nent as well as a subcutaneous proliferation of ectatic
phase. Immunohistochemical cellular markers that indi-
cate proliferation include type IV collagenase, vascular
The term ‘cavernous' is confusing. At times this term is
endothelial growth factor, basic fibroblast growth factor,
used to refer to the deep dermal component of a heman-
and other endothelial markers (CD31 and von Willebrand
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.37
Hemangioma on forehead of infant appeared at 2
Figure 2.38
(A) Clinical appearance of deep hemangioma with
weeks of age and gradually enlarged. (A) Appearance at 9 weeks of
overlying telangiectasias on lateral trunk of 3-month-old girl. (B) After
age. (B) Clinical appearance 4 months after initial laser treatment
five treatments with PDL at a fluence of 7 J/cm2. Note complete
and 1 month after fifth treatment with PDL at a fluence of 7 J/cm2.
resolution of hemangioma with only very faint persistence of
Treatments were given at 3-week intervals. Although some persistence
overlying telangiectatic component. Hypopigmented area was present
of the lesion is seen clinically as a faint pink macule, parents elected
before any treatment commenced.
to discontinue treatment. (Reprinted from Fitzpatrick RE: AmericanJournal of Cosmetic Surgery 9:107, 1992. With permission fromAmerican Academy of Cosmetic Surgery.)
the basement membrane, further thickening the vessel
factor).148 Growth is particularly rapid during the first 6
wall. Therefore, although response to laser treatment
months of life but may continue until 12 months of age.
occurs, it may not be as dramatic as in thinner-walled
Gradual spontaneous involution begins between the sixth
hemangiomas of infancy.
and tenth month.39,149,150 These stages are not distinct,
Studies of the natural history of hemangiomas reveal
because proliferation continues while involution slowly
that complete resolution occurs in 50% of children by age
begins to dominate. The first sign of regression is a change
5 and in 70% by age 7, with continued improvement in
in color from crimson to dull purple. Clinical studies indi-
the remaining children until ages 10 to 12.39,40,143,150–152
cate that involution proceeds on the same time schedule
However, 15% to 25% of lesions do not completely invo-
for both deep and superficial hemangiomas.142,147 Lesions
lute, and lesions that do not show significant signs of
located on the nose and lips are thought to involute more
regression by age 6 to 8 years are not likely to regress com-
pletely.152,153 Rate and extent of resolution are unrelated to
Adult hemangiomas are composed of mature, capillary-
lesion size147 (Fig. 2.40).
sized vessels approximately 100 mm in diameter, resem-bling dermal venules with virtually no endothelialgrowth.151 Basement membrane thickness ranges from 0.6
Complications and Adverse Sequelae
to 14 mm because of multiple superimposed layers of basallamina. At times an ingrowth of dermal collagen fibers is
Complications from the proliferative phase include ulcer-
seen in the vessel wall. Pericytic cells are also immersed in
ation (5% to 11% of patients) and infection, which is more
Cutaneous and Cosmetic Laser Surgery
Figure 2.40
(A) Hemangioma on left cheek of 15-month-old child.
Figure 2.39
Extensive hemangioma on right forehead and upper
Lesion arose at 3 weeks of age and increased in size over first year
eyelid of 9-month-old girl. (A) Clinical appearance before any therapy.
before beginning a short resolution. Patient was treated with oral
(B) Clinical appearance 7 months after systemic prednisolone therapy
prednisolone at 6 weeks of age until 6 months of age without
per protocol described in this text, plus six separate treatments with
noticeable effect on size of hemangioma. (B) Appearance at 3 years
PDL at a fluence of 7 J/cm2. CT scan demonstrated no intracranial
of age. Note redundant skin with some superficial atrophic scarring
connection with hemangioma. Severe orbital dystopia will be
and persistent superficial telangiectasia.
corrected when her bones are more fully developed at age 6. Inaddition, periorbital atrophy and loss of subcutaneous tissue in areaof hemangioma will be corrected with surgical excision after bonycorrection has been achieved. (Reprinted from Fitzpatrick RE:
Skeletal distortion is rare but may occur from a mass
American Journal of Cosmetic Surgery 9:107, 1992. With permission
effect on underlying bone. Lesions of the nasal tip often
from the American Academy of Cosmetic Surgery.)
distort underlying cartilage (Fig. 2.42). Deviation of facialbones or orbital enlargement may occur.157
Lesions of the upper eyelid may obstruct the visual axis,
causing deprivation amblyopia with failure to develop
common on the lip and genitoanal areas, where abrasion
binocular vision.158 Interruption of vision in infancy for as
is common149,154 (Fig. 2.41). Bleeding from trauma is an
briefly as 1 to 2 weeks can cause permanent damage, with
annoying and relatively common problem that usually
longer periods of obstruction being more harmful. Upper
responds to pressure. At times, superficial ulcerations
eyelid lesions may also distort the growing cornea by
appearing on buttock, sacral, or lip skin in infants can
direct pressure producing refractive errors (strabismic
precede the development of hemangiomas by a few weeks.
amblyopia).159 Large hemangiomas of the lower eyelid may
The reason for this evolution in some infants is
result in similar problems, but even the smallest heman-
gioma within the upper eyelid can cause visual distur-
A retrospective analysis of 60 pediatric patients showed
bances.160 Therefore all children with hemangiomas
that in the 37% of ulcerations that were treated with the
involving either the upper or the lower eyelid should be
PDL, 50% showed definite improvement, 18% showed no
referred to an ophthalmologist promptly, even if vision
response and 5% showed worsening. Other forms of treat-
appears normal.
ment included surgical excision (3%), interferon (8%),
Other functional problems may occur because of the
systemic antibiotics (43%) and systemic corticosteroids
sheer bulkiness of lesions in critical locations causing
obstruction. Lesions of the nose may interfere with breath-
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.42
(A) Five-month-old boy with 1.5-cm hemangioma on
tip of nose and overlying 4 ¥ 8-mm strawberry-red mark in center oflesion, which is very fluctuant. Hemangioma was present at birth andis continuing to enlarge. (B) Approximately 3 years later, after fourtreatments with pulsed dye laser at a fluence beginning initially at6.75, then 7.5, 8.0, and 8.5 J/cm2. In addition to laser treatment,patient received intralesional injection of triamcinolone, with a total of10 mg given over five injections Although nose has assumed normalappearance, it is still slightly boggy and increases in size with hotweather and when patient cries. (Reprinted from Fitzpatrick RE:American Journal of Cosmetic Surgery 9:107, 1992. With permissionfrom American Academy of Cosmetic Surgery.)
Extensive facial hemangiomas may also be associated
with cardiac and abdominal anomalies. Associated anom-alies can be right-sided aortic arch coarctation, a supra-umbilical midabdominal raphe defect, associated laryngeal
Figure 2.41
(A) Three-month-old girl with hemangioma on right
and duodenal hemangiomas, and posterior brain fossa
lower lip that was flat and pink at birth and slowly enlarged duringfirst 2 months before rapid enlargement over last 4 weeks, at which
abnormalities such as Dandy–Walker malformations.164–168
point it interfered with eating and was bleeding. (B) Clinical response
The constellation of findings consisting of large facial
2 years later and 1 year after last treatment with PDL. Total of nine
hemangiomas, posterior fossa malformations, arterial
laser treatments were given at 4- to 6-week intervals with a fluence of
anomalies, coarctation of the aorta and cardiac defects, and
7 J/cm2. Within 2 weeks of first laser treatment, definite improvement
eye abnormalities has been given the acronym PHACE syn-
with rapid resolution of lesion was seen, as well as near-immediate
drome.168 This syndrome represents a spectrum of malfor-
resolution of pain associated with eating. Slight depression appears in
mations of varying degrees of severity caused by a common
midportion of lower lip underlying previous hemangioma. However,there is no distortion of the vermilion border. (Reprinted from
morphogenetic event or events in utero.167,169
Fitzpatrick RE: American Journal of Cosmetic Surgery 9:107, 1992.
The facial hemangiomas seen in the PHACE syndrome
With permission from American Academy of Cosmetic Surgery.)
are usually plaque like in quality and cover at least one der-matome; 88% occur in females. Infants who have largefacial hemangiomas should be evaluated with head cir-cumference measurements and neurologic studies, includ-ing brain imaging with cranial ultrasound and MRI scans.
ing. Lesions in the subglottic airway may obstruct the
These patients also appear at risk for developing airway
larynx, causing life-threatening asphyxiation.161 Lesions
abnormalities and should be evaluated closely for cardiac
may obstruct the external auditory canal and cause a mild
defects as well.
to moderate hearing loss. Lesions in the anogenital area
Sacral hemangiomas have also been associated with
may cause obstruction, pressure, or tenderness when ulcer-
several anomalies, including imperforate anus, genitouri-
ated and inflamed.
nary abnormalities (absent or hypoplastic kidney, abnor-
Medical complications include the Kasabach–Merritt
mal genitalia), and tethered spinal cord without neurologic
syndrome162: generalized bleeding from profound throm-
bocytopenia associated with a large hemangioma or exten-
In a study of 175 cases of severe superficial heman-
sive hemangiomatosis. Congestive heart failure is a
giomas, defined as lesions involving large surface areas,
potentially lethal complication in an infant with multiple
symptomatic visceral hemangiomas were present in 11.4%
cutaneous and visceral hemangiomas.163
and associated malformations in 6.9%.172 The authors
Cutaneous and Cosmetic Laser Surgery
found that MRI was the best imaging modality to detect
ill-advised attempts at treatment that have resulted in
the deep growth. Hemangiomas give signals that are dif-
excessive scarring.176
ferent from lymphatic or arteriovenous malformations. It
Surgical intervention,177 X-ray therapy,72,176,178 sclero-
may also indicate the rapidity of blood flow movement.
therapy,176,179 diathermy,180 CO2 snow cryotherapy,181
Color-flow duplex ultrasonography was helpful but could
and electrocautery182 have all been attempted, resulting in
not accurately distinguish a hemangioma from an AVM
significant scarring. A basal cell carcinoma (BCC) present-
because both may have a high-flow pattern.
ing 58 years after thorium X applications to a birthmark
As described later, many hemangiomas resolve sponta-
when the patient was 3 years old has been reported.183
neously without any form of treatment. However, even
Thorium X, a rich source of alpha radiation, was frequently
when resolution is complete, the skin that remains after
used for treating hemangiomas until the 1960s.184,185 BCC
involution exhibits mild atrophy, has a wrinkled texture
developing after thorium X treatment of hemangiomas has
with a few telangiectatic vessels, or is paler than the sur-
also been reported.186 Angiosarcomas have also occurred
rounding skin.141 Resolution that is considered cosmeti-
after radiation treatment of hemangiomas, with a review
cally acceptable has been said to occur in 70% to 82% of
of the literature uncovering 11 such cases.187 The progno-
patients.154 However, in an analysis of 298 hemangiomas,
sis is poor, with a 10% 5-year survival rate.
80% that had not involuted by age 6 left a significant resid-
Thus these prior methods of treatment, because of
ual cosmetic deformity.173 In addition, 38% of those lesions
unacceptable side effects and lack of efficacy, have been
that had completely resolved by age 6 left a significant
replaced with laser therapy as a destructive and antiprolif-
residual deformity. Scarring after ulceration generally
erative modality and with other antiproliferative medical
leaves a white, flat area of fibrosis. When the hemangioma
treatments. As discussed later, some patients require
has been large, there is often redundant skin and a resid-
multiple forms of therapy.
ual subcutaneous fibrofatty tissue mass.141 Therefore, our
When the pathogenesis of these lesions is considered,
opinion, supported by the clinical studies detailed next, is
an antiangiogenic agent that would prevent proliferation
to treat lesions at the first available opportunity.
and induce involution of the entire lesion is the most
In addition to medical complications, including persist-
logical therapeutic approach.188 At present, the only
ent scarring, the psychologic consequences are real. A child
treatment of this type is the use of systemic steroids with
develops a sense of self-awareness between 18 and 24
or without interferon-alpha.
months of age. This awareness may be transmitted to psychosocial developmental disorders. Parental guilt,anger, and disappointment as well as over protectiveness
Systemic and Intralesional
are common. These feelings may affect the dynamics of
the entire family. A study consisting of interviews of the parents of 25 children aged 6 months to 8 years with
If a hemangioma is steroid responsive (30%–60% of
facial hemangiomas at least 1 cm in diameter reported neg-
cases),188,172 the result is often immediate and dramatic.176
ative social stigmatization in the majority of families.174
A treatment schedule of 2 to 3 mg/kg/day of prednisone,
For 32% of parents this interfered with outings. Common
rapidly tapered, when the tumor has reduced in size, is
feelings that the parents expressed were grief, loss, isola-
given for a cycle of 4 to 6 weeks, followed by a rest period.
tion and guilt. For the children, social sensitivity occurred
Complications have been few.188 In patients who fail to
after age 4. This study underscores a family's need for
respond, however, increasing the dose of prednisone to 4
more than just medical management of an infant's
to 5 mg/kg/day may trigger growth of the lesion.172 Loss of
appetite and diminished growth rate have been observedbut resolve quickly when steroids are discontinued.
However, because the proliferative activity of the tumor
continues for 6 to 12 months, the need for prolongedsteroid suppression persists, with rebound growth of the
Treatment of hemangiomas has been oriented toward
hemangioma occurring when treatment is discontinued.
waiting for natural regression to occur since Lister's article
This often results in an escalating steroid dosage or aban-
in 1938 outlined a predictable course for these lesions.152
donment of this regimen because of concern regarding
However, contemporaries of Lister recognized the effec-
potential steroid side effects.
tiveness of early treatment before and during the pro-
A second steroid schedule has been used throughout
liferative stage of growth.175 Therapeutic intervention
the entire proliferative phase of the hemangioma. Pred-
historically has been reserved for lesions causing recurrent
nisolone is given in a dosage of 3 to 5 mg/kg/day for 2 to
bleeding, ulceration, infection, or serious distortion of
4 weeks until control of growth of the hemangioma is
facial features and lesions that interfere with normal
achieved. An alternate-day schedule is then instituted by
physiologic functions, such as breathing, hearing, eating,
doubling the dose on the ‘on' day and eliminating the
vision, and bladder and bowel function. Despite these
alternate-day dosage gradually over 2 weeks. Prednisolone
therapeutic guidelines, there is tremendous pressure on
is then tapered every 2 weeks by 5 mg if the hemangioma
anguished, concerned parents observing an enlarging
does not enlarge.189 This treatment schedule is maintained
hemangioma on their child, particularly when it is a facial
for 6 to 10 months. In 43 infants treated, only one infant
lesion of any size. Because of this, there have been many
had transient side effects. However, it is advisable to use
Laser Treatment of Cutaneous Vascular Lesions
emphasizes the need for close monitoring of patients whorequire systemic steroids.
Intralesional steroids have been used successfully in
the treatment of periorbital hemangiomas.191,192 Soft tissueatrophy is common but seems to be temporary.191 Intra-lesional steroid injection carries a risk of hemorrhage orhematoma in the retrobulbar space, which is a threat tovision.167 It may cause occlusion of the central retinalartery and damage to the optic nerve.193 When the heman-gioma extends posterior into the orbital cone, systemicsteroids should be considered.176
A study of 70 children with 74 hemangiomas treated
with intralesional corticosteroids showed more than 75%reduction in volume in 58% of patients, 50% to 75%improvement in 22%, 25% to 50% improvement in 12%,and less than 25% improvement in 8%.194 No patient hadregrowth after treatment with a mean follow-up of 14months. The authors used doses of 10 to 120 mg per injec-tion of triamcinolone and betamethasone acetate (1.5–18 mg/injection) given in a total volume of 0.5 to 6.0 mL,
Figure 2.43
(A) Large hemangioma over right side of face of 4-
depending on the hemangioma's size. Side effects included
week-old girl. This lesion began as a red macule 5 mm in diameter,
temporary cushingoid facies and hypopigmentation, each
first noted over right cheek 2 days after birth. Hemangioma spreadrapidly over left forehead, cheek, neck, and eyelid over only 2 to 3
in two patients. No apparent correlation existed between
days. Patient was initially treated with pulsed dye laser (PDL) alone at
response and lesion location, size, patient gender, or age.
a fluence of 7.25 J/cm2. However, when seen 2 weeks later, although
Enjolras and Mulliken195 advise giving 3 to 5 mg/kg/pro-
there was definite improvement in laser-treated site, hemangioma
cedure (either intralesional or systemic) of triamcinolone
was continuing to enlarge rapidly, involving entire orbit and right
with or without cortisone acetate. They believe that
parotid and neck area. Subcutaneous masses were present, and
intralesional and systemic corticosteroids have a similar
prednisolone was begun at a dosage of 4.5 mg/kg/day. Laser
response rate. They advise caution when injecting
treatment was continued at a fluence of 7.5 J/cm2 at 2-week intervals. Almost immediately after institution of oral prednisolone,
hemangiomas of the upper eyelid because particles in the
hemangioma stopped growing and began a resolution phase.
steroid suspension can potentially occlude retinal or
Prednisolone dosage was tapered 2 weeks after institution. When it
choroidal microvessels or the central retinal artery.
was discontinued totally on pediatrician's advice 6 weeks later,hemangioma again began to enlarge. We therefore restartedprednisolone therapy, increasing it to a dosage of 5 mg/kg/day to
initiate resolution of hemangioma. During this time, lesion wascontinually treated at 2- to 3-week intervals with PDL at fluences
Interferon is the second-line drug for treating severe, large
ranging from 7.0 to 7.5 J/cm2. Interestingly, on alternate-day
hemangiomas. Interferon-alpha2a (IFN-a2a) is a potent,
prednisolone therapy, patient's parents noticed hemangioma
fairly well-tolerated cytokine that requires liver and hema-
increasing in size during the day off therapy, with it regressing in size
tologic monitoring during therapy. Long-term treatment
during the day on prednisolone therapy. Prednisolone therapy was
carries the risk of thyroid dysfunction and neurologic com-
tapered over 2 years before being discontinued. (B) Patient, now 11
plications. Interferon is known to inhibit angiogenesis,
months old, has marked resolution of hemangioma. At this point she
probably through inhibition of vascular smooth muscle
is continuing to have prednisolone therapy and has had total of ninetreatments with PDL, with last treatment 4 months previous to this
cells and capillary endothelial cells.196
photograph. (C) Patient is now 7 years old without recurrence.
In a study of 20 patients with vision-threatening
(Reprinted from Fitzpatrick RE: American Journal of Cosmetic Surgery
hemangiomas resistant to corticosteroids, 18 patients had
9:107, 1992. With permission from American Academy of Cosmetic
more than 50% regression after an average of 8 months of
the lowest effective dose of steroid for the shortest period
until the hemangioma enters the regression phase (Fig. 2.43).
At times, especially when treating large or extensive
Adverse effects of systemic corticosteroids include
hemangiomas and conditions such as diffuse neonatal
immunosuppression, osteoporosis, hypertension, cushin-
hemangiomatosis, multiple modalities may be required. In
goid features, hypothalamic–pituitary–adrenal (HPA) axis
these patients, systemic prednisone with or without inter-
suppression, impaired glucose tolerance, growth suppres-
feron, embolization of hemangiomas, surgical debulking
sion and ocular complications. One study of 22 patients
and excision of the dermal component, and PDL treatment
with hemangiomas treated with an average corticosteroid
to superficial lesions may all be used198 (Fig. 2.44).
dose of 2.23 mg/kg/day for an average of 28.1 weeks found
Although one might believe that the laser is cosmetic, its
irritability, fussiness or insomnia in 73%, hypertension
use in these patients prevents cutaneous hemorrhage and
in 45% and HPA suppression in 87%.190 This review
facilitates routine skin care.
Cutaneous and Cosmetic Laser Surgery
a hemangioma are its depth of penetration, energy fluence,and pulse duration. Lasers with short wavelengths (<585 nm) can only deliver sufficient energy required forthermocoagulation to a depth less than 1 mm. Pulse dura-tions necessary range from 0.5 to 10 ms, depending on thesize of the ectatic vessels. Energy fluences should be greaterthan 6 J/cm2. With these parameters, many differentpulsed-light modalities have demonstrated efficacy.
Argon Laser
The argon laser199–201 has been used effectively to treat
hemangiomas. The potential benefit of the argon laser
is limited by its depth of penetration into the dermis
(<1 mm) and its tendency to cause hypertrophic scarring
through nonspecific thermal injury, as reported in children
with PWS.25 However, with PWSs thicker than 1 mm, non-
specific thermal injury may be advantageous with a physi-cian experienced in the laser's use. In addition, onephysician noted atrophic, hypopigmented scarring inthree of five cases of hemangioma treated with the argonlaser.202
Nd:YAG Laser
The Nd:YAG laser at 1064 nm can penetrate deeply into
tissue (2–8 mm) but produces widespread tissue injury
because of its nonspecific absorption. This tends to result
in scar formation. The Nd : YAG laser has been successful
in shrinking large symptomatic lesions through non-
specific thermal injury,203 but the risk of scarring, which
always occurs to some extent,204 must be weighed against
potential benefits.
Figure 2.44
(A) Venous malformation involving lip and mucosa
Some physicians believe the Nd : YAG laser should be
before treatment. (B) After therapy with Nd:YAG laser delivered as
limited to treating mucous membrane lesions (e.g., oral
two separate treatments 6 weeks apart. Laser was used with 600-mmfilter in noncontact mode and energy fluence of 30 W delivered in
mucosa). Treatment technique also makes a difference.
0.2-s pulses to shrink malformation. This was followed 6 weeks later
Treatment using 3- to 4-mm-diameter spots with 6- to 8-
by surgical resection. This photograph is clinical appearance 6
mm untreated lesion between spots has been advocated as
months after surgery. (Courtesy Milton Waner, MD.)
a method to decrease scarring while still effectively debulk-ing a lesion.
Early therapeutic intervention with laser photocoagula-
In 160 patients treated with the Nd : YAG laser at energy
tion of the vessels of a hemangioma when it is in a thin,
fluences of 400 to 1600 J/cm2 in a continuous manner with
flat stage or on initial presentation as localized telangiec-
pulses of 0.5 s, 13% had excellent results, 55% had a reduc-
tasia is advocated to minimize enlargement of the tumor,
tion in hemangioma size by more than 50%, 35% had a
ulceration, bleeding, and obstruction of vital organs.30,32
reduction in lesion size by less than 50%, and 2% had
This may be effective theoretically if the laser is capable of
negligible results.205 Ten percent of patients had scarring
reaching all the vessels present. However, the notion that
from superficial necrosis. The incidence of textural or pig-
superficial photocoagulation will initiate regression of the
mentary changes to the overlying skin was not reported.
entire hemangioma, including adjacent untreated tumor,
The authors noted an advantage of surgical resection of
does not appear to be true. Our experience, along with that
bulky tumors after thermocoagulation was obtained with
of several colleagues, is that the deep portion of heman-
the Nd : YAG laser.
giomas beyond the depth of laser penetration continues to
Dr Berlien in Germany has successfully used the
proliferate despite involution of the superficial compo-
Nd : YAG laser to coagulate tissue, with its fluence being
nents treated by laser.28,30,32
transferred to the bulk of the tumor through a 600-mm barefiber at 8 to 10 W on a continuous mode until tumor coag-
ulation occurs. The fiber is introduced through an 18-gaugeTeflon cannula, and the skin is monitored with a thermal
The necessary requirements for any laser or pulsed light
probe and cooled to minimize thermal injury to the epi-
source to thermocoagulate effectively the ectatic vessel of
dermis. The fiber is slowly extracted at a rate approximat-
Laser Treatment of Cutaneous Vascular Lesions
ing 0.5 mm/s, and monthly treatments are given to allow
with 8% maintaining this reduction at 6 months. To
resorption of coagulated tissue. Postoperative edema and
achieve these results, 50% required two treatments and
some pain occur for about 6 hours. A study of 100 pedi-
8% three treatments; 33% of hemangiomas ulcerated after
atric patients treated over 3 years with intralesional 532 nm
therapy. Thus this aggressive form of treatment is best
KTP and 1064 nm Nd : YAG laser in continuous mode for
reserved for large, voluminous hemangiomas that have
one to four treatments showed a 90% reduction in size in
functional significance (e.g. airway obstruction, visual
446 patients and 50–90% reduction in 56 patients of the
hemangiomas with no difference between the two lasers
The 532 nm Nd:YAG laser can be used on the skin to
used.206 Surgical resection was required in 76 patients.
treat superficial hemangiomas. A retrospective study of 50
Thus, the intralesional laser decreased the size of the lesion
infants with 62 superficial hemangiomas found that com-
to permit a smaller, more cosmetic resection of the
plete regression occurred in 41% of lesions with PDL treat-
ment versus 30% with the 532 nm Nd : YAG laser.214 The
Use of the sapphire-tip contact probe with the Nd : YAG
532 nm Nd : YAG laser was used at 20 J/cm2 with a 50-ms
laser allows excision of vascular lesions with control of
pulse through a 5-mm-diameter spot. The PDL was a 0.45-
bleeding and offers another therapeutic approach in
ms, 585 nm laser with a 7-mm spot size.
debulking the lesion.207,208 However, a retrospective analy-sis of 11 patients treated with this technique demonstratedonly three ‘aesthetically acceptable' results despite four
Carbon Dioxide Laser
separate treatments.209 In contrast, Afpelberg210 has
The CO2 laser is also useful as a scalpel to excise and debulk
reported total removal without complications or side
vascular tissue with minimal blood loss. It has been used
effects in 16 patients treated in this manner. The difference
to excise laryngeal lesions causing airway obstruction,30,161
in treatment results may be related to preoperative prepa-
oral hemangiomas,215–217 and facial lesions.218 Because this
ration of the patient with intralesional corticosteroids or
treatment modality has also been associated with a higher
waiting until the lesion is in a stable or regressive stage
rate of scarring, it is not recommended for cutaneous
before proceeding with surgical correction.
lesions and is most efficacious in treating internal and
Cryogen spray cooling of the epidermis overlying
mucosal hemangiomas.30 In our practice the UPCO2 laser
hemangiomas may protect the epidermis and papillary
has been used to resurface scarred hemangiomas and
dermis while achieving deep tissue photocoagulation
tighten those with redundant and stretched tissue with
during Nd : YAG laser irradiation.211 A preliminary study on
excellent results (Fig. 2.45).
the highly vascularized chicken comb demonstrated 6.1-mm-deep photocoagulation while preserving epidermalintegrity. To be effective, however, surface temperature
Pulse Dye Laser
monitoring must occur simultaneously with laser treat-
Although initially developed to treat vessels present in
ment and cooling to prevent epidermal damage. Therefore
PWS, the similar vessel diameter and depth in heman-
this technique may allow treatment of thick hemangiomas
giomas explains the PDL's clinical efficacy. The depth of
with a deep component.
penetration of 585 nm is 0.6 to 1.2 mm, which limits its
One study detailing the treatment of 61 deep vascular
efficacy to relatively superficial lesions. Its safety and speci-
malformations with a contact cooling 1064 nm Nd : YAG
ficity are the reasons for its appeal. Early studies have
laser showed that 50% of treated lesions demonstrated
demonstrated efficacy in hastening resolution of lesions
55% or greater resolution after a single treatment session
beyond the proliferative phase,28,32 as well as slowing or
with 35% having less than 50% improvement and 20%
arresting proliferative growth.28,30,32 The PDL is best uti-
having 100% resolution.212 Unfortunately, many of the
lized and most effective in eradicating lesions in a macular
lesions presented in this paper did not have specified treat-
stage before proliferation.30,32
ment parameters.
The PDL will not affect hemangioma vasculature below
1.5 mm, because proliferation of deeply situated heman-gioma vessels proceeds despite involution of superficial
532 nm Potassium Titanyl Phosphate (KTP) and
Nd : YAG Laser
Of 50 patients (mean age 13 months) treated with
The KTP laser used through an intralesional 0.6-mm-
the PDL an average of 3.8 times, 53% had significant
diameter bare fiber has been found to shrink heman-
improvement in color without an appreciable reduction in
giomas.213 With this technique the fiber is passed through
lesion bulk.219 Four of seven patients with flat lesions com-
a 20-gauge needle positioned in the center of the heman-
pletely cleared after two treatments, with the remaining
gioma. Laser energy is then delivered at 15 J until shrink-
three patients achieving satisfactory results requiring no
age is seen or the overlying skin begins to feel warm to the
further treatment. Eight flat, ulcerated hemangiomas
touch. However, this is inaccurate and potentially danger-
healed completely with PDL treatment after one to three
ous because once the overlying skin is warm, excessive
treatments. Ten patients with slightly raised lesions
nonspecific damage has probably occurred. In a series of
required a mean of 5.2 treatments to achieve clearance in
12 patients 1 month to 31/2 years of age, 92% had a greater
four and a good degree of flattening and improvement
than 50% reduction in the hemangioma size at 3 months,
in the remaining six patients. Red raised lesions with a
Cutaneous and Cosmetic Laser Surgery
2- to 4-week intervals at fluences of 6.0 to 6.5 J/cm2.
Almost 70% healed within 2 weeks after a single laser treatment.
Garden et al222 have reported that the best results occur
when the hemangioma is elevated 3 mm or less and advisetreatment in the first weeks of life. They studied 33 heman-giomas in 24 patients 2 weeks to 7 months of age in whom
a 93.9% lightening occurred in the superficial lesions in4.1 treatment sessions. Seven lesions 4 mm or more inthickness lightened 83.7% in seven treatment sessions.
They found that compressing the hemangioma with a glassslide to bring the deeper vascular component closer to thesurface did not increase efficacy.
A report on treating 68 infants with 100 hemangiomas
within 12 weeks of their development further confirmsPDL efficacy.223 Seventy-three lesions required a singletreatment and 27 up to five treatments; 23% of lesionsshowed complete remission, 55% showed partial remis-sion, and 14% stopped growing. Only 8% of lesions con-tinued to grow despite treatment. This efficacy occurredwith virtually no serious adverse sequelae.
Proliferative hemangiomas causing functional impair-
ment in seven patients 8 to 24 weeks of age showed significant reduction in size, normalization of color, andresolution of superficial ulceration.224 Lesions were treatedat fluences of 7.0 to 9.25 J/cm2 at 4- to 8-week intervals(two to six times) until the hemangioma completelyregressed or stopped regressing further. As reported in theprevious studies, all patients with ulceration responded totreatment. Four of seven patients had complete resolutionof the hemangioma. No adverse events or complicationswere noted.
Our experience is similar to that of most reports.225 The
following is a summary of our experience with a total of34 infants and children. We used the PDL at fluences of6.25 to 8.0 J/cm2 at 2- to 3-week intervals with spots over-
Figure 2.45
(A) Appearance of hemangioma in 5-year-old girl.
(B) Clinical appearance 30 years later. Note flaccid, wrinkled skin.
lapping 10% to 15% to produce an endpoint of homoge-
(C) Six months after resurfacing with Coherent Ultra Pulse CO2 Laser.
nous darkening of the entire lesion until one of the
Three passes were given at 500 mJ through 3-mm collimated spot size
following circumstances: resolution occurred, the treat-
ment was judged to be ineffective, or the parents discon-tinued treatment for unrelated reasons. Treatment wasoffered to all parents of children with hemangiomas after
significant subcutaneous component showed elimination
explanation of the natural resolution history of the lesions
of the red coloration and any ulcerations without change
and the potential benefits and risks of laser therapy and
in lesion bulk.
steroid therapy. Some patients had medical reasons for
Morelli and Weston220 reported their 2-year experience
treatment (visual obstruction, interference with feeding or
in treating 55 lesions. Five patients with hemangiomas less
breathing, or lesional bleeding), but treatment was also
than 3 cm3 treated once or twice with the PDL in the pro-
offered to patients with lesions that caused concern
liferative phase had a good response with arrest of lesion
because of proliferative activity, tissue distortion, or cos-
growth. An additional five patients with large lesions
greater than 20 cm3 had an excellent response and resolu-
Seventeen hemangiomas had only a superficial compo-
tion of lesions in those who continued treatment. Twenty
nent, with 13 of these less than 1 cm in diameter, averag-
patients with superficial and deep lesions had resolution
ing 7 mm. The lesions appeared as a red macule initially,
of the superficial component only. Purely nodular older
at an average age of 15 days. The lesions typically began
lesions resisted treatment. Ulcerated painful heman-
to proliferate, causing parental concern. The average age at
giomas, when treated, heal and become painless, provid-
first treatment was 5.5 months. The most common reason
ing the best indication for treatment. Morelli et al221
for the delay in treatment was difficulty in finding an
reported a total of 37 infants with ulcerated hemangiomas
appropriate treatment center. The average patient received
treated with the PDL between 2 and 40 weeks of age.
3.6 treatments over 3.3 months. The average fluence used
All ulcerations healed with one to three treatments at
was 6.8 J/cm2. Nine patients (53%) cleared completely.
Laser Treatment of Cutaneous Vascular Lesions
Table 2.8
Superficial Capillary Hemangiomas: PDL Treatment
age lesion
age first
if not clear
Table 2.9
Mixed Superficial/Deep Capillary Hemangioma: Early PDL Treatment
age lesion
age first
Table 2.10
Mixed Superficial/Deep Capillary Hemangioma: Late PDL Treatment
age lesion
age first
Those lesions not clearing had an average improvement of
age of 41 months. These nine lesions received an average
67% (Table 2.8).
of 3.5 treatments, using an average fluence of 7.2 J/cm2
Seventeen infants had hemangiomas classified as mixed,
and a treatment interval averaging 9 weeks. Two patients
with both a superficial and a deep component. All these
received intralesional steroids in an attempt to shrink
lesions were large, some encompassing the entire side of
the persistent deep component of the lesion. These nine
the face. Eight lesions were treated relatively early, during
patients achieved a much more modest degree of improve-
the proliferative phase, and nine lesions were treated later,
ment than the other eight patients treated early, with the
during the involution-dominant phase (Table 2.9).
superficial component improving an average 62% and the
Those hemangiomas treated early had appeared at an
deep component only 32% (Table 2.10).
earlier age (average age 6 days). Treatment was initiated at
Most authors report cessation of the proliferative phase
an average age of 4.5 months and continued for an average
as a consequence of treatment with the PDL.30,31,222 Con-
7.4 months, during which time 4.5 treatments were
troversy has surrounded the question of whether early
administered, using an average fluence of 6.8 J/cm2. Four
treatment can prevent the proliferative phase and espe-
patients (50%) received adjunctive therapy with steroids
cially whether it may have an effect on the deep com-
during this period, two receiving systemic prednisolone
ponent that the PDL cannot reach.226 Indeed, the case
and two receiving intralesional steroids. Interestingly, in
reported by Glassberg et al30 showed deep proliferation
these four patients, treatment with steroids abruptly halted
despite cutaneous involution during PDL therapy. Mul-
the proliferation of the deep component and resulted in
liken176 reported his experience of the same phenomenon.
shrinking this deep portion, but it had no visible effect on
This was our experience as well: four patients with super-
the superficial component. Likewise, the PDL treatment
ficial and deep components showed response of only the
abruptly halted proliferation and resulted in clearance of
superficial component to treatment with the PD laser,
the superficial component without apparent effect on the
while the deep component continued to proliferate. Inter-
deep component, which continued to proliferate unless
estingly, the opposite was true as well; only the deep com-
steroids were used. Treatment resulted in near-complete
ponent was responsive to steroid therapy, the superficial
(96%) clearing of the superficial component in all patients
component being unaffected. Glassberg et al30 also have
and an average improvement of 73% in the deep compo-
mentioned this point.
nent (Table 2.9).
The cases reported by Glassberg et al,30 Sherwood and
Lesions treated later in their course appeared at an
Tan,31 and us well illustrate that the PDL can rapidly and
average age of 40 days but were not treated until an average
dramatically halt the proliferative superficial component.
Cutaneous and Cosmetic Laser Surgery
In two of these three series, the hemangiomas were very
extensive and were responsive throughout the superficialcomponent. Because the depth of penetration is limited, aseries of treatments is usually necessary gradually to reachdeeper layers of vessels. We recommend a treatment sched-ule of every 2 to 3 weeks for three to six treatments, usinga fluence of 5 to 7 J/cm2 for most lesions. Lesions with onlya superficial component are very responsive to treatmentbecause 90% will completely involute (see Fig. 2.37). Thosewith a deep component and a superficial component mayrequire intralesional steroid therapy for the deep compo-nent and laser therapy for the superficial component (seeFigs 2.42 and 2.43). Widespread and deep lesions mayrequire laser therapy and systemic steroids to arrest thegrowth of the deep component. Treatment at the earliestopportunity is critical in determining success in thesecases.
Kauvar227 presented the 1.5-ms pulsed, 595 nm PDL in
Figure 2.46
(A) Fifteen-year-old girl with facial hemangioma.
the treatment of ten hemangiomas. Two superficial lesions
(B) Appearance after 6 treatments with the PDL. (Courtesy Gerald
cleared in two or three treatments, and eight lesions with
both a superficial and a deep component cleared in two tofour treatments. In the latter group, the superficial com-ponent cleared completely, but with only a 10% to 50%
Intense Pulsed-light Treatment
reduction in the deep component. Kauvar recommends thefollowing parameters:
In addition to monochromatic light, noncoherent lighthas also been found to be effective in treating heman-
infants: 7-mm-diameter spot, 7 J/cm2
giomas when used within an adequate wavelength range
adults: 7-mm-diameter spot, 8 J/cm2
and with proper fluence and pulse duration. We have used
hypertrophic lesions: 7-mm-diameter spot, 9 J/cm2.
the IPL with a cutoff filter at 550, 570, or 590 nm to treat
A total of 165 children with 225 separate hemangiomas
multiple hemangiomas with excellent results (Fig. 2.47).
treated with the PDL with a mean of two treatments were
We have used this light source to treat evolving as well as
separated out as superficial or mixed hemangiomas.228 Flat
long-established tumors. One 66-year-old patient devel-
cutaneous hemangiomas had an excellent response in 32%
oped a hemangioma after trauma 15 years before treat-
and good results in 52%. Of the mixed hemangiomas, 39%
ment. One treatment with a 570-nm cutoff filter at
had a response of the superficial component with contin-
80 J/cm2 given as a double pulse of 9 and 13 ms separated
ued proliferation in 61% and no change in the dermal
by a 50-ms delay resulted in 100% resolution. The lesion
component. The authors concluded that early treatment is
immediately became purpuric, then crusted before com-
only effective for superficial lesions and did not prevent
pletely involuting (Fig. 2.48).
progression of deep or mixed lesions. The drawback of this
Foster and Gold230 reported 90% involution of a 7.5 ¥
study was that the investigators used an old PDL at
4-cm ulcerated cavernous hemangioma on the abdomen
585 nm, 300-ms pulse and a 5-mm spot beam without epi-
of an 11-week-old black infant. They used a 550-nm cutoff
dermal cooling allowing for only 5–7 J/cm2 to be delivered.
filter at a fluence of 38 J/cm2 in a triple-pulse mode (T1
One would suspect that the use of a 595 nm PDL with a
3 ms, T2 2 ms, T3 1.7 ms) with a 10-ms delay between pulses.
7- to 10-mm-diameter spot and epidermal cooling would
Two additional treatments at 2-week intervals with a 570-
allow deeper therapeutic effects to be obtained.
nm cutoff filter at fluences of 30 and 38 J/cm2 using a triple
The largest study on the treatment of childhood heman-
pulse (T1 4 ms, T2 3 ms, T3 2 ms) with a 20-ms delay between
giomas evaluated 548 children with 692 hemangiomas
pulses resulted in maximal resolution with minimal
who were treated with the PDL.229 After 1 to 12 treatments
(mean 2.5) further growth was stopped in 96.6% of allhemangiomas; 13.8% achieved a complete remission with
a significant regression in another 14.9%. Small and super-ficial hemangiomas responded best to treatment. As with
Surgical therapy should be considered for certain lesions.
previous studies, the drawback of this study was the use of
Hemangiomas of the vermilion border, mucous mem-
a first-generation PDL with a pulse duration of 0.45 ms and
branes, and nasal tip are very slow to involute and may
a spot size of 5 to 7 mm. A mean fluence of 8.4 J/cm2 was
seriously interfere with a child's self-esteem. In these cases,
used. Adverse effects even with this high fluence were rare
excision before entering school may be considered.176
with 7% developing temporary pigmentary changes and
Microsurgical techniques with hemostatic lasers have been
4% developing small atrophic scars. The age at the start of
successful at debulking lesions and coagulating vessels
treatment was not important in this study (Fig 2.46).
to allow for easier surgical excision.231 The use of tissue
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.48
(A) This 66-year-old male had a 15-year history of
hemangioma on forehead before treatment. (B) Immediately aftertreatment with PhotoDerm VL (see text). (C) Nine days aftertreatment an eschar developed, which healed without scarring 3 months later. (D) Complete resolution 4 weeks later.
Figure 2.47
(A) Persistent hemangioma/venous malformation
the only antiangiogenic agents currently available are cor-
despite four treatments with argon laser (0.5-s pulses at 2 W) and one
ticosteroids and that they may afford dramatic benefit
treatment with the PDL (8 J/cm2 with 5-mm-diameter spot size).
without significant risks as one might expect. When this
(B) After three treatments with Photoderm VL with 590-nm cutoff
therapeutic approach is considered in light of the recent
filter, double pulse of 4.2 and 7.7 ms with 300-ms delay between
information available regarding laser responsiveness of the
pulses at 65, 71, and 69 J/cm2, respectively.
superficial component, we conclude that the PDL shouldbe used at the earliest sign of a capillary hemangioma andcertainly as soon as active proliferation begins. In con-junction with this treatment, intralesional or systemic
expansion further improves excision cosmesis.232 Facial or
steroids should be given to halt the proliferation of the
neck hemangiomas that have incompletely resolved with
deep component when that portion proceeds despite laser
sagging skin or excessive fibrofatty residuum may be
therapy. The risks of therapy have been demonstrated to
Ideally, a team approach should be used in treating
Extensive hemangiomas that have not responded to
extensive hemangiomas. Multiple specialties, including
other treatments have reduced with IPL treatment.235
but not limited to vascular surgeons, radiologists, derma-
The recommendations of the American Academy of Der-
tologists, plastic surgeons, and pediatricians, may combine
matology's Guidelines of Care Committee for treatment of
their expertise to optimize patient care. Apfelberg et
hemangiomas are summarized as follows236:
al207,234 have reported the use of the Nd:YAG laser with sapphire tip in conjunction with intralesional steroids and
1. Prevent or reverse life-threatening or function-
also in a team approach using superselective embolization
before resection.
2. Prevent permanent disfigurement left by residual skin
3. Minimize psychosocial stress.
4. Avoid scarring procedures.
Mulliken140 correctly calls for a biologic approach to the
5. Prevent or treat ulcerative lesions to minimize scarring,
treatment of hemangiomas of infancy. He points out that
infection, and pain.
Cutaneous and Cosmetic Laser Surgery
Pyogenic granuloma (PG) is an acquired vascular lesion, a
The term ‘telangiectasia' refers to superficial cutaneous
true neoplasm distinct from granulation tissue, usually
vessels visible to the human eye.248 These vessels measure
solitary, 0.5 to 2.0 cm in diameter, bright red, and pedun-
0.1 to 1.0 mm in diameter and represent a dilated venule,
culated.237–239 The surface is soft, bleeding easily with
capillary, or arteriole. Telangiectasia that are arteriolar in
trauma. It may become ulcerated and develop a granulo-
origin are small in diameter, bright red in color, and do not
matous surface with a brown or black crust. Lesions usually
protrude above the skin surface. Those that arise from
appear suddenly and may enlarge rapidly. There is no
venules are wider, blue in color, and often protrude above
history of preceding trauma or infection in most patients
the skin surface. Telangiectasia arising at the capillary loop
(75%),240 although this typically is assumed.241 These
are often initially fine, red lesions but become larger and
lesions also frequently occur as a superimposed growth on
purple or blue with time because of venous backflow from
the surface of a PWS.158 Repeated episodes of bleeding and
increasing hydrostatic pressure.249
unresponsiveness to electrocautery have been reported in
Telangiectasia have been subdivided into four classifica-
up to 50% of patients.240 This may be secondary to
tions based on clinical appearance: (1) simple or linear, (2)
the extension of vascular proliferation deep into the
arborizing, (3) spider, and (4) papular250 (Fig. 2.49). Red
dermis, often with a unique lobular arrangement of
linear and arborizing telangiectasia are very common on
the face, especially the nose, midcheeks, and chin. These
The argon laser,243 as well as the CO2 laser,244 has been
lesions are also seen relatively frequently on the legs. Blue
shown to be effective in treating PGs. The PG is photo-
linear and arborizing telangiectasia are most often seen on
coagulated until the entire lesion blanches and turns a
the legs but also may be present on the face. Spider telang-
dusty gray color. Treatments are repeated at 3- to 4-week
iectasia are described in the next section. Papular telang-
intervals as needed.
iectasia are frequently part of genetic syndromes, such as
PG lesions have been unpredictably responsive to the
Osler–Weber–Rendu disease, and also are seen in collagen
PDL.78 They have been shown to respond,38,241,245,246 but in
vascular diseases.
most cases lesions are too thick for the laser to penetrate
All forms of telangiectasia are thought to occur through
throughout the lesion in one treatment. Tan and Kurban245
the release or activation of vasoactive substances under the
use a glass slide to compress the superficial ectatic vessels
influence of a variety of factors, such as anoxia, estrogen,
and use the laser through the glass to treat the deeper com-
corticosteroids (topical or systemic), various chemicals,
ponent of this lesion. This maneuver presumably allows
multiple types of bacterial or viral infection, and multiple
treatment of deep vessels, after which the slide is removed
physical factors, with resultant capillary or venular neo-
and the treatment is repeated to coagulate more superfi-
genesis.249 Box 2.2 lists the associated diseases and causes
cially located vessels. When effective by itself, the PDL
of telangiectasia.
must be used with multiple, 100% overlapping pulses toturn the lesion deep purple. As previously demonstrated,this technique produces nonselective photo-thermolysisand is therefore no different than CW argon, copper vapor,or 577-nm dye lasers.
A study of 18 patients with PG treated with the PDL
demonstrated both symptomatic and clinical clearing in16 patients with excellent cosmetic results.247 Seven of thelesions had been previously treated with electrosurgery orexcision. The authors flattened the lesions with a glass slideand used fluences of 6.5 to 9.0 J/cm2 in an overlappingmanner to cover the lesion completely. Treatments wererepeated up to four times to achieve success. Treatmentoutcomes were excellent, but two postoperative photosshowed textural changes that resembled a scar, althoughthis was not noted by the authors.
We have not been able to achieve uniform success in
treating PG lesions with or without the diascopy maneu-ver with the PDL. In addition, multiple treatments areimpractical because of the ease with which lesions aretraumatized between sessions. We therefore recommendshave excision of the lesion's papular component if a his-tologic specimen is necessary or CO2 vaporization of thelesion followed by PDL therapy to the remaining flatmacular lesion if necessary.
Figure 2.49
Four types of telangiectasia. (A) simple; (B) arborized;
(C) spider; and (D) popular. (Modified from Goldman MP, BennettRG. J Am Acad Dermatol 1987; 17:167.)
Laser Treatment of Cutaneous Vascular Lesions
Causes of Cutaneous Telangiectasia
Telangiectasia macularis eruptiva perstans
Carcinoma telangiectasia (metastatic tumors)
Ataxia telangiectasia
Component of a primary cutaneous disease
Sturge–Weber syndrome
Maffucci syndrome
Basal cell carcinoma
Congenital poikiloderma (Rothmund–
Merkel cell tumor
Thomson syndrome)
Necrobiosis lipoidica diabeticorum
Poikiloderma vasculare atrophicans
Cockayne syndrome
Capillaritis (purpura annularis telangiectodes)
Hereditary hemorrhagic telangiectasia
Xeroderma pigmentosum
Pseudoxanthoma elasticum
Essential progressive telangiectasia
Generalized essential telangiectasia
Superficial epithelium with sebaceous differentiation
Familial (autosomal dominant)Acquired (hormonal or infectious stimulation)
Unilateral nevoid telangiectatic syndrome
Diffuse neonatal hemangiomatosis
Corticoid induced
Hereditary benign telangiectasia
Cushing syndrome/diseaseIatrogenic (from systemic, topical, or intralesional use)
Acquired disease with secondary cutaneous
Estrogen therapy (usually with high dose)
component: Collagen vascular diseases
Lupus erythematosus (especially periungual)
Actinic dermatitis
Progressive systemic sclerosis (especially periungual, and
with the calcinosis, Raynaud, esophageal dysmotility,
Postsurgical, especially in suture lines under tension and
sclerodactyly, and telangiectasia [CREST] syndrome)
after rhinoplasty
Modified from Goldman MP, Bennett RG Treatment of telangiectasia: a review. Journal of the American Academy of Dermatology 1987;17:167.
Telangiectasia of the face are most often seen in patients
Carbon Dioxide Laser
with fair complexion (Fitzpatrick types I and II skin). These
The CO2 laser has been used for treatment of facial telang-
lesions are especially common on the nasal alae, nose, and
iectasia.251 Because tissue destruction is nonselective with
midcheeks and are probably caused by persistent arteriolar
this laser, occurring by vaporization of water within cells,
vasodilatation resulting from vessel wall weakness. The
the skin surface and dermis overlying the telangiectasia are
vessels dilate further when damage to the surrounding
destroyed as well as the vessel. Because of this nonselective
connective and elastic tissue occurs from factors such as
action, the CO2 laser has no advantage over electrosurgery
chronic sun exposure or use of topical steroids. These
in the treatment of telangiectasia.
lesions have a definite familial or genetic component.
Rosacea may be an accompanying condition.
Telangiectasia: Clinical Variants
The argon laser has often been used for treatment of facialtelangiectasia. Treatment parameters have varied, with laser
Linear Facial Telangiectasia
powers of 0.8 to 2.9 W; exposure times of 50 ms, 0.2 s, and
These telangiectasia often carry an unjustified social stigma
0.3 s; and continuous output with spot sizes of 0.1 and
implying alcoholism. Thus patients are understandably
1 mm. Although the success rate has been reported to be
distressed by this otherwise benign condition. Treatment
good to excellent in 65% to 99% of patients treated,2,252
of large vessels on the nasal ala or nasal alar groove is dif-
pitted and depressed scars, hypopigmentation, hyperpig-
ficult and often leads to noticeable scarring when conven-
mentation, and recurrence of veins have been noted.3,253
tional methods are used. We have found the PDL to be a
One area of particular concern is the nasal alae and
powerful and effective tool in the treatment of these
nasolabial creases, where depressed scarring is relatively
lesions, virtually without the risk of scarring or other per-
common, although resolution gradually occurs over
manent skin changes.
Cutaneous and Cosmetic Laser Surgery
Adverse healing may occur with the argon laser because
painful than the ATDL. Swelling and erythema was similar
of nonspecific thermal damage to perivascular tissue and
between the two lasers, but hyperpigmentation was greater
the overlying epidermis. This is caused by competition for
with the ATDL. Excellent clearance occurred with the LPDL
absorption of laser fluence from epidermal melanin and
in 78% of patients compared with 28% with the ATDL.
extensive radial diffusion and dissipation of heat from thetarget blood vessels.254 Both these factors result in rela-
Copper Vapor/Copper Bromide Laser
tively nonspecific thermal destruction. To minimize these
The CVL or CBL operates at two specific wavelengths,
effects, a small beam size (100 mm) has been advocated to
578 nm (yellow) and 511 nm (green), and delivers a ‘quasi-
trace vessels precisely,83,255,256 and a low power or pulsing
continuous wave' composed of pulsed laser light energy in
with a 50-ms shutter has been recommended.255,257,258 As
20-ns pulses at a frequency of 15,000 pulses per second.
mentioned previously, these parameters must be moni-
This train of pulses interacts with tissue in the same
tored closely, because the successful treatment of vascular
manner as a continuous beam because of the accumula-
lesions with the argon laser requires experience and artful
tion of heat with the large number of pulses delivered.
expertise. As described previously, the use of a very small
Because of resulting thermal diffusion, it may be necessary
beam (100 mm) greatly increases the scattering of laser
to gate the pulse electronically with 20- to 50-ms second-
photons within the dermis and limits treatment to the
ary pulses or to use a scanning device.
most superficial of dermal vessels. With these parameters,
The ability to pulse the CVL between 20 and 50 ms
many physicians find the argon laser effective in treating
allows this laser to work within the thermal relaxation time
facial telangiectasia, with only minimal risk of adverse
of many telangiectasia (Fig. 2.50). When the laser is used
healing. This application is its most successful use.
To limit heat diffusion with the argon laser, robotized
scanning laser handpieces have been used (see earlier
section on treatment of PWS in adults). The use of thisdevice has been reported to be successful in the treatmentof facial and leg telangiectasia when the telangiectasiaoccur in densely interlacing mats.259 This technique iseffective and greatly reduces the risk of adverse response,but it is not well suited for individual or widespread isolated telangiectasia.
Argon-Pumped Tunable Dye Laser
The argon-pumped tunable dye laser (ATDL) is a CW laser,
although mechanically shuttered pulses as short as 20 ms
are achievable. Beam size may be varied from 50 mm to
6.0 mm. Yellow light (577–595 nm) is usually chosen for
treatment of vascular lesions. The tracing technique using
a 100-mm beam with this laser has been used extensively
and advocated by Scheibner and Wheeland255 as a tech-
nique that produces good to excellent results with minimal
risk in a variety of cutaneous vascular lesions,83,256 includ-ing facial telangiectasia.260 The proper endpoint of treat-ment is disappearance of the vessel, not blanching,blistering, or charring of the overlying skin. Because thetracing hand motion cannot be accurately quantified,treatment parameters are difficult to teach except by directmonitoring. In addition, this technique is more tediousand time-consuming than the PDL, even when onebecomes a skilled operator. Multiple treatments are usuallyrequired, with hypopigmentation rarely occurring.261 Thelaser also may be used with a robotized scanning device iflarge areas of matted telangiectasia are to be treated.259,262However, multiple treatments are required, including spotvessel tracing to eliminate the hexagonal appearance.
A prospective, side-by-side comparison of the ATDL with
the PDL in 14 patients found better efficacy with the
Figure 2.50
This man with telangiectasias was treated for about
PDL.263 The ATDL used was a modified ophthalmic laser at
20 minutes on two occasions with a copper bromide laser. (A) Beforetreatment. (B) After treatment. First treatment was at 2.2 W and
585 nm, focused to a 0.1-mm circular spot with a power of
0.7 mm spot size, with 20-ms pulses (11.4 J/cm2) for smaller vessels
0.7 to 0.8 W at a pulse duration of 0.1 s (Coherent Medical,
and 30-ms pulses (17.1 J/cm2) for larger vessels. Second treatment
now Lumenis). Treatment times were about three times
2 months later was at 2 W with 20-ms pulses for all remaining vessels
longer for the ATDL than the PDL. The PDL was more
(10.4 J/cm2). (Courtesy Sue McCoy, MD.)
Laser Treatment of Cutaneous Vascular Lesions
with these refinements, it is somewhat safer and more
pulse duration varied from 7 to 60 ms, the treatment
effective than the argon laser for treatment of facial telang-
endpoint was vessel spasm without epidermal blanching.
iectasia and has the advantage of leaving only very minor
About 45% of patients required one treatment, and 35% of
superficial crusts overlying treated vessels, in contrast to
patients required two treatments. The remaining 20% of
the very visible, dark purpuric impact spots of the PDL.264
patients received three to five treatments. Treatment ses-
A comparison of the CVL with the PDL in 10 adults with
sions ranged from 5 to 60 minutes, with the average treat-
facial telangiectasia resistant to electrosurgical therapy
ment requiring 16.7 minutes. Moderate erythema occurred
demonstrated no difference in efficacy or adverse seque-
in most patients and lasted 2 to 3 hours. Swelling and
lae.265 However, Dinehart et al188 report a 10% incidence
crusting were rare. There were no reports of scarring or
of transient hyperpigmentation. Whether this represents
melanin or hemosiderin is unknown.
McCoy et al270 postulate that the reduced effectiveness
Of 33 patients with facial telangiectasia treated with the
in treating vessels less than 100 mm results from the
CVL, 69% had good to excellent results and 19% had poor
thermal relaxation time being much less than the deliv-
results.266 Treatment occurred with a 1-mm-diameter spot
ered pulse durations. The reduced effectiveness in vessels
size at pulse durations of 50 to 200 ms at energy densities
greater than 300 mm results from absorption of laser fluence
from 8 to 32 J/cm2 or continuous until vessel blanching
in the superficial portion of the vessel with ‘protection' to
occurred. Best results were seen on the cheeks, with poor
the deeper endothelium. McCoy recommends using sclero-
results on the nose or nasolabial folds. Atrophic scarring
therapy for vessels greater than 300 mm in diameter.
was reported on nasal lesions (7 of 33 patients), and edema
When used with the modulating influence of a second-
lasting 1 to 3 days occurred on the cheeks and lower
ary shuttered pulse or scanning device, the risk of adverse
response is further minimized without significant change
Thibault267 reported the results of a patient question-
in clinical effectiveness. In a comparison of 144 patients
naire of 180 patients treated with the CVL with or without
treated with the point-by-point technique and 105
sclerotherapy for facial telangiectasia. He used a CVL (Vis-
patients treated by the Hexascan, French investigators
Erase 3 w, Visiray Pty Ltd., Hornsby, NSW, Australia) with
determined that the incidence of hypertrophic scarring
a 200-mm fiber delivering a spot size of 150 to 400 mm. A
was less than 1% in the Hexascan group versus 7% in the
clinical endpoint of vessel blanching was used, which
point-by-point group. Treatment time was also reduced to
required a power of 600 to 700 mW impinging on the
20% of the point-by-point time. Patients treated by Hexa-
vessel for less than 1 s delivered continuously. Patients
scan also experienced less pain, and local anesthesia was
developed blistering 24 to 48 hours after treatment, with
rarely required.271 The balance between these two results
crusting lasting 7 to 17 days. Edema usually lasted 3 days.
(vessel obliteration and adverse healing) must be weighed
Almost half of patients treated with the CVL alone devel-
and the treatment technique altered to be consistent with
oped hypopigmentation. Good results were seen in 47% of
the goals of treatment.
vessels treated with the CVL alone, and these patients weresatisfied with treatment, versus 86% of patients who hadcombination treatment with CVL and sclerotherapy being
532 nm KTP Laser
satisfied with treatment.
The Starpulse KTP laser (LaserScope) operates at 532 nm
Waner et al268 compared the CVL with the PDL in 12
and emits a train of 1-ms Q-switched pulses at 25 kHz.
patients with facial telangiectasia. Treatment times and
What distinguishes this system from other frequency-
patient satisfaction were equivalent. Postoperative swelling
doubled Nd : YAG lasers is that the arc lamp is modulated
and prolonged healing occurred with the PDL. The CVL
and can provide pulse durations ranging from 1 to 50 ms.
used was the Vasculase (Metalaser), with a spot size of
This produces very-high-energy pulses that can be pro-
150 mm at a power of 0.35 to 0.55 W chopped at 0.2-s inter-
jected onto the skin in spots ranging in diameter from 0.25
vals. Waner concludes that both lasers have equal efficacy,
to 4.0 mm while still maintaining fluences within the 5
with most patients preferring the CVL.
to 10 J/cm2 therapeutic range. The combination of high-
McCoy269 has reported her results in treating 570
energy pulses and ability to adjust pulse duration to match
patients with facial telangiectasia. Similar results were
the thermal relaxation time of blood vessels allows
reported in another study of 23 patients evaluated in a
modulated KTP lasers to remove vascular lesions with less
blinded manner.270 In both studies, greater than 75% clear-
purpura than the PDL (Figs 2.51 and 2.52). One study
ance was achieved in 70% of patients, 50% to 75% clear-
demonstrated a 75–100% improvement in 94% of patients
ance in 17%, and less than 50% clearance in 13%. Vessels
using 16–22.5 J/cm2 through a 500- to 700-mm spot with
on the cheeks cleared much better than nasal telangiecta-
pulse durations of 15–30 ms. Telangiectasia were treated
sia, which only had an excellent response in 26% of
with complete vessel blanching or visible intravascular
patients. Vessels less than 100 mm or greater than 300 mm
did not respond as well as vessels 100 to 300 mm in diam-
A comparison study of the KTP laser (Starpulse) with
eter. McCoy used the CBL at 578 nm only (Norseld CuB D-
the PDL found less swelling, pain, bruising, and redness
10, Adelaide, South Australia) in a train of 30-ns pulses at
with the KTP laser.273 The Starpulse was used with a 2-mm-
16 kHz. The average power through the 600-mm fiber was
diameter spot size, 17 J/cm2, 10 ms. The PDL was used with
2 W. The fiber was used in a slightly defocused mode to
a 3-mm-diameter spot size at 6.8 J. No significant difference
produce a 0.9-mm-diameter spot. Although the chopped
in efficacy was found between the two lasers.
Cutaneous and Cosmetic Laser Surgery
Figure 2.51
(A) Perinasal telangiectasia before treatment. (B) Two
Figure 2.52
(A) Facial telangiectasia before treatment. (B) Three
weeks after treatment with Orion potassium titanyl phosphorus (KTP)
months after treatment with Orion KTP at 40 W, 4-mm Dermastat
at 5 W, tracing vessels once with 250-mm spot Dermastat with 10-ms
with 20-ms exposure duration, 1 pps. (Courtesy Burton E Silver, MD.)
exposure duration, 5 pulses per second (pps). (Courtesy Burton ESilver, MD.)
This laser has been effective in treating facial telangiecta-sia that have sometimes proved to be recalcitrant to
Q-switched Nd:YAG laser, 532 nm
treatment with other laser modalities260 (Fig. 2.54). Our
Even without consideration for the thermal relaxation
experience in using this modality both with and without
time of vascular lesions, the 532-nm laser has been shown
epidermal cooling has been variable but positive.
to be effective in clearing facial telangiectasia. Ten patients
An evaluation of four different 532nm Nd:YAG lasers
treated with the Nd:YAG laser at 532 nm in the Q-switched
showed improvement with all four lasers without signifi-
mode with 5-ns pulses at 1 to 2 and 3 to 4 J/cm2 demon-
cantly different adverse effects. One was a Q-switched laser
strated clearance.274 Excellent results occurred in 30% of
with a train of 5–10 ms pulses producing a pulse duration
patients treated at 1 to 2 J/cm2 versus 70% of patients
of 10–400 ms; a second machine used a quasi-continuous
having excellent results when treated at 3 to 4 J/cm2.
train of pulses delivering pulse durations of 10–25 ms; a
However, at 3 to 4 J/cm2, purpura occurred in all patients,
third system was a diode-pumped system delivering 1–
with hyperpigmentation developing in 20% at 30 days and
100 ms pulses and the fourth laser delivered 2–10 ms
10% at 60 days. The effectiveness and pigmentation were
probably caused by the explosive effects to the absorption
To decrease pain associated with the use of 532 nm
of the laser energy in a very short time (5 ns) with rupture
lasers, cold clear gel has been demonstrated under a cooled
of the vessel and release of hemosiderin.
sapphire window to decrease pain and erythema. Coolingthe skin did not decrease efficacy.276
Long-pulse Nd:YAG laser, 532 nm
By extending the pulse duration to within the thermal
Pulse Dye Laser
relaxation time of the vessel treated, purpura is avoided
The clinical treatment technique involves delivering a
without loss of efficacy (see earlier discussion) (Fig. 2.53).
train of pulses overlapping 10% to 20%, tracing the vessels
Laser Treatment of Cutaneous Vascular Lesions
Depth (mm)
Figure 2.53
Temperature distribution achieved in treating 0.1-mm-
diameter, 0.2-mm deep vessel with 532-nm laser with 2-ms pulseduration, 5-mm-diameter spot size, 15 J/cm2 with epidermal coolingto 4°C. (Courtesy ESC Medical, Inc.)
to be treated with a 3-, 5-, 7- or 10-mm delivery spot, andtreating an area of interlacing telangiectasia with overlap-ping spots to cover the involved area. Delivery energiesrange from 5 to 8 J/cm2 with a 0.45-ms pulse and higherwith longer duration pulses as described below and areadjusted according to lesion response and location. Dis-
Figure 2.54
(A) Facial telangiectasia on cheek of 60-year-old
ruption of blood flow occurs with resultant purpura which
woman before treatment. (B) Two months after single treatment with
varies with pulse duration and fluence but without exces-
Versapulse, 532 nm at 10-ms pulse duration, 3-mm-diameter spot
sive swelling or crusting. Treatments of telangiectasia are
size, 10 J/cm2 with epidermal cooling to 4°C.
generally done without the use of test spots, althoughthese may be used if the patient or physician is unsure andwants to observe response in a small area. If test spots areused, the entire length of the vessel must be treated to
erated by our patient population, who were informed in
eliminate the vessel (Fig. 2.55).
advance of its occurrence. They could therefore modify
One study reports a 77% response rate to PDL treatment
their social and business schedules accordingly. Typically,
of adult facial linear telangiectasia.38 Although vessel diam-
makeup foundation was used to camouflage the purpura
eter was not measured, larger blue vessels were less respon-
approximately 5 days after treatment. A small number of
sive than smaller red vessels. The poorer response to
patients experienced other transient skin changes at the
treatment of these larger vessels may result from: (1) the
treatment site. Even though no anesthesia was used,
thermal relaxation time in larger-diameter telangiectasia
patients experienced only mild to moderate discomfort in
being longer than the pulse duration of the laser; and (2)
93% of the cases. Most patients (62%) underwent only one
the deoxygenated Hb absorbing at a lower wavelength of
treatment, although further improvement and a better
the laser (545 nm.)
response were seen in patients with more than one treat-
Our published series shows 97.5% of 182 patients
ment. The largest percentage of patients had an excellent
achieving good to excellent results (14% good, 83.5%
response with fluences above 7 J/cm2 (93% versus 78%).
excellent), indicating resolution of more than 50% of the
There was a noticeable trend toward increasing effec-
treated lesions in one or two treatments277 (Figs 2.56 and
tiveness with increasing fluence. Our experience is that
2.57). In 152 of these patients, more than 75% of their
vessels larger than 0.2 mm in diameter require multiple
telangiectasia was removed. Scarring did not occur in
treatments. Vessels larger than 0.4 mm in diameter are
any patient, and skin texture remained unchanged. As
responsive to PDL treatment when the pulse duration is
expected, all patients experienced a transient bluish purple
discoloration at the treatment site that resolved sponta-
A method for using the PDL without purpura formation
neously in 10 to 14 days. This discoloration was well tol-
consists of giving two to three pulses over the telangiecta-
Cutaneous and Cosmetic Laser Surgery
Figure 2.55
(A) Facial rosacea and telangiectasia in 32-year-old
woman before treatment. (B) Three years after single treatment with
PDL at 7 J/cm2 delivered through 5-mm-diameter spot.
sia at lower fluences (pulse stacking). Typically, with a 595nm PDL, a 10-mm-diameter spot size is used at 4–5 J/cm2. Multiple pulses even with short pulse durations(0.45 ms) allow a gradual increase in intravascular temper-ature to effect selective photothermolysis without vesselrupture. The only drawback is that multiple treatments arerequired. One study demonstrated a good effect using theabove-mentioned parameters with a 1.5-ms pulse. Patientsrequired 7.4 ± 2.3 weekly treatments for vessel resolutionto occur. A second study compared the PDL used atpurpura-free parameters (595 nm, 10-ms pulse, 10-mm spotsize dynamic cooling with a 30-ms spray and 20-ms delay
Figure 2.56
(A), Bright-red linear telangiectasia 0.1 mm in diameter
at 7.5 J/cm2) as a single pulse on one side of the face with
over nasal alar crease in 46-year-old man. (B) Three months after
three or four pulses on the other side. Although side effects
PDL treatment at 7 J/cm2. Note complete resolution of nasal alar
were similar with both single and stacked pulses, vessel
telangiectasia. (C) One year after laser treatment. Note persistent
clearance was 74.3% in the pulse stacked side and 58.5%
resolution of nasal telangiectasia.
in the single pulse side.278
Another method of achieving purpura-free resolution of
telangiectasia with the PDL is to increase the pulse
9 J/cm2. This study divided the face so that one side was
duration. One study used a 7-mm-diameter spot at 10-ms
treated with purpura-free settings and the other side
pulse duration with a dynamic cooling device delivering
with purpuric settings. The study demonstrated that in
a 30-ms spray with a 20-ms delay. One pass was used at a
82% of the patients, the purpuric side had better
fluence 1 J/cm2 lower than a purpuric fluence, typically
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.57
(A) Extensive telangiectasia bilaterally on cheeks of 56-
year-old woman. (B) Six months after one treatment with pulsed dyelaser at 7.25 J/cm2. Total of 40 5-mm impact pulses were given toentire left cheek.
940 nm Diode-Pumped Laser
This laser (Medilas D SkinPulse; Dornier MedizinLaser
GmbH, Germering, Germany) has been reported to be
effective at clearing 1- to 3-mm-diameter periocular vessels
in 86% of patients when used at 141 J/cm2, 20-ms pulse
through a 3-mm-diameter spot size.280
1064 nm Long Pulse Nd:YAG Laser
In an effort to treat deeper, larger and bluer vessels, a longer
wavelength system has been developed. Using this wave-
length requires fluences over ten times that used with 532
Figure 2.58
(A) Nasal telangiectasia 1–2 mm in diameter.
nm to 595 nm lasers since the absorption of Hb and HbO2
(B) Complete resolution after 1 treatment with the LP 1064nm
at 1064 nm is ten times less. These higher fluences neces-
CoolTouch Varia. A 3.5-mm-diameter spot size was used at 200 J/cm2
sitate epidermal cooling. One laser uses dynamic cryogen
with a 25-ms pulse duration and 25 ms of cryogen cooling given
cooling to achieve epidermal protection. A study using the
immediately after the laser pulse. A total of 22 laser pulses were
CoolTouch laser (New Star Lasers, Rosemont, CA) found
greater than 75% improvement in 97% of treated sites witha 125–150 J/cm2 fluence through a 6-mm-diameter spotand 25-ms pulse duration for small diameter vessels and
Another long-pulse 1064 nm laser using precooling
75- to 100-ms pulse durations for reticular veins (Fig. 2.58).
through a copper contact probe demonstrated moderate to
All treated reticular veins including periorbital and tem-
significant vessel improvement in 80% of patients.282 This
poral veins resolved 100%. One or two passes were required
laser (CoolGlide Excell, Cutera, Burlingame, CA) is used
to achieve vessel spasm or coagulation.281
at 120–170 J/cm2 with a 3-mm-diameter spot size and 5–
Cutaneous and Cosmetic Laser Surgery
with this IPL may be due to the lower fluence used (13 and 22 J/cm2) and single pulse durations of 10, 15 or 30 ms.
Spider Telangiectasia
Spider telangiectasia represents telangiectasia with a
central feeding arteriole. They typically appear in pre-
school and school-age children. The peak incidence
appears to be between ages 7 and 10,285 and as many as
40% of girls and 32% of boys less than 15 years old have
at least one lesion.286,287 The incidence in healthy adults
is about 15%.288 The difference between these stated
incidences implies that 50% to 75% of childhood lesions
regress. However, this is not easily observable because most
lesions seem to persist without change and become a
source of cosmetic concern when present on the face.
Treatment in the past included electrocautery and the
argon laser. Both modalities have the disadvantages ofbeing painful and prone to causing punctate scarring. Inaddition, recurrence is common if treatment is done lightlyto avoid scarring. The PDL has proved to be a very effec-tive treatment for these benign lesions.289,290
Figure 2.59
Multiple depressed 3mm scars at the point of
treatment with the CoolGlide (now Cutera) 1064-nm long pulse laser(see arrows).
Pulse Dye Laser
Geronemus289 reported 100% success without any adverse
sequelae in 12 children treated with the PDL for facial
40-ms pulses until vessel blanching or coagulation occurs.
spider telangiectasia. We retrospectively evaluated the
These patients received two treatments 4 weeks apart to
response to treatment with the PDL in 23 children with 55
achieve therapeutic efficacy. Cutaneous blistering and scar-
spider telangiectasia.290 Lesions were treated at energy
ring with 2- to 3-mm depressions and/or hypopigmenta-
fluences of 6.5 to 7.5 J/cm2 (mean 6.9 J/cm2). One or two
tion was reported in this study and is not uncommonly
pulses were given to the central punctum of the ‘spider',
seen by this author with the use of this laser (Fig. 2.59). It
with additional pulses with a 10% overlap given to the
is presumed that pre- and postcontact cooling can be insuf-
radiating ‘arms' of the lesion if the lesion was greater than
ficient in some patients.
5 mm in diameter. Local anesthesia was not used. Seventypercent of lesions resolved completely with one treatment.
Intense Pulsed Light
Twelve lesions required a second treatment for complete
This high-energy pulsed light source described previously
resolution. The remaining five lesions not treated a second
is very effective in treating facial telangiectasia. Advantages
time had an average clearance of 78% (Fig. 2.62). The three
are the almost complete lack of purpura and adverse seque-
patients with five lesions who did not have a second treat-
lae. Energy fluences of 25 to 45 J/cm2 are required for vessel
ment were either satisfied with the degree of resolution
ablation given as a double pulse of 2.4 to 6.0 ms each with
from their first treatment or were unavailable for further
a 10–30-ms delay between pulses. A 550 nm, 560 nm or 570
nm cutoff filter works best. Lesions usually clear in one
Spider telangiectasia respond equally as well in adults,
treatment in 90% of patients.283 Lesions are treated with
with 93% of patients having total resolution with one
one or two pulses until initial vessel spasm or slight
treatment between 6.5 and 7.0 J/cm238 (Fig. 2.63).
purpura occurs (Figs 2.60 and 2.61). The only potential side
With the PDL, lesions become purpuric immediately
effects are slight purpura, which lasts 2 to 4 days, or epi-
after laser treatment. Purpura resolves within 7 to 10 days.
dermal desquamation when treatment is performed on
We have not seen permanent pigmentary changes or
tanned or type III or IV skin. Epidermal desquamation in
scarring. Although adverse effects from treating spider
pigmented patients can be avoided by changing the filter
telangiectasia are extremely rare, one case of granuloma
to a longer wavelength or increasing the delay time
telangiectaticum (pyogenic granuloma) after argon laser
between double or triple pulses (see Chapter 8).
therapy has been reported.291 This complication occurred
A dual-mode filtering IPL (Ellipse Flex, Danish Derma-
3 months after the central vessel was treated at 5 W, 50 ms,
tologic Development, Hoersholm, Denmark) that restricts
with a 0.5-mm-diameter spot size. The authors speculated
the filtered light to between 555 and 950 nm (median
that laser trauma, in addition to the lack of complete
wavelength of 705 nm) has been show to provide more
destruction of the spider telangiectasia endothelium, led
than 50% reduction in facial telangiectasia in 79% of
to a focal capillary proliferation. This effect has also been
patients after one to four treatments; 37.5% of patients had
reported with laser treatment of PWS, with development
greater than 75% improvement.284 The decreased efficacy
of ‘hemangiomas' within the treated areas.
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.60
(A) Extensive facial telangiectasia on 55-year-old woman after automobile airbag impacted her face. (B) Eight weeks after
treatment with PhotoDerm VL, 550-nm cutoff filter, 40 J/cm2, double pulse of 2.4 and 4.0 ms with 10-ms delay. Note 50% improvement.
(C) Four weeks after second PhotoDerm VL treatment with 570-nm cutoff filter, 44 J/cm2 delivered in two pulses of 2.4 and 4.0 ms with 10-msdelay. (D) Complete resolution 8 weeks after third and fourth treatments, with parameters similar to those in (C).
Figure 2.61
(A) Facial telangiectasia before treatment. (B) After two treatments with PhotoDerm VL with 570- or 550-nm cutoff filters,
37 J/cm2 given as double pulse of 3 and 4 ms with 10-ms delay between pulses. (Courtesy Beverly Kemsley, MD, Calgary, Canada.)
Cutaneous and Cosmetic Laser Surgery
Figure 2.62
(A) Two spider telangiectasia on left inner canthal area
Figure 2.63
(A) This 44-year-old man had an 18-month history of
and outer canthal area of 10-year-old girl for 6 months. (B) Four
enlarging spider telangiectasia on right cheek. (B) Six months after
weeks after single treatment with PDL at 7 J/cm2. Note complete
single treatment with PDL at 7 J/cm2. A total of six 5-mm pulses were
resolution of telangiectasia without any evidence of cutaneous
given, with complete resolution of telangiectasia.
changes. (Reprinted from Fitzpatrick RE: American Journal of CosmeticSurgery 9:107, 1992. With permission from American Academy ofCosmetic Surgery.)
532 nm KTP or Nd:YAG Laser
An additional nearly painless treatment modality is the
long-pulse 532 nm laser. Parameters found to be efficacious
in treating spider telangiectasia are a 3- to 4-mm-diameter
spot with an energy fluence of 12 to 14 J/cm2 delivered in
a 10-ms pulse. The treatment is almost painless because the
laser beam is delivered through a double-chambered clear
quartz crystal cooled in its center to 4°C with water (Fig.
2.64). The quartz cooling device is placed over the lesion
on the skin surface, and the laser fluence is delivered one
to three times until the lesion blanches. This laser has an
efficacy of almost 100% for this treatment in our practice.
The KTP laser (Orion/Aura, Laserscope, Palo Alto, CA)
Figure 2.64
Cooling head is attached to handpiece of Versapulse
has been found to be effective when used with a 1- to
532-nm laser and placed on skin over target vascular lesion. Laser
2-mm diameter spot size, at 13–10 J/cm2 respectively with
energy is then given through this cooling device.
a 5–8 ms pulse duration.
paper to match the lesion's diameter. A simple sheet of
Intense Pulsed Light
white paper, cardboard, or self-sticking label is sufficient
The IPL has also been useful in treating these lesions.
to block out the light delivered through the quartz or sap-
Because one needs only to treat the lesion, we use a hole
phire light guide. Fluences of 35 to 40 J/cm2 delivered in a
punch or scissors to cut out an open area in a sheet of white
double pulse of 2.4 and 4.0 ms with a 10-ms delay through
Laser Treatment of Cutaneous Vascular Lesions
Rosacea
Rosacea is essentially a cutaneous vascular disorder. It is
best thought of not as a disease but as a typology of a
cluster of patients with a characteristic combination of
cutaneous stigmata consisting of telangiectasia, papules,
pustules, and rhinophyma.293 A comprehensive review of
the literature finds that of the 18 histologic studies on
rosacea, 14 showed an increase in Demodex mites.294 It is
hypothesized that these mites may play a role in the
inflammation of rosacea.295 Studies have demonstrated
thermal destruction of these mites after IPL therapy,
which may contribute to the therapeutic effects of
IPL.295,296 Telangiectasia represents the later phase of
vascularization and probably results from a reduction in
mechanical integrity of the upper dermal connective
tissue, allowing a passive dilatation of capillaries. Inflam-
mation and associated angiogenesis may contribute to
the telangiectasia. Interestingly, facial temperature ishigher in rosacea and this has been associated with a dif-ference in the nature and behavior of skin bacteria, par-ticularly coagulase-negative staphylococci.297 Therefore,the elimination of excessive blood vessels may not onlydecrease the erythematous appearance of rosacea but alsomodify the bacterial flora further decreasing the cutaneouserythema.
Pulse Dye Laser
Rosacea-associated telangiectasia and erythema respond
well to treatment with the PDL. We have reported good to
excellent results in 24 of 27 patients (89%).298,299 In addi-
tion to the cosmetic improvement resulting from elimina-
Figure 2.65
Extensive vascular ectasia on both feet of 54-year-old
tion of the vascular component of this disorder, PDL
woman. (A) Before treatment. (B) Six months after initial treatment
treatment also appears to alter the pathophysiology of this
and 3 months after second treatment with PDL at 7.25 J/cm2 using
condition because a decrease in papule and pustule activ-
84 and 115 pulses, respectively. (From Goldman MP. Sclerotherapy:
ity occurs in up to 59% of patients (Fig. 2.67). After PDL
treatment of varicose and telangiectatic leg veins, 2nd edn. St Louis:
treatment, patients who responded to treatment with elim-
ination of the vascular component required less or notopical or systemic antibiotic therapy to maintain disease
a 550-nm cutoff filter usually gives near 100% efficacy with
resolution.299 Anecdotal reports from Tan and Kurban245
almost no pain. Even small children and male adults tol-
confirm our more formal evaluations. An additional study
erate the treatment without complaint. Lesions are rarely
of 12 patients with rosacea treated with the PDL to one
purpuric and usually without adverse sequelae, making
cheek with the other as a control at purpuric settings of
this modality a treatment of choice.
5-mm diameter, 5.5–6.5 J/cm2, 0.45-ms pulse withoutcooling also demonstrated a 75% reduction in telangiecta-sia is 11 of 12 patients and a decrease in erythema of 50%
Generalized Essential Telangiectasia
after three treatments.300
This telangiectasia generally occurs on the legs but may
The efficacy of the first-generation PDL was reproduced
also involve other cutaneous surfaces. Various treatments
in a study of a 6 ms PDL at 595 nm with fluence between
have been proposed, with variable efficacy.249 Tan and
7 and 9 J/cm2 and cryogen spray cooling.301 Here, two of
Kurban245 reported successful treatment with the PDL at
12 patients had over 75% improvement with one treat-
fluences of 6 J/cm2. We also treated four patients with the
ment. Another two had 50–75% improvement; five had
PDL at fluences ranging from 6.0 to 7.5 J/cm2. Two patients
25–50% improvement. These parameters did not produce
responded with total resolution, but two patients had
almost no improvement in their appearance (Fig. 2.65).
A questionnaire rating of Dermatology Life Quality
Therefore we believe that intrinsic factors in these patients
Index and symptoms of flushing, burning, itching,
may preclude predictable results. We recommend per-
dryness, swelling and skin sensitivity was performed on
forming a patch test for such patients.
16 patients treated with a PDL for their rosacea.302 PDL
IPL treatment has also been found to be effective
parameters of treatment were 10 J/cm2, 7-mm-diameter
spot, 1.5-ms pulse duration with cryogen skin cooling
Cutaneous and Cosmetic Laser Surgery
Figure 2.66
(A) Essential telangiectasia before treatment in a 38-year-old women. (B) After one treatment with the Lumenis Vasculite IPL at
32 J/cm2 with a 570 cut-off filter and a double pulse of 2.4 and 4.0 ms with a 10-ms delay. (Reprodued with permission from Goldman MP,Bergan JB: Sclerotherapy treatment of varicose and telangiectatic leg veins, 3rd Ed. Mosby, 52 Louis, 2001.)
treatment. Seven patients had less symptoms and only onepatient was unchanged. Superficial nerve fiber density andnumber of substance P immunoreactive nerve fibers weredecreased.
In assessing the overall success and potential risk of each
laser used in the treatment of facial telangiectasia withrosacea, we believe that the PDL provides effective and relatively risk-free results. However, purpura resulting aftertreatment is an inconvenience that must be recognized inadvance in scheduling treatment. In addition, one studyof ten patients showed that only 50% had less papulopus-tular lesions after an average of 2.4 treatments with param-eters of the PDL previously described above.304 However,even in this ‘negative' paper, two of the ten treated pa-tients had excellent results when evaluated 5 years aftertreatment.
Figure 2.67
(A) 24-year-old women with rosacea on the cheeks.
532 nm KTP Laser
(B) One month after the second of two treatments with the PDL.
A study of 47 patients treated with the KTP 532 nm laserwith variable-sized handpieces depending on the type of
of 40–50-ms spray after a 20-ms delay. Two treatments
telangiectasia showed good results.305 Matted telangiecta-
were performed. The Dermatology Quality of Life Index
sia were treated with a 4-mm-diameter spot and 20-ms
improved in each patient from an average score of 7.8
pulse width at an average energy of 0.7 W. Vessels 0.1 to
before treatment to 3 after the first treatment and 1.9 after
0.3 mm in diameter were treated with a 0.25-mm-diameter
the second treatment (75.6%). Symptoms of burning,
spot size and 20-ms pulse width at an average energy of
flushing, stinging and itching improved by at least 57%
0.12 W. Vessels greater than 0.3 mm in diameter were
after treatment. Therefore, a cosmetic improvement is not
treated with a 1-mm-diameter spot and 10-ms pulse width
the only benefit of laser treatment of rosacea.
at an average energy of 0.2 W. Vessels were treated by
The improvement in symptoms was reproduced in
tracing them with the laser until they disappeared. This
another study of 32 patients with rosacea treated with
required one to several tracings. Of the 47 patients, 38%
the first-generation 0.45-ms, 5-mm-diameter PDL with
had more than 70% resolution of their telangiectasia, and
purpura lasting 5–14 days.303 These patients all had a posi-
32% required a second treatment to achieve the same
tive lactic acid (Stinger) test indicating skin sensitivity. 24
result. The only adverse effect consisted of linear crusting
of the 32 patients became stinger negative after one PDL
along the path of the telangiectasia.
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.68
(A) Erythematous cheeks of 66-year-old woman before
treatment. (B) After three treatments with PhotoDerm VL using 570-nm cutoff filter and 12-ms pulse at 52 J/cm2; followed 4 weeks laterby 590-nm cutoff filter and 12-ms pulse at 55 J/cm2; then followed 4weeks later by 550-nm cutoff filter at 44 J/cm2 given as double pulseof 4.2 and 7.7 ms with 20-ms delay.
Intense Pulsed Light
The IPL has also been found to be effective in treating
rosacea. As described previously, this light source has the
advantage of relatively quick vessel elimination without
Figure 2.69
(A) Diffusely red telangiectatic nose of 58-year-old
significant purpura or crusting (Figs 2.68 and 2.69). Scan-
male. (B) After one treatment with Lumenis 1 intense pulsed lightusing 560-nm cutoff filter at 20 J/cm2 given as double pulse of 3, 5
ning Doppler evaluation demonstrated a 30% decrease in
and 3.5 ms with a 10-ms delay.
blood flow after five IPL treatments.306 In addition, a 21%decrease in erythema intensity as well as a 29% decreasein the actual size of the cheek with telangiectasia was noted
2.4 ms and 4.0 ms with a 10-ms delay and an energy
in this study of four patients. A larger study of 60 patients
density of 30 J/cm2 (26J/cm2 with the QuantumSR) and
were treated with the IPL with pulse durations of 4.3 to
3.0 ms and 3.0 ms at 18 J/cm2 with the Lumenis One).
6.5 ms and energy density of 25–35 J/cm2.307 A mean clear-
Patients are retreated every 3–4 weeks until clear. We have
ance of 77.8% was achieved and maintained for a follow-
found that most patients clear in two or three treatments.
up period averaging 51.6 months. An additional study of
A certain percentage of patients (20%) do not respond to
32 consecutive patients treated with an average of 3.6 IPL
the IPL and need to be treated with the PDL. Most of
treatments similar to the above-mentioned parameters
our patients need to return for retreatment every year
showed that 83% of patients had reduced redness, 75%
experienced reduced flushing and 64% noted fewer acnebreakouts.308
Poikiloderma of Civatte
Poikiloderma of Civatte is a variant of telangiectasia
involving the neck and upper chest and occurring
We treat the entire facial area affected by the erythematous
from accumulated ultraviolet exposure and associated
rosacea. Short pulses appear to be most effective. We typi-
photosensitization of various chemicals, most notably
cally use a 550 or 560 cutoff filter with a double pulse of
fragrances.309 Poikiloderma consists of a combination
Cutaneous and Cosmetic Laser Surgery
of telangiectasia, irregular pigmentation, and atrophic
changes of the skin. Histologic changes on biopsy confirmthis clinical combination. These changes are best treatedby addressing the telangiectatic and pigmentary compo-nents simultaneously.
In the past, poikiloderma treatment focused on the
telangiectatic component and solely on various lasermodalities. Fair efficacy was reported, but treatments werelengthy because of the large surface areas involved andmultiple sessions required. The argon laser is only partiallysuccessful and frequently results in areas of hypopigmen-tation with a low incidence of scarring. The 532 nm KTPlaser has also been reported to be successful in a onepatient case report.310
Pulse Dye Laser
The PDL, as reported by Tan and Kuran,245 Wheeland and
Applebaum,311 and us, has good efficacy at fluences of 6 to
7 J/cm2 with a 5-mm-diameter spot size. Our recommendedfluence at a 7-mm-diameter is 5 to 6 J/cm2. Problems withthe PDL include extensive purpura, multiple treatmentsbecause of large surface area, and mottled response withcircular imprints (Figure 2.70). In addition, by mainly tar-geting the vascular component, these treatments often dolittle to change the hyperpigmentation. Scarring has alsobeen reported when the PDL was used at fluences greaterthan 6 J/cm2.312
We recommend that if the PDL is used to treat poikilo-
derma of Civatte, it be used with a 10-mm-diameter spotsize, 0.45- to 0.5-ms pulse duration if pigmentation isprominent and 1.5- to 2-ms pulse duration if erythema isprominent. Fluence should be just strong enough to give aminimal purpuric response and epidermal cooling shouldbe performed. Patients must be told that three to five
Figure 2.70
(A) Extensive poikilodermic changes from sun damage
treatments will be necessary and that the treated area
on central chest of 36-year-old woman. (B) After three treatmentswith FLPDL at a fluence of 7 J/cm2 during each treatment session.
may appear to be polka-dotted until all treatments are given.
Between 200 and 300 5-mm impact pulses were given at eachsession. Note excellent clearing of poikilodermic changes. Somewhat
Intense Pulsed Light
mottled pigmentation remains between laser impact sites and on
Our results using an IPL have been very favorable. With
peripheral aspect of poikiloderma.
this pulsed light system, the target is both vascular and epidermal and dermal melanin. Multiple wavelengths are
second expanded study of 135 patients randomly selected
used, usually with a 515-nm filter first. This filter is used
with typical changes of poikiloderma of Civatte on the
with a single pulse of 3 ms at a fluence of 22 to 25 J/cm2.
neck and/or upper chest were treated one to five times
These treatment parameters are effective in removing
every 4 weeks with the IPL. Clearance of over 75% of
epidermal melanin and very superficial telangiectasia. A
telangiectasias and hyperpigmentation comprising
second or third treatment spaced at least 4 weeks apart is
poikiloderma was observed. Incidence of side effects was
usually necessary and typically uses a 550-nm cutoff filter
5% including pigment changes. In many cases, improved
with a double pulse of 2.4 and 4 ms with a 10-ms delay.
skin texture was noted both by physician and patient
This is helpful in treating slightly larger or deeper telang-
(Figs 2.71 and 2.72).315,316
iectasia. A total fluence from 35 to 42 J/cm2 is usually
Approximately 75% improvement occurs after one treat-
necessary to achieve an optimal clinical result. With these
ment. Side effects include transitory erythema from 24 to
parameters, few or no side effects have been noted in
72 hours. Purpura occurs only 10% of the time and only
our patients (Fig. 2.71). This experience has been detailed
with some pulses in variable locations. This purpura is dif-
in a study in which 66 patients with typical changes of
ferent from that seen with the PDL in that it is intravas-
poikiloderma of Civatte on the neck were treated with IPL
cular and resolution occurs within 3 to 5 days. A slight
at various settings every 4 weeks until desired improve-
stinging pain during treatment is easily tolerated for up to
ment occurred. A 50%–75% improvement in the extent of
60 pulses per session. No anesthesia is required, and the
telangiectasias and hyperpigmentation comprising entire neck and chest area can be treated during one treat-poikiloderma was observed in an average of 2.8 treat-
ment session. Patients must be informed that ‘foot-prints'
ments. Incidence of hypopigmentation was 5%.313,314 A
representing the shape of the contact crystal may be
Laser Treatment of Cutaneous Vascular Lesions
Figure 2.71
(A) Poikiloderma of Civatte in 44-year-old male before
treatment. (B) One month after a second treatment with IPL using a550-nm cutoff filter at 40 J/cm2 given as a double pulse of 2.4 and4.0 msec separated by a 10 msec delay.
Figure 2.73
(A) Progressively enlarging venous lake on lower lip of
a 78-year-old women for 2 years. (B) Three months after single
treatment with PDL at 7.5J/cm2 with a total of four 4-mm impacts.
present after the first and even second treatment and is anormal response.
Venous Lake
Venous lakes are dilated ‘lake-like' venules in the upperdermis typically seen on the lips or ears of elderly patients.
These lesions are dark-blue to purple, soft, raised nodules,usually 2 to 10 mm in diameter.317 Patients generallyrequest treatment because of concern over possible medicalconsequences and recurrent bleeding with trauma or forcosmetic improvement.
Treatment of venous lakes of the lips and ears has been
effective with the argon laser, requiring from one to four
Figure 2.72
(A) Poikiloderma of Civatte in 52-year-old male before
treatment sessions.318,319 Use of laser spot size less than
treatment. (B) Six months after third treatment with PhotoDerm VL
1 mm in diameter may promote excessive bleeding.320
using 550-nm cutoff filter at 37 to 40.5 and 43.5 J/cm2 given over
Lesions less than 5 mm in diameter almost always heal
three treatments as a double pulse of 2.4 and 2.4 ms with 10-ms
without scarring, whereas those larger than 5 mm healed
delay between pulses.
with scarring in 21% of patients treated in one study.321Treatment of venous lakes with electrocautery has beenunsatisfactory.322 The PDL has been reported to be suc-cessful in treating venous lake lesions (Fig. 2.73).245,269
Cutaneous and Cosmetic Laser Surgery
Figure 2.74
(A) Venous lake present for more than 20 years on the
lower lip. (B) Four weeks after second treatment with intense pulsedlight at 590-nm cutoff filter, 38J/cm2 given as a double pulse of 3 msand 2 ms with a 10-ms delay.
When using the PDL, selective photothermolysis is not thegoal. Multiple pulses over the same area with or withoutdiascopy are usually required for efficacy. This has resultedin epidermal and perivascular thermal damage as previ-ously described. A tunable dye laser at 577 nm used withdiascopy in a continuous wave at 1 W also gives excellent
Figure 2.75
(A) A 32-year-old woman who had a venous lake on
cosmetic results.323
the lower lip for 18 years and requested removal for cosmeticreasons. One treatment was given with a long-pulse Nd:YAG laser
The IPL has also been found to be effective in resolving
with a 7-mm diameter spot size, a pulse duration of 45 ms and a
venous lakes in one or two treatments. Treatment param-
fluence of 120 J/cm2. Six pulses were given. (B) Complete resolution
eters are similar to those used for treating hemangiomas
4 months after treatment. (Courtesy of Don Groot, MD.)
(Fig. 2.74). The long-pulse Nd:YAG is also suitable for treat-ing this vascular lesion. Figure 2.75A shows a 32-year-oldwoman who had had a venous lake on the lower lip for 18
from epidermal hyperproliferation in response to viral
years and requested removal for cosmetic reasons. One
genome incorporation into epidermal cellular DNA. To
treatment was given with a long-pulse Nd:YAG laser with
maintain a proliferative growth, neovascularization is stim-
a 7-mm-diameter spot size, a pulse duration of 45 ms and
ulated. This is reflected histologically in prominent, dilated
a fluence of 120 J/cm2; six pulses were given. Figure 2.75B
blood vessels in dermal papillae.325 Theoretically, vapor-
shows complete resolution 4 months after treatment.
ization and coagulation of the new capillaries should haltviral replication and promote verrucae resolution. Toproduce vascular coagulation, the CVL has been reported
to treat genital warts effectively.326
Nemeth and Reyes326 postulated that using epidermal
Verrucae represent benign tumors of epidermal cells
melanin as the ‘surrogate' target produces epidermal-
induced by the human papillomavirus (HPV). They occur
dermal separation with removal of the wart. In a population
in about 10% of adults and children.324 Verrucae develop
of resistant warts, only 12 of 137 patients failed to respond
Laser Treatment of Cutaneous Vascular Lesions
to treatment with a CVL. However, one-third of patients
higher clearance rate because they were usually treated up
developed recurrent lesions at the 6-month follow-up.
to three times, whereas never-treated were usually only
In an effort to coagulate deeper vessels, using a laser with
treated once or twice. These patients generally preferred
a longer wavelength should be effective. Therefore we have
PDL treatment to cryosurgery, with only 2 of 32 patients
used the PDL to treat common verrucae. Lesions are treated
having residual pain lasting 1.5 days to 1.5 weeks.
at a fluence of 7.0 to 7.5 J/cm2 with single pulses to flat warts
Increasing the fluence of the PDL to 8.1 to 8.4 J/cm2 has
and two to four pulses to hypertrophic verrucae. Paring
increased clearance. Of 97 verrucae treated at this higher
down the warts is recommended but not to the point of
fluence, 70% had 100% clearance.331
bleeding, which would cause the laser light to be absorbed
A prospective randomized study of PDL vs. conventional
by surface blood. Lesions are treated until they appear gray.
treatment on 40 adults with 194 warts showed a 70%
They then become black after 24 hours. Patients are
response with conventional and 66% response with PDL
retreated every 1 to 2 weeks until resolution. We combined
treatment. Conventional treatment was LN2 with 2, 15
a limited series of patients with those of Webster et al327 at
second freeze thaw time. PDL treatment was with a 5-mm-
Jefferson Medical College and found that flat warts were
diameter spot, 9–9.5 J/cm2 given in two pulses. Both groups
most responsive to treatment, with 71% resolving com-
also performed 17% salicylic acid treatments daily to their
pletely in an average of 2.4 treatments. Palmar and plantar
warts. A mean number of two treatments was necessary in
warts had a 65% total resolution response in 2.3 treatments,
both groups to achieve clearance. There appeared to be a
and periungual warts only cleared completely in 33% of
slight advantage of the PDL in the treatment of recalcitrant
patients despite an average of 3.3 treatments. Some warts
cleared after one treatment, and some required several
Unfortunately, not all studies report the same degree of
treatments. There was no significant difference in the
significant efficacy with PDL therapy.332 A study of 27
number of treatments or fluence used. Very few warts failed
patients with 79 recalcitrant palmoplantar, digital, periun-
to show some response to treatment, with smaller warts
gual, and body lesion warts found that 36% of the patients
responding more quickly than larger warts. None of our
had complete resolution of their warts and 59% partially
patients developed postprocedural debility.
responded. Of the total number of warts treated, only 21%
Tan et al328 treated 39 patients with verrucae recalcitrant
completely resolved.333 Of those warts that cleared, 40%
to multiple treatment modalities with the PDL after paring
recurred within 4 months with a mean follow-up of 7
the warts at fluences ranging from 6.25 to 7.5 J/cm2. As
months. Exact treatment parameters and techniques were
with our patients, excellent resolution occurred, warts
not noted in this abstract, but this study from a major
totally clearing in 72% of patients after an average of
laser center casts doubt on the absolute efficacy of PDL
1.68 treatments. The more rapid response was most likely
related to enhanced efficacy by allowing deeper vascular
Reasons for the difference in therapeutic response is
coagulation through paring the surface of the verrucae.
unknown. Because of a wart's tendency toward sponta-
Only one of the patients had a recurrence in the 5- to
neous resolution, however, a blinded controlled study of
6-month follow-up period. The authors examined 15
PDL versus sham laser is necessary. Nevertheless, the effi-
patients histologically and found marked agglutination
cacy of this treatment, in addition to its ease and lack of
of RBCs accompanied by vessel wall necrosis. Necrotic
scarring, is encouraging. In addition to the proposed mech-
keratinocytes surrounded these vessels.
anism of vascular coagulation for wart destruction, direct
Kauvar et al329 have reported the highest efficacy with
thermal effects from treatment may be significant.
PDL treatment: 93% overall efficacy in treating 142
Nonlaser-induced hyperthermia has been demonstrated
patients with 703 verrucae that had been recalcitrant
to result in regression of warts in a high percentage of
to previous treatment with various modalities, including
patients.334 The mechanism of action for heat may be related
liquid nitrogen and CO2 laser vaporization. Warts were
to direct epidermal protein coagulation or coagulation of
pared and hemostasis obtained with aluminum chloride 3
nutritive blood vessels or to the subsequent inflammatory
weeks before treatment. When warts cleared, they did so
response to thermal injury. Other lasers have been used to
in an average of 2.5 treatments (range 2–5). In addition to
produce local hyperthermia, and the term ‘laserthermia' has
an enhanced therapeutic response from therapy, 60% of
been used specifically with Nd:YAG laser therapy.335,336 As
patients reported adverse effects that were minimal enough
with PDL treatment, Nd:YAG laserthermia has advantages
to prevent a change in their daily activities, and 70%
over CO2 laser treatment because the skin remains intact and
thought PDL treatment was less painful than liquid nitro-
bleeding usually does not occur. Thirty-one patients with
gen cryosurgery. Interestingly, the study found no differ-
recalcitrant warts (previous unsuccessful cryosurgery, kera-
ence in efficacy among 7, 8, 9, or 10 J/cm2 fluences, as well
tolytic treatment, antimitotic therapy, and excision alone or
as no apparent difference between two to five pulses and
in combination) were treated with the Nd:YAG at 10 W with
six to ten pulses. The authors concluded that each wart
8-mm-diameter spot size and irradiation time of 20 s. These
should receive two or three pulses at a fluence of 6 to
parameters resulted in heating of wart tissue to 40°C for
7 J/cm2 at 3-week intervals.
30 s. Patients were treated up to three times at 3-week inter-
An additional study of 156 warts in 32 individuals
vals. At 9-month follow-up, 77% of patients cleared com-
treated with the PDL at 8 J/cm2 showed resolution of 68%
pletely without scarring or recurrence.337 Local anesthesia
of recalcitrant warts and 47% of never-treated warts with
was not necessary because patients felt only a slight burning
an average of 1.78 treatments.330 Recalcitrant warts had a
sensation during and after treatment.
Cutaneous and Cosmetic Laser Surgery
The frequency doubled Nd:YAG laser has also been found
6. Garden JM, Tan OT, Kerschmann R et al. Effect of dye laser
to be effective in the treatment of flat warts.338 This laser was
pulse duration on selective cutaneous vascular injury. J
used with a 3-mm-diameter spot size at 2.5 J/cm2 with mul-
Invest Dermatol 1986; 87:653.
tiple 10 ns pulses. Lesions were treated until whitening
7. Tanghetti E, Sierra RA, Sherr EA, Mirkov M. Evaluation of
occurred and all lesions turned into eschars after a few days.
pulse-duration on purpuric threshold using extended pulsepulsed dye laser (Cynosure V-Star). Lasers Surg Med 2002;
In the seven patients treated, hyperpigmentation developed
but all lesions resolved without adverse effects.
8. Tan OT, Murray S, Kurban A. Action spectrum of vascular spe-
Finally, the treatment of warts in sensitive areas such as
cific injury using pulsed irradiation. J Invest Dermatol 1989;
the anogenital region is less traumatic with the PDL than
with conventional techniques.339
9. van Gemert MCJ et al. Can physical modeling lead to an
optimal laser treatment strategy for port-wine stains? In
Wolbarsht ML, ed. Laser applications in medicine andbiology, vol 5. New York: Plenum; 1991.
The warts are first paired down just before bleeding occurs.
10. Pickering TW, van Gemert MJC. 585 nm for the laser treat-
If bleeding occurs it must first be stopped with pressure.
ment of port-wine stains: a possible mechanism. Lasers Surg
The PDL is used with a 5-mm-diameter spot size. A 2-ms
Med 1991; 11:616 (letter).
pulse, at 9 J/cm2 is given with slight epidermal cooling (air
11. Gilchrist BA, Rosen S, Noe JM. Chilling port-wine stains
cooling at 2). Two to four pulses are given until the lesion
improves the response to argon laser therapy. Plast ReconstrSurg 1982; 69:278.
turns grey. Treatments are repeated every 2 weeks.
12. Dreno B, Patrice T, Litoux P et al. The benefit of chilling in
argon laser treatment of port-wine stains. Plast Resconstr
Surg 1985; 75:42.
13. Pope K, Kask G. Epidermal temperature evaluation during
Another system that incorporates both vascular specificity
dynamic spray cooling, contact cooling, and ice. Presented
and thermal effects is the IPL. As previously described, this
at the 20th Annual meeting of the ASLMS, Reno, Nevada,
system produces deep vascular coagulation and thermal
effects. We have achieved excellent success with this
14. Nelson JS, Milner TE, Anvari B et al. Dynamic epidermal
cooling during pulsed laser treatment of port-wine stain.
With the IPL, the wart is exposed through a hole-punch
Arch Dermatol 1995; 131:695.
in a white index card. The IPL crystal is then placed on the
15. Nelson JS, Milner TE, Anvari B. Dynamic epidermal cooking
wart which is exposed through the hole-punch. IPL energy
in conjunction with laser-induced photothermolysis of port-
does not penetrate through the white index card and the
wine stain blood vessels. Lasers Surg Med 1996; 19;224.
16. Edris A, Choi B, Aguilar G, Nelson JS. Measurements of laser
treatment is not painful. With the IPL a 550-, 560-, or 570-
light attenuation following cryogen spray cooling spurt ter-
nm filter is used, with a double pulse of 2.4 ms and 4.0 ms
mination. Laser Surg Med 2003; 32:143–7.
or 3.0 ms and 3.0 ms (with the Lumenis One) with a 10-ms
17. Greve B, Hammes S, Raulin C. The effect of cold air cooling
delay between the pulses, 45 J/cm2 (30 J/cm2 with the
on 585 nm pulsed dye laser treatment of port-wine stains.
Lumenis One) is used; one to three pulses are given until
Dermatol Surg 2001; 27:633–636.
the wart turns gray.
18. Raulin C, Hammes S. Cold air in laser therapy: First experi-
ences with a new cooling system. Lasers Surg Med 2000;
19. Tan OT, Sherwood K, Gilchrest BA. Treatment of children
This chapter is extensively updated from: Goldman MP,
with port-wine stains using the flashlamp-pulsed tunable dye
Fitzpatrick RE: ‘Laser Treatment of Cutaneous Vascular
laser. N Engl J Med 1989; 320:416.
Lesions' in Cutaneous Laser Surgery: The Art and Science
20. Garden JM, Tan OT, Parrish JA. The pulsed dye laser: its use
of Selective Photothermolysis 2nd Ed. Goldman MP,
at 577 nm wavelength. J Dermatol Surg Oncol 1987; 13:134.
Fitzpatrick RE (eds) Mosby, St. Louis, 1999.
21. Garden JM, Polla LL, Tan OT. The treatment of port-wine
stains by the pulsed dye laser. Analysis of pulse duration andlong-term therapy. Arch Dermatol 1988; 124:889.
22. Garden JM et al. The pulsed dye laser for the treatment of
port-wine stains. J Dermatol Surg Oncol 1986; 12:757.
1. Solomon H et al. Histopathology of the laser treatment of
23. Glassberg E et al. The flashlamp-pumped 577 nm pulsed
port-wine lesions. J Invest Dermatol 1968; 50:141.
tunable dye laser: clinical efficacy and in vitro studies. J Der-
2. Apfelberg DB, Maser MR, Lash H. Argon laser management
matol Surg Oncol 1988; 14:1200.
of cutaneous vascular deformities: a preliminary report. West
24. Rios WR. Flashlamp-excited dye laser: treatment of vascular
J Med 1976; 124:99.
cutaneous lesions. Facial Plast Surg 1989; 6:167.
3. Dolsky RL. Argon laser skin surgery. Surg Clin North Am
25. Morelli JG, Tan OT, Garden J. Tunable dye laser (577 nm)
64:861, 1984.
treatment of port-wine stains. Lasers Surg Med 1986; 6:94.
4. Anderson RR, Parrish JA. Microvasculature can be selectively
26. Reyes BA, Geronemus R. Treatment of port-wine stains
damaged using dye lasers: a basic theory and experimental
during childhood with the flashlamp-pumped pulsed dye
evidence in human skin. Lasers Surg Med 1981; 1:263.
laser. J Am Acad Dermatol 1990; 23:1142.
5. Anderson RR, Parrish JA. Selective photothermolysis: precise
27. Ruiz-Esparza J, Goldman MP, Fitzpatrick RE, Lowe NJ, Behr
microsurgery by selective absorption of pulsed radiation.
KL. Flashlamp-pumped dye laser for port-wine stains. Lasers
Science 1983; 220:524.
Surg Med 1993; 19(11):1000–3.
Laser Treatment of Cutaneous Vascular Lesions
28. Goldman MP, Fitzpatrick RE, Ruiz-Esparza J. Treatment of
51. Roach ES. Congenital cutaneovascular syndromes. In: Vinkin
port-wine stains (capillary malformation) with the flash-
PV, Bruyn GW, Klawans HL, eds. Handbook of clinical neu-
lamp-pumped pulsed dye laser. J Pediatr 1993; 122:71.
rology: vascular diseases, vol 2. Amsterdam: Elsevier; 1989.
29. Glassberg E et al. The flashlamp-pumped 577 nm pulsed
52. Stern R. Syndromes associated with port-wine stains. In:
tunable dye laser: clinical efficacy and in vitro studies. J Der-
Arndt K, Noe J, eds. Cutaneous laser therapy: principles and
matol Surg Oncol 1988; 14:1200.
methods. New York: Wiley & Sons; 1983.
30. Glassberg E et al. Capillary hemangiomas. Case study of a
53. Jessen RT, Thompson S, Smith EB. Cobb syndrome. Arch Der-
novel laser treatment and a review of therapeutic options. J
matol 1977; 113:1587.
Dermatol Surg Oncol 1989; 15:1214.
54. Mulliken JB. Classification of vascular birthmarks. In:
31. Sherwood KA, Tan OT. Treatment of a capillary hemangioma
Mulliken JB, Young AG, eds. Vascular birthmarks, heman-
with the flashlamp-pumped dye laser. J Am Acad Dermatol
giomas and malformations. Philadelphia: WB Saunders; 1988.
1990; 22:136.
55. Tay Y-K, Morelli J, Weston WL. Inflammatory nuchal-
32. Ashinoff R, Geronemus RG. Capillary hemangiomas and
occipital port-wine stains. J Am Acad Dermatol 1996; 35:
treatment with the flashlamp-pumped pulsed dye laser. Arch
Dermatol 1991; 127:202.
56. Noe JM et al. Port-wine stains and the response to argon laser
33. Dinehart SM, Flock S, Waner M. Beam profile of the flash-
therapy: successful treatment and the predictive role of color,
lamp-pumped pulsed dye laser: support for overlap of expo-
age, and biopsy. Plast Reconstr Surg 1980; 65:130.
sure spots. Lasers Surg Med 1980; 15:277.
57. Geronemus RG, Ashinoff R. The medical necessity of evalu-
34. Jackson BA, Arndt KA, Dover JS. Are all 585 nm pulsed
ation and treatment of port-wine stains. J Dermatol Surg
dye lasers equivalent? J Am Acad Dermatol 1996; 34:
Oncol 1991; 17:76.
58. Wilson BB, Brewer PC, Cooper PH. Spontaneous improve-
35. Archauer BM, Vander Kam VM, Padilla III JF. Clinical expe-
ment of port-wine stain. Cutis 1995; 55:93.
rience with the tunable pulsed-dye laser (585 nm) in the
59. Holloway KB, Ramos-Caro FA, Brownlee RE Jr et al. Giant
treatment of capillary vascular malformations. Plast Recon-
proliferative hemangiomas arising in a port-wine stain. J Am
str Surg 1993; 92:1233.
Acad Dermatol 1994; 31:675.
36. Glassberg E et al. The flashlamp-pumped 577 nm pulsed
60. Wagner KD, Wagner RF. The necessity for treatment of child-
tunable dye laser: clinical efficacy and in vitro studies. J Der-
hood port-wine stains. Cutis 1990; 45:317.
matol Surg Oncol 1988; 14:1200.
61. Malm M, Calberg M. Port-wine stain: a surgical and psycho-
37. Swinehart JM. Hypertrophic scarring resulting from flash-
logical problem. Ann Plast Surg 1988; 20:512.
lamp-pumped pulsed dye laser surgery. J Am Acad Dermatol
62. Lanigan SW, Cotterill JA. Psychological disabilities amongst
1991; 25:845.
patients with port-wine stains. Br J Dermatol 1989; 121:209.
38. Gonzalez E, Gange RW, Momtaz K. Treatment of telangiec-
63. Hansen K, Kreiter CD, Rosenbaum M, Whitaker DC, Arpey
tases and other benign vascular lesions with the 577 nm
CJ. Long-term psychological impact and perceived efficacy
pulsed dye laser. J Am Acad Dermatol 1992; 27:220.
of pulsed-dye laser therapy for patients with port-wine stains.
39. Jacobs AH, Walton RG. The incidence of birthmarks in the
Dermatol Surg 2003; 29:49–55
neonate. Pediatrics 1976; 58:218.
64. Ashinoff R, Geronemus RG. Flashlamp-pumped pulsed dye
40. Pratt AG. Birthmarks in infants. Arch Dermatol 1953; 67:302.
laser for port-wine stains in infancy: earlier versus later treat-
41. Warner M. Personal communication, 1996.
ment. J Am Acad Dermatol 1991; 24:467.
42. Barsky SH et al. The nature and evolution of port-wine
65. Tan E, Vinciullo C. Pulsed dye laser treatment of port-wine
stains: a computer-assisted study. J Invest Dermatol 1980;
stains. Dermatol Surg 1996; 22:119.
66. Masclarelli PC. Living with a port-wine birthmark. In: Tan
43. Motley RJ, Lanigan SW, Katugampola GA. Videomicroscopy
OT, ed. Management and treatment of benign cutaneous
predicts outcome in treatment of port-wine stains. Arch Der-
vascular lesions. Philadelphia: Lea & Febiger; 1992.
matol 1997; 133:921.
67. Troilius A, Wrangsjo B, Ljungren B. Patients with port-wine
44. Susan JO et al. The ‘tomato catsup' fundus in Sturge–Weber
stains and their psychosocial reactions after photothermo-
syndrome. Arch Ophthalmol 1974; 92:69.
lytic treatment. Dermatol Surg 2000; 26:190–196.
45. Mulliken JB. Capillary (port-wine) and other telangiectatic
68. Kurwa HA, Mills CM, Langian SW. Improvement in the
stains. In: Mulliken JB, Young AE, eds. Vascular birthmarks,
psychological impact of a port wine stain after successful
hemangiomas, and malformations. Philadelphia: WB
pulsed dye laser therapy. J Dermal Ther 1999; 10:277–
Saunders; 1988.
46. Bebin EM, Gomez MR. Prognosis in Sturge–Weber disease:
69. McClean K, Hanke CW. The medical necessity for treatment
comparison of unihemispheric and bihemispheric involve-
of port-wine stains. Dermatol Surg 1997; 23:663.
ment. J Child Neurol 1988; 3:181.
70. Clodius I. Excision and grafting of extensive facial haeman-
47. Paller AS. The Sturge–Weber syndrome. Pediatr Dermatol
giomas. Br J Plast Surg 1977; 30:185.
1987; 4:300.
71. Clodius I. Surgery for the extensive facial port-wine stain?
48. Iwach AG et al. Analysis of surgical and medical manage-
Aesthetic Plast Surg 1985; 9:61.
ment of glaucoma in Sturge–Weber syndrome. Ophthalmol-
72. Wilson GM, Kilpatrick R, Eckert H. Thyroid neoplasms fol-
ogy 1990; 97:904.
lowing irradiation. Br Med J 1958; 2:929.
49. Uram M, Zubillaga C. The cutaneous manifestations of
73. Hidano A, Ogihara Y. Cryotherapy with solid carbon dioxide
Sturge–Weber syndrome. J Clin Neurol Ophthalmol 1982;
in the treatment of nevus flammeus. J Dermatol Surg Oncol
1977; 3:213.
50. Tallman B, Tan OT, Morelli JG et al. Location of port-
74. Conway H, Montroy RE. Permanent camouflage of capillary
wine stains and the likelihood of ophthalmic and/or
hemangiomas of the face by interdermal injection of insol-
central nervous system complications. Pediatrics 1991;
uble pigments (tattooing): indications for surgery. NY State
J Med 1965; 65:876.
Cutaneous and Cosmetic Laser Surgery
75. Thomson HG, Wright A. Surgical tattooing of the port-wine
97. Raulin C, Schroeter C, Weiss RA, Keiner M, Werner S. Treat-
stain: operative technique, results and critique. Plast Recon-
ment of port-wine stains with a noncoherent pulsed light
str Surg 1971; 48:113.
source. Arch Dermatol 1999; 135:679–683.
76. Cosman B. Clinical experience in the laser therapy of port-
98. Cliff S, Misch K. Treatment of mature port wine stains with
wine stains. Lasers Surg Med 1980; 1:133.
the photoderm VL. J Cutan Laser Ther 1999; 1:101–104.
77. Bailin PL. Treatment of port-wine stains with the CO2 laser:
99. Bjerring P, Christiansen K, Troilius A. Intense pulsed light
early results. In: Arndt KA, Noe JM, Rosen S, eds. Cutaneous
source for the treatment of dye laser resistant port-wine
laser therapy: principles and methods. Chichester, UK: Wiley
stains. J Cosmetic Laser Ther 2003; 5:7–13.
& Sons; 1983.
100. Hellbrugge G, Stockmeier M, Henschel R, Drosner M. Port
78. Buecker JW, Ratz JL, Richfield DF. Histology of port-wine
wince stains: comparison of intense pulsed light with pulsed
stains treated with carbon dioxide laser. J Am Acad Derma-
dye laser. Lasers Surg Med 2004; S16:14.
tol 1984; 10:1014.
101. Ho WS, Ying SY, Chan PC, Chan HH. Treatment of portwine
79. Ratz JL, Bailin PL, Levine HL. CO2 laser treatment for port-
stains with intense pulsed light: a prospective study. Derma-
wine stains: a preliminary report. J Dermatol Surg Oncol
tol Surg 2004; 30:887–891.
1982; 8:1039.
102. Zachariea H. Delayed wound healing and keloid formation
80. Lanigan SW, Cotterill JA. The treatment of port-wine stains
following argon laser treatment or dermabrasion during
with the carbon dioxide laser. Br J Dermatol 1990; 123:229.
isotretinoin treatment. Br J Dermatol 1988; 118:703.
81. Landthaler M, Haina D, Brunner R. Neodymium-YAG laser
103. Rubenstein R, Roenigk HH, Stegman S et al. Atypical keloids
therapy for vascular lesions. J Am Acad Dermatol 1986; 14:
after dermabrasion of patients taking tretinoin. J Am Acad
Dermatol 1986; 15:280.
82. Walker EP, Butler PH, Pickering JW. Histology of port-wine
104. Bernestein LJ, Geronemus RG. Keloid formation with the
stains after copper vapor laser treatment. Br J Dermatol 1989;
585-nm pulsed dye laser during isotretinoin treatment. Arch
Dermatol 1997; 133:111.
83. Scheibner A, Applebaum J, Wheeland RG. Treatment of
105. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions
port-wine hemangiomas in children. Lasers Surg Med (Suppl)
following pulsed tunable dye laser treatment of port-wine
stains in 701 patients. Br J Dermatol 1997; 136:725.
84. Dixon J, Huether S, Rotering R. Hypertrophic scarring in
106. Renfro L, Geronemus RG. Anatomical differences of port-
argon laser treatment of port-wine stains. Plast Reconstr Surg
wine stains in response to treatment with the pulse dye laser.
1984; 73:771.
Arch Dermatol 1993; 129:182.
85. Hobby LW. Argon laser treatment of superficial vascular
107. Eubanks LE, McBurney EI. Videomicroscopy of port-wine
lesions in children. Lasers Surg Med 1986; 6:16.
stains: correlation of location and depth of lesion. J Am Acad
86. Brauner GJ, Schliftman A. Laser surgery for children. J Der-
Deramtol 2001; 44:948–51.
matol Surg Oncol 1987; 13:178.
108. Yohn JJ, Huff JC, Aeling JL et al. Lesion size is a factor
87. Brauner G, Schliftman A, Cosman B. Evaluation of argon
for determining the rate of port-wine stain clearing follow-
laser surgery in children under 13 years of age. Plast Recon-
ing pulsed dye laser treatment in adults. Cutis 1997; 59:
str Surg 1991; 87:37.
88. Morelli JG, Weston WL. Pulsed dye laser treatment of port-
109. Lanigan SW. Poor response of port wine stains on lower limb
wine stains in children. In: Tan OT, ed. Management and
to pulsed tunable dye laser therapy. Lasers Surg Med 1995;
treatment of benign cutaneous vascular lesions, Philadel-
phia: Lea & Febiger; 1992.
110. Taylor MB. Flashlamp laser treatment of port-wine stain
89. Morelli JG, Weston WL, Huff JV et al. Initial lesion size as a
hemangiomas and other vascular lesions using a defocused
predictive factor in determining the response of port-wine
‘fuzzy spot'. Lasers Surg Med 1991; Suppl 3:68.
stains in children treated with the pulsed dye laser. Arch
111. Lucassen GW, Verkruysse W, Keijzer M et al. Light distribu-
Pediatr Adolesc Med 1995; 149:1142.
tions in port-wine stain model containing multiple cylindri-
90. Alster TS, Wilson F. Treatment of port-wine stains with the
cal and curved blood vessels. Lasers Surg Med 1996; 18:
flashlamp-pumped dye laser: extended clinical experience in
children and adults. Ann Plast Surg 1994; 32:474.
112. Fiskerstrand EJ, Dalaker M, Norvang LT. Laser treatment of
91. Orten SS, Waner M, Flock S et al. Port-wine stains: an assess-
port-wine stains: a study comparing therapeutic outcome
ment of 5 years of treatment. Arch Otolaryngol Head Neck
with morphologic characteristics of the lesions. Acta Derm
Surg 1996; 122;1174.
Venerol (Stockh) 1995; 75:92.
92. Eppley BL, Sadove M. Systemic effects of photothermolysis
113. Fiskerstrand EJ, Svaasand LO, Kopstad G et al. Photother-
of large port-wine stains in infants and children. Plast Recon-
mally induced vessel-wall necrosis after pulsed dye laser
str Surg 1994; 93:1150.
treatment: lack of response in portwine stains with small
93. Pao D. Personal communication, 1994.
sized or deeply located vessels. J Invest Dermatol 1996;
94. Levine VJ, Geronemus RG. Adverse effects associated with
the 577- and 585-nm pulsed dye laser in the treatment of
114. Hohenleutner U, Hilbert M, Wlotzke U et al. Epidermal
cutaneous vascular lesions: a study of 500 patients. J Am
damage and limited coagulation depth with the flashlamp-
Acad Dermatol 1995; 32:613.
pumped pulsed dye laser: a histochemical study. J Invest Der-
95. Lanigan SW. Port-wine stains on the lower limb: response
matol 1995; 104:798.
to pulsed dye laser therapy. Clin Exp Dermatol 1996;
115. Orten SS, Waner M, Flock S, Roberson PK, Kincannon J. Port-
wine stains: an assessment of 5 years of treatment. Arch Oto-
96. Koster PHL, Horsr CMAM van der, Bossuyt PMM, Gemert
laryngol Head Neck Surg 1996; 122(11):1174–9.
MJC van. Prediction of portwine stain clearance and required
116. Smaller RB, Rosen S. Port-wine stains: a disease of altered
number of flashlamp pumped pulsed dye laser treatments.
neural modulation of blood vessels. Arch Dermatol 1986;
Lasers Surg Med 2001; 29:151–155.
Laser Treatment of Cutaneous Vascular Lesions
117. Rydh M, Malm M, Jernbeck J et al. Ectatic blood vessels in
135. Dierickx CC, Farinelli WA, Flotte T et al. Effect of long-pulsed
port-wine stains lack innervation: possible role in patho-
532 nm ND-YAG laser on port-wine stains (PWS). Lasers Surg
genesis. Plast Reconstr Surg 1991; 87:419.
Med 1996; Suppl 8:188.
118. Kauvar AN, Geronemus RG. Repetitive pulse dye laser treat-
136. Woo WK, Jasim ZF, Handley JM. Evaluating the efficacy of
ments improve persistent port-wine stains. Dermatol Surg
treatment of resistant port-wine stains with variable-pulse
1995; 21:515.
595-nm pulsed dye and 532-nm Nd:YAG lasers. Dermatol
119. Kane KS, Smoller BR, Fitzpatrick RE et al. Pulsed dye laser-
Surg 2004; 30:158–162.
resistant port-wine stains. Arch Dermatol 1996; 132:839.
137. Chan H, Chan E, Kono T, Ying SY, Wai-Sun H. The use of
120. Lo JS, Sgouros GN, Laohs FE et al. Basal cell carcinoma within
variable pulse width frequency doubled Nd:YAG 532 nm laser
a nevus flammeus: report of a case. Int J Dermatol 1991;
in the treatment of port-wine stain in Chinese patients.
Dermatol Surg 2000; 26:657–661.
121. Chang C-J, Nelson JS. Cryogen spray cooling and higher
138. Ahcan U, Zorman P, Recek D, Ralca S, Mararon B. Port
fluence pulsed dye laser treatment improve port-wine stain
wine stain treatment with a dual-wavelength Nd:YAG and
clearance while minimizing epidermal damage. Dermatol
cryogen spray cooling: a pilot study. Lasers Surg Med 2004;
122. Geronemus RG. High-fluence modified pulsed dye laser
139. Yang MU, Yaroslavsky AN, Farinelli WA, Flotte TJ, Rius-Dias
photocoagulation with dynamic cooling of port-wine stains
F, Tsao SS, Anderson RR. Long-pulsed Nd:YAG laser treatment
in infancy. Arch Dermatol 2000; 136:942–943.
of portwine stains. J Am Acad Dermatol 2005; 52:480–490.
123. Kelly KM, Nanda VS, Nelson JS. Treatment of port-wine stain
140. Mulliken JB. A plea for a biologic approach to hemangiomas
birthmarks using the 1.5-msec pulsed dye laser at high flu-
of infancy. Arch Dermatol 1991; 127:243 (editorial).
ences in conjunction with cryogen spray cooling. Dermatol
141. Mulliken JB. Diagnosis and natural history of hemangiomas.
Surg 2002; 28:309–313.
In: Mulliken JB, Young AE, eds. Vascular birthmarks, heman-
124. Bencini PL. The multilayer technique: a new and fast
giomas and malformations. Philadelphia: WB Saunders;
approach for flashlamp-pumped pulsed (FLPP) dye laser
treatment of port-wine stains (preliminary report). Dermatol
142. Finn MC, Glowacki J, Mulliken JB. Congenital vascular
Surg 1999; 25;786–789.
lesions: clinical application of a new classification. J Pediatr
125. Lorenz S, Brunnberg S, Landthaler M, Hohenleutner U.
Surg 1983; 18:894.
Regading the multilayer technique for treatment of PWS.
143. Jacobs AH. Strawberry hemangiomas: the natural history of
Dermatol Surg 2001; 27:90.
the untreated lesion. Calif Med 1957; 86:8.
126. Bernstein EF. Treatment of a resistant port-wine stain with
144. Holmdahl K. Cutaneous hemangiomas in premature and
the 1.5-msec pulse duration, tunable, pulsed dye laser. Der-
mature infants. Acta Paediatr 1955; 44:370.
matol Surg 2000; 26:1007–1009.
145. Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma:
127. Greve B, Raulin C. Prospective study of port wine stain
evidence of accelerated involution. J Pediatr 1996; 128:329.
treatment with dye laser: Comparison of two wavelengths
146. Rositto A, Ranalletta M, Drut R. Congenital hemangioma of
(585 nm vs. 595 nm) and two pulse durations (0.5 milli-
eccrine sweat glands. Pediatr Dermatol 1993; 10:341.
seconds vs. 20 milliseconds). Lasers Surg Med 2004; 34:168–
147. Bowers RE, Graham EA, Tomlinson KM. The natural history
of the strawberry nevus. Arch Dermatol 1960; 82:667.
128. Laube S, Taibjee S, Lanigan S. Treatment of resistant port
148. Takahashi K, Mulliken JB, Kozakewich HPW et al. Cellular
wine stains with the V Beam® pulsed dye laser. Lasers Surg
markers that distinguish the phases of hemangioma during
Med 2003; 33:282–287.
infancy and childhood. J Clin Invest 1994; 93:2357.
129. Scherer K, Lorenz S, Wimmershoff M, Landthaler M,
149. Margileth AM, Museles M. Cavernous hemangiomas in chil-
Hohenleutner U. Both the flashlamp-pumped dye laser and
dren: diagnosis and conservative management. JAMA 1965;
the long-pulsed tunable dye laser can improve results in
port-wine stain therapy. Br J Dermatol 2001; 145:79–84.
150. Bivings L. Spontaneous regression of angiomas in children:
130. Kimel S, Svaasand LO, Hammer-Wilson MJ, Nelson JS. Influ-
twenty-two years' observation covering 236 cases. J Pediatr
ence of wavelength on response to laser photothermolysis of
1954; 45:643.
blood vessels: implications for port wine stain therapy. Lasers
151. Tuder RM, Young R, Karasek M et al. Adult cutaneous heman-
Surg Med 2003; 33:288–295.
giomas are composed of new replicating endothelial cells.
131. Chang C-J, Kelly KM, van Gemert MJC, Nelson JS. Compar-
J Invest Dermatol 1987; 89:594.
ing the effectiveness of 585-nm vs. 595-nm wavelength
152. Lister WA. The natural history of strawberry naevi. Lancet
pulsed dye laser treatment of port wine stains in conjunc-
1938; 1:1429.
tion with cryogen spray cooling. Lasers Surg Med 2002;
153. Baker ER, Manders E, Whitney CW. Growth of cavernous
hemangioma with puberty. Clin Pediatr 1985; 24:596.
132. Randeberg LL, Bonesronning JH, Dalaker M, Nelson JS.
154. Moroz B. The course of haemangiomas in children. I:
Methemoglobin formation during laser induced photo-
Ryan TJ, Cherry GW, eds. Vascular birthmarks: pathogenesis
thermolysis of vascular skin lesions. Lasers Surg Med 2004;
and management. New York: Oxford University Press;
133. Svaasand LO, Aguilar G, Viator JA, Randeberg LL, Kimel S,
155. Liang MG, Frieden IJ. Perineal and lip ulcerations as the pre-
Nelson JS. Increase of dermal blood volume fraction reduces
senting manifestation of hemangioma of infancy. Pediatrics
the threshold for laser-induced purpura: Implications for
1997; 99:256.
port wine stain laser treatment. Lasers Surg Med 2004;
156. Kim HJ, Colombo M, Frieden IJ. Ulcerated hemangiomas:
clinical characteristics and response to therapy. J Am Acad
134. Raulin C, Hellwig S, Schonermark MP. Treatment of a non-
Dermatol 2001; 44:962–72.
responding port-wine stain with a new pulsed light source
157. Williams HB. Facial bone changes with vascular tumors in
(Photoderm VL). Lasers Surg Med 1997; 21:203.
children. Plast Reconstr Surg 1979; 63:309.
Cutaneous and Cosmetic Laser Surgery
158. Thomson G, Stigmar G et al. Hemangiomas of the eyelid:
181. Brain RT, Calvan CD. Vascular naevi and their treatment. Br
visual complications and prophylactic concepts. Plast Recon-
J Dermatol 1952; 64:147.
str Surg 1979; 63:641.
182. Matthews DN. Hemangiomata. Plast Reconstr Surg 1968;
159. Robb RM. Refractive errors associated with hemangiomas of
the eyelids and orbit in infancy. Am J Ophthalmol 1977;
183. Scerri L, Navaratnam A. Basal cell carcinoma presenting as
a delayed complication of thorium X used for treating a
160. Malm M. Port-wine stain: laser treatment and evaluation of
congenital hemangioma. J Am Acad Dermatol 1994; 31:
results. Stockholm: Kongl Carolinska Medico Chirargiska
Institutet; 1987.
184. Lomholt S. Alpha and beta ray therapy in dermatology. Br J
161. Healy GB et al. Treatment of subglottic hemangioma
Dermatol 1936; 48:567.
with the carbon dioxide laser. Laryngoscope 1980; 90:
185. Bowers RE. Treatment of haemangiomatous naevi with
thorium X. Br Med J 1951; 1:121.
162. Kasabach HH, Merritt KK. Capillary hemangioma with
186. Courtermarche AD. Thorium X induced BCC: a case report.
extensive purpura. Am J Dis Child 1940; 59:1063.
Br J Plast Surg 1961; 14:254.
163. McLean RH et al. Multinodular hemangiomatosis of the liver
187. Caldwell JB, Ryan MT, Benson PM et al. Cutaneous angiosar-
in infancy. Pediatrics 1972; 49:563.
coma arising in the radiation site of a congenital heman-
164. Patel SD, Cohen BA, Kan JS et al. Extensive facial heman-
gioma. J Am Acad Dermatol 1995; 33:865.
giomas associated with cardiac and abdominal abnormali-
188. Dinehart S, Waner M, Flock S. The copper vapor laser for
ties. J Am Acad Dermatol 1997; 36:636.
treatment of cutaneous vascular and pigmented lesions. J
165. Igarashi M, Uchida H, Kaji T. Supraumbilical midabdominal
Dermatol Surg Oncol 1993; 19:370.
raphe and facial cavernous hemangiomas. Clin Genet 1985;
189. Sybert V. Personal communication, 1992.
190. George ME, Sharma V, Jacobson J, et al. Adverse effects of
166. Schneeweiss A, Blieden LC, Shem-Tov A et al. Coarctation of
systemic glucocorticosteroid therapy in infants with heman-
the aorta and congenital hemangioma of the face and neck
giomas. Arch Dermatol 2004; 140:963–9.
and aneurysm or dilatation of a subclavian or innominate
191. Kushner BJ. The treatment of periorbital infantile heman-
artery. Chest 1982; 82:186.
giomas with intralesional corticosteroid. Plast Reconstr Surg
167. Reese V, Frieden IJ, Paller AS et al. Association of facial
1985; 76:517.
hemangiomas with Dandy–Walker and other posterior fossa
192. Nelson LB, Melick JE, Harley RD. Intralesional corticosteroid
malformations. J Pediatr 1993; 122:379.
injections for infantile hemangiomas of the eyelid. Pediatrics
168. Frieden IJ, Reese V, Cohen D. PHACE syndrome. Arch Der-
1984; 74:241.
matol 1996; 132:307.
193. Ellis PO. Occlusion of the central retinal artery after retro-
169. Gorlin RJ, Kantaputra P, Aughton DJ et al. Marked female
bulbar corticosteroid injection. Am J Ophthalmol 1983;
predilection in some syndromes associated with facial
hemangiomas. Am J Med Genet 1994; 52:130.
194. Chowdri NA, Darzi MA, Fazili Z et al. Intralesional corticos-
170. Goldberg NS, Hebert AA, Esterly NB. Sacral hemangiomas
teroid therapy for childhood cutaneous hemangiomas. Ann
and multiple congenital abnormalities. Arch Dermatol 1986;
Plast Surg 1994; 33:46.
195. Enjolras O, Mulliken JB. The current management of vascu-
171. Albright AL, Gartner JC, Wiener ES. Lumbar cutaneous
lar birthmarks, Pediatr Dermatol 1993; 10:311.
hemangiomas as indicators of tethered spinal cords. Pedi-
196. Brousty-Brye D, Zelter BR. Inhibition of cell motility by inter-
atrics 1989; 83:977.
feron. Science 1980; 208:516.
172. Enjolras O, Gelbert F. Superficial hemangiomas: associations
197. Ezekowitz RA, Mulliken JB, Folkman J. Interferon alpha-2a
and management. Pediatr Dermatol 1997; 14:173.
therapy for life-threatening hemangiomas of infancy. N Engl
173. Finn MC, Glowacki J, Mulliken JP. Congenital vascular
J Med 1992; 326:1456.
lesions: clinical applications of a new classification. J Pediatr
198. Stratte EG, Tope WD, Johnson CL et al. Multimodal man-
Surg 1983; 18:894.
agement of diffuse neonatal hemangiomatosis. J Am Acad
174. Tanner JL, et al. Growing up with a facial hemangioma:
Dermatol 1996; 34:337.
parent and child coping and adaptation. Pediatrics 1998;
199. Apfelberg DB, Greene RA, Maser MR. Results of argon laser
exposure of capillary hemangiomas of infancy: preliminary
175. Modlin JJ. Capillary hemangiomas of the skin. Surgery 1955;
report. Plast Reconstr Surg 1981; 67:188.
200. Hobby LW. Further evaluation of the potential of the argon
176. Mulliken JB. Treatment of hemangiomas. In: Mulliken JB,
laser in the treatment of strawberry hemangiomas. Plast
Young AE, eds. Vascular birthmarks, hemangiomas and mal-
Reconstr Surg 1983; 71:481.
formations. Philadelphia: WB Saunders; 1988.
201. Achauer BM, VanderKam VM. Argon laser treatment of
177. Cherry GW. A review of the role of surgery in the treatment
strawberry hemangioma in infancy. West J Med 1985;
of hemangiomas and port-wine stains. In: Ryan TJ, Cherry
GW, eds. Vascular birthmarks: pathogenesis and manage-
202. Apfelberg DB. Cases 5.6, 5.7, 5.9, 5.10, 5.11. In: Apfelberg
ment. New York: Oxford University Press; 1987.
DB, ed. Atlas of cutaneous laser surgery. New York: Plenum;
178. Bek V, Zahn K. Cataract as a late sequela of contract roent-
gen therapy of angiomas in children. Acta Radiol 1960;
203. Shapshay SM, David LM, Zeitels S. Neodymium-YAG laser
photocoagulation of hemangiomas of the head and neck.
179. Tan OT, Gilchrest BA. Laser therapy for selected cutaneous
Laryngoscope 1987; 97:323.
vascular lesions in the pediatric population: a review. Pedi-
204. Apfelberg DB. Cases 6.9, 6.10, 6.19, 6.20. In: Apfelberg DB,
atrics 1988; 82:652.
ed. Atlas of cutaneous laser surgery. New York: Plenum; 1992.
180. MacCollum DW. Treatment of hemangiomas. Am J Surg
205. Preeyanont P, Nimsakul N. The Nd:YAG laser treatment of
1935; 29:32.
hemangioma. J Clin Laser Med Surg 1994; 12:225.
Laser Treatment of Cutaneous Vascular Lesions
206. Burnstein FD, Simms C, Cohen SR et al. Intralesional therapy
24th Annual meeting of the American Society of Dermato-
of extensive hemangiomas in 100 consecutive pediatric
logic Surgery, Boston, MA; May 1997.
patients. Ann Plast Surg 2000; 44:188–94.
228. Poetke M, Philipp C, Berlien HP. Flashlamp-pumped pulsed
207. Apfelberg DB, Maser MR, Lash H. YAG laser resection of com-
dye laser for hemangiomas in infancy. Arch Dermatol 2000;
plicated hemangiomas of the hand and upper extremity.
J Hand Surg 1990; 15A:765.
229. Hohenleutner S, Badur-Ganter E, Landthaler M, Hohenteut-
208. Apfelberg DB, Maser MR, Lash H. High- and low-tech solu-
ner U. Long-term results in the treatment of childhood
tions for massive cavernous hemangioma of the face. Lasers
hemangiomas with the flashlamp-pumped pulsed dye
and leeches to the rescue. Ann Plast Surg 1989; 23:341.
laser: An evaluation of 617 cases. Lasers Surg Med 2001;
209. Thompson HG, Lonigan M. The Cyrano nose: a clinical
review of hemangiomas of the nasal tip. Plast Reconstr Surg
230. Foster TD, Gold MH. The successful use of the PhotoDerm
1979; 63:155.
VL in the treatment of a cavernous hemangioma in a dark-
210. Afpelberg DB. Argon and YAG laser photocoagulation and
skinned infant. Minim Invas Nurs 1996; 10:102.
excision of hemangiomas and vascular malformations of the
231. Fujino T. Microsurgical technique in resection of haeman-
nose. West J Med 1995; 163:122.
gioma in infants. Ann Plast Surg 1985; 13:190.
211. Hoffman WL, Anvari B, Said S et al. Cryogen spray cooling
232. Argenta L. Controlled tissue expansion in reconstructive
during Nd:YAG laser treatment of hemangiomas. Dermatol
surgery. Br Plast Surg 1984; 37:520.
Surg 1997; 23:635.
233. Grabb WC et al. Facial hamartomas in children: neuro-
212. Groot D, Rao J, Johnston P, Nakatsui T. Algorithm for using
fibroma, lymphangioma and haemangioma. Plast Reconstr
a long-pulsed Nd:YAG laser in the treatment of deep cuta-
Surg 1980; 66:509.
neous vascular lesions. Dermatol Surg 2003; 29:35–42.
234. Apfelberg DB, Lane B, Marx MP. Combined (team) approach
213. Achauer BM, Vanderkam VM, Celikoz B. Intralesional bare
to hemangioma management: arteriography with super-
fiber laser treatment of hemangioma. Lasers Surg Med 1997;
selective embolization plus YAG laser/sapphire-tip resection.
Plast Reconstr Surg 1991; 88:71.
214. Raulin C, Greve B. Retrospective clinical comparison of
235. Raulin C, Raulin SJ, Hellwig S, Schonermark MP. Treatment
hemangiomas treatment by flashlamp-pumped (585 nm) and
of benign venous malformation with an intense pulsed light
frequency-doubled Nd:YAG (532 nm) lasers. Lasers Surg Med
source (Photoderm VL). Eur J Dermatol 1997; 7:279.
2001; 28:40–43.
236. Frieden IJ, Eichenfield LF, Esterly NB et al. Guidelines of care
215. Kaplan I, Sharon U. Current laser surgery. Ann NY Acad Sci
for hemangiomas of infancy. J Am Acad Dermatol 1997;
1976; 267:247.
216. Labandter H, Kaplan I. Experience with a continuous laser in
237. Pasyl KA. Classification and histopathological features of hae-
the treatment of suitable cutaneous conditions. Preliminary
mangiomas and other vascular malformations. In: Ryan TJ,
report. J Dermatol Surg Oncol 1977; 3:527.
Cherry GW, eds. Vascular birthmarks: pathogenesis and man-
217. Apfelberg DB, Maser MR, Lash H et al. Benefits of carbon
agement. New York: Oxford University Press; 1987.
dioxide laser in oral hemangioma excision. Plast Reconstr
238. Bucci FA, Wiener BD. The pyogenic granuloma. Ariz Med
Surg 1985; 75:46.
1984; WLI:794.
218. Apfelberg DB, Maser MR, Lash H. Review and usage of argon
239. Cooper PH, Mills SE. Subcutaneous granuloma pyogenicum:
and carbon dioxide lasers for pediatric hemangiomas. Ann
lobular capillary hemangioma. Arch Dermatol 1982; 118:
Plast Surg 1984; 12:353.
219. Scheepers JH, Quaba AA. Does the pulsed tunable dye laser
240. Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular
have a role in the management of infantile hemangiomas?
capillary hemangioma): a clinicopathologic study of 178
Observations based on 3 years' experience. Plast Reconstr
cases. Pediatr Dermatol 1991; 8:267.
Surg 1995; 95:305.
241. Goldberg DJ, Sciales CW. Pyogenic granuloma in children.
220. Morelli JG, Weston WL. Pulsed dye laser treatment of
J Dermatol Surg Oncol 1991; 17:960.
hemangiomas. In: Tan OT, ed. Management and treatment
242. Mills SE, Cooper PH, Fechner RE. Lobular capillary heman-
of benign cutaneous vascular lesions. Philadelphia: Lea &
gioma: the underlying lesion of pyogenic granuloma, a study
Febiger; 1992.
of 73 cases from the oral and nasal mucous membranes.
221. Morelli JG, Tan OT, Yohn JJ. Treatment of ulcerated heman-
Am J Surg Pathol 1980; 4:471.
giomas in infancy. Arch Pediatr Adolesc Med 1994; 148:1104.
243. Arndt KA. Argon laser therapy of small cutaneous vascular
222. Garden JM, Babus AD, Paller AS. Treatment of cutaneous
lesions. Arch Dermatol 1982; 118:220.
hemangiomas by the flashlamp-pumped pulsed dye laser:
244. Blickenstaff RD et al. Recurrent pyogenic granuloma with
prospective analysis. J Pediatr 1992; 120:555.
satellitosis. J Am Acad Dermatol 1989; 21:1241.
223. Maier H, Neumann R. Treatment of strawberry marks with
245. Tan OT, Kurban AK. Noncongenital benign cutaneous vas-
flashlamp-pumped pulsed dye laser in infancy. Lancet 1996;
cular lesions: pulse dye laser treatment. In: Tan OT, ed. Man-
agement and treatment of benign cutaneous vascular lesions.
224. Barlow RJ, Walker NPJ, Markey AC. Treatment of prolifera-
Philadelphia: Lea & Febiger; 1992.
tive haemangiomas with the 585 nm pulsed dye laser. Br J
246. Glass AT, Milgram S. Flashlamp-pumped dye laser treatment
Dermatol 1996; 134:700.
for pyogenic granuloma. Cutis 1992; 49:351.
225. West T, Fitzpatrick RE, Goldman MP, Alster T. Treatment of
247. Gonzalez S, Vibhagool C, Falo LD Jr et al. Treatment of pyo-
capillary hemangiomas with the Candela flashlamp-pumped
genic granulomas with the 585-nm pulsed dye laser. J Am
pulsed dye laser and corticosteroids. Dermatol Surg 1998.
Acad Dermatol 1996; 35:428.
226. McDaniel DH, Mordon S. Hexascan: a new robotized scan-
248. Merlen JF. Red telangiectasias, blue telangiectasias. Soc Franc
ning laser handpiece. Cutis 1990; 45:300.
Phlebol 1970; 22:167.
227. Kauvar A. Long-pulse and high energy pulsed dye laser treat-
249. Goldman MP, Bennett RG. Treatment of telangiectasia: a
ment of port-wine stains and hemangiomas. Presented at
review. J Am Acad Dermatol 1987; 17:167.
Cutaneous and Cosmetic Laser Surgery
250. Redisch W, Pelzer RH. Localized vascular dilatations of the
271. Mordon SR et al. Comparison study of the ‘point-by-point
human skin: capillary microscopy and related studies. Am
technique' and the ‘scanning technique' for laser treatment
Heart J 1949; 37:106.
of port-wine stain. Lasers Surg Med 1989; 9:398.
251. Kaplan I, Peled I. The carbon dioxide laser in the treatment
272. Cassuto DA, Ancona DM, Emanuelli G. Treatment of
of superficial telangiectases. Br J Plast Surg 1975; 28:214.
facial telangiectasias with a diode-pumped Nd:YAG laser at
252. Achauer BM, VanderKam VM. Argon laser treatment of the
532 nm. J Cutan Laser Ther 2000; 2:141–6.
face and neck: 5 years' experience. Lasers Surg Med 1987;
273. Hevia O. New laser treatment for facial telangiectasias: a ran-
domized study. Cosmetic Dermatol 1997; 10:53.
253. Lyons GD, Owens RE, Moriney DF. Argon laser destruction
274. Goldberg DJ, Marcus J. The use of the frequency-doubled Q-
of cutaneous telangiectatic lesions. Laryngoscope 1981;
switched Nd:YAG laser in the treatment of small cutaneous
vascular lesions. Dermatol Surg 1996; 22:841.
254. Greenwald J et al. Comparative histological studies of the
275. Goldberg DJ, Meine JG. A comparison of four frequency-
tunable dye (at 577 nm) laser and argon laser: the specific
doubled Nd:YAG (532 nm) laser systems for treatment of
vascular effects of the dye laser. J Invest Dermatol 1981;
facial telangiectases. Dermatol Surg 1999; 25:463–7.
276. Kauvar ANB, Frew KE, Friedman PM, Geronemus RG.
255. Scheibner A, Wheeland RG. Argon-pumped tunable dye laser
Cooling gel improves pulsed KTP laser treatment of facial
therapy for facial port-wine stain hemangiomas in adults:
telangiectasia. Lasers Surg Med 2002; 30:149–53.
a new technique using small spot size and minimal power.
277. Fitzpatrick RE et al. Treatment of facial telangiectasia with
J Dermatol Surg Oncol 1989; 15:277.
the flash pump dye laser. Lasers Surg Med 1991; Suppl 3:70.
256. Scheibner A, Wheeland RG. Use of the argon-pumped
278. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking
tunable dye laser for port-wine stains in children. J Derma-
improve the results of treatment with variable-pulse pulsed-
tol Surg Oncol 1991; 17:735.
dye lasers? Dermatol Surg 2004; 30:163–7.
257. Cosman B. Role of retreatment in minimal-power argon
279. Alam M, Dover JS, Arndt KA. Treatment of facial telangiec-
laser therapy for port-wine stains. Lasers Surg Med 1982;
tasia with variable-pulse high-fluence pulsed-dye laser:
comparison of efficacy with fluences immediately above
258. Fitzpatrick RE, Feinberg JA. Low dose argon laser therapy of
and below the purpura threshold. Dermatol Surg 2003;
port-wine stains. ICALEO ‘83 Proceedings; 1984; 6:47.
259. Rotteleur G et al. Robotized scanning laser handpiece for the
280. Eremia S, Willoughby M. A prospective study of periocular
treatment of port-wine stains and other angiodysplasias.
reticular veins treated with a 940-nm diode pumped laser.
Lasers Surg Med 1988; 8:283.
Cosmet Dermatol 2002; 15:19–21.
260. Orenstein A, Nelson JS. Treatment of facial vascular lesions
281. Eremia S, Li CY. Treatment of face veins with a cryogen spray
with a 100 mm spot 577 nm pulsed continuous wave dye
variable pulse width 1064 nm Nd:YAG laser: a prospective
laser. Ann Plast Surg 1989; 23:310.
study of 17 patients. Dermatol Surg 2002; 28:244–247.
261. Apfelberg DB. Cases 7.6, 7.7, 7.22, 7.30. In: Apfelberg DB,
282. Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm
ed. Atlas of cutaneous laser surgery. New York: Raven;
neodymium:YAG laser treatment of facial telangiectasia.
Dermatol Surg 2003; 29:56–58.
262. McDaniel DH, Mordon S. Hexascan: a new robotized scan-
283. Goldman MP. Optimal management of facial telangiectasia.
ning laser handpiece. Cutis 1990; 45:300.
Am J Clin Dermatol 2004; 5:423–434.
263. Broska P, Martinho E, Goodman MM. Comparison of the
284. Bjerrimg P, Christian K, Troilius A. Intense pulsed light source
argon tunable dye laser with the flashlamp pulsed dye laser
for treatment of facial telangiectasias. J Cosmetic & Laser
in treatment of facial telangiectasia. J Dermatol Surg Oncol
Ther 2001; 3:169–73.
1994; 20:749.
285. Alderson MR. Spider naevi: their incidence in healthy school
264. Waner M, Dinehart S. Lasers in facial plastic and recon-
children. Arch Dis Child 1963; 38:286.
structive surgery. In: Davis RK, Shapstray S, eds. Lasers in
286. Wenzl JE, Burgert EO. The spider nevus in infancy and child-
otolaryngology/head and neck surgery. Philadelphia: WB
hood. Pediatrics 1964; 33:227.
Saunders; 1989.
287. Oster J, Nielsen A. Nuchal naevi and intrascapular telang-
265. Dinehart SM, Waner M. Comparison of the copper vapor and
iectases. Acta Paediatr Scand 1970; 59:416.
flashlamp-pulsed dye laser in the treatment of facial telang-
288. Bean WB. Vascular spiders and related lesions of the skin.
iectasia. J Am Acad Dermatol 1991; 24:116.
Springfield, IL: Thomas; 1958.
266. Neumann RA, Leonhartsberger H, Bohler-Sommereger K
289. Geronemus RG. Treatment of spider telangiectases in chil-
et al. Results and tissue healing after copper-vapour laser (at
dren using the flashlamp pumped pulsed dye laser. Pediatr
578 nm) treatment of port-wine stains and facial telangiec-
Dermatol 1991; 8:61.
tasias. Br J Dermatol 1993; 128:306.
290. Goldman MP, Fitzpatrick RE, Ruiz-Esparza J. Treatment of
267. Thibault PK. Copper vapor laser and microsclerotherapy
spider telangiectasia in children. Contemp Pediatr 1993;
of facial telangiectases. J Dermatol Surg Oncol 1994; 20:
291. de Rooij MJM, Neumann HAM. Granuloma telangiectaticum
268. Waner M, Dinehart SM, Wilson MB et al. A comparison of
after argon laser therapy of a spider nevus. Dermatol Surg
copper vapor and flashlamp pumped dye lasers in the treat-
1995; 20:354.
ment of facial telangiectasia. J Dermatol Surg Oncol 1993;
292. Raulin C, Weiss RA, Schohermark MP. Treatment of essential
telangiectasias with an intense pulsal light source (Photo-
269. McCoy SE. Copper bromide laser treatment of facial telang-
Derm VL). Dermatol Surg 1997; 23:941.
iectasia: results of patients treated over five years. Lasers Surg
293. Wilkin JK. Rosacea: pathophysiology and treatment. Arch
Med 1997; 21:329.
Dermatol 1994; 130:359.
270. McCoy S, Hanna M, Anderson P et al. An evaluation of the
294. Schmidt NF, Gans EH. Demodex and rosacea, I: the preva-
copper-bromide laser for treating telangiectasia. Dermatol
lence and numbers of Demodex mites in rosacea. Cosmetic
Surg 1996; 22:551.
Dermatol 2004; 17:497–502.
Laser Treatment of Cutaneous Vascular Lesions
295. Hernandez-Perez E, Ibiett EV. Gross and microscopic find-
317. Bean WB, Walsh JR. Venous lakes. Arch Dermatol 1956;
ings in patients submitted to nonablative full face resur-
facing using intense pulsed light. Dermatol Surg 2002;
318. Lanthaler M, et al. Laser therapy of venous lakes (Bean–
Walsh) and telangiectasia. Plast Reconstr Surg 1984; 73:78.
296. Prieto VG, Sadick NS, Lloreta J, et al. Effects of intense pulsed
319. Pasyk KA. Case 5.27: venous lake at the lower lip. In:
light on sun-damaged human skin, routine, and ultrastruc-
Apfelberg DB, ed. Atlas of cutaneous laser surgery. New York:
tural analysis. Lasers Med Surg 2002; 30:82–5.
Raven; 1992.
297. Dahl MV, Ross AJ, Schlievert PM. Temperature regulates bac-
320. Orenstein A. Case 5.28: venous lake of the lip. In: Apfelberg
terial protein production: possible role in rosacea. J Am Acad
DB, ed. Atlas of cutaneous laser surgery. New York: Raven;
Dermatol 2004; 50:266–72.
298. Fitzpatrick RE et al. Treatment of facial telangiectasia with
321. Neumann RA, Knobler RM. Venous lakes (Bean–Walsh) of
the flash pump dye laser. Lasers Surg Med 1991; Suppl 3:70.
the lips: treatment experience with the argon laser and 18
299. Lowe NJ et al. Flashlamp pumped dye laser for rosacea-
months follow-up. Clin Exp Dermatol 1990; 15:115.
associated telangiectasia and erythema. J Dermatol Surg
322. Alcalay J, Sandbank M. The ultrastructure of cutaneous
Oncol 1991; 17:522.
venous lakes. Int J Dermatol 1987; 26:645.
300. Clark SM, Lanigan SW, Marks R. Laser treatment of erythema
323. Rosio TJ. Case 7.31: venous lake. In: Apfelberg DB, ed. Atlas
and telangiectasia associated with rosacea. Lasers Surg Med
of cutaneous laser surgery. New York: Raven; 1992.
2002; 17:26–33.
324. Rowson KEK, Mahy BWJ. Human papova (wart) virus. Bac-
301. Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed
teriol Rev 1967; 31:100.
dye laser treatment of rosacea-associated telangiectasia
325. Lever WF, Schaumberg-Lever G. Diseases caused by viruses.
using subpurpuric clinical threshold. Dermatol Surg 2004;
In: Lever WF, ed. Histopathology of the skin. Philadelphia:
Lippincott; 1983.
302. Tan SR, Tope WD. Pulsed dye laser treatment of rosacea
326. Nemeth AJ, Reyes BA. Copper vapor laser treatment of
improves erythema, symptomotology, and quality of life. J
genital and body warts. Lasers Surg Med 1950; 10:52.
Am Acad Dermatol 2004; 51:592–9.
327. Webster GF, Satur N, Goldman MP et al. Treatment of recal-
303. Lonne-Rahm S, Nordlund K, Edstrom DW, Ros AM. Laser
citrant warts using the pulsed dye laser. Cutis 1996; 56:230.
treatment of rosacea: a pathoetiological study. Arch Derma-
328. Tan OT, Hurwitz RM, Stafford TJ. Pulsed dye laser treatment
tol 2004; 140:1345–9.
of recalcitrant verrucae: a preliminary report. Lasers Surg
304. Berg M, Edstrom DW. Flashlamp pulsed dye laser did not
Med 1993; 13:127.
cure papulpustular rosacea. Lasers Surg Med 2004; 34:266–8.
329. Kauvar AB, McDanial DH, Geronemus RG. Pulsed dye laser
305. Silver BE, Livshots YL. Preliminary experience with the
treatment of warts. Arch Fam Med 1995; 4:1035.
KTP/532 nm laser in the treatment of facial telangiectasia.
330. Jacobsen E, McGraw R, McCagh S. Pulsed dye laser efficacy
Cosmetic Dermatol 1996; 9:61.
as initial therapy for warts and against recalcitrant verrucae.
306. Mark K, Sparacio RM, Voigt A, et al. Objective and quantita-
Cutis 1997; 59:206.
tive improvement of rosacea-associated erythema after
331. Jain A, Storwick GS. Effectiveness of the 585 nm flashlamp-
intense pulsed light treatment. Dermatol Surg 2003; 9:600–4.
pulsed tunable dye laser (PTDL) for treatment of plantar
307. Schroeter CA, Below SH, Neumann HAM. Effective treatment
verrucae. Laser Surg Med 1997; 21:500.
of Rosacea using intense pulsed light systems. Dermatol Surg
332. Huilgol SC, Barlow RJ, Markey AC. Failure of pulsed dye
2005 (in press).
laser therapy for resistant verrucae. Clin Exp Dermatol 1996;
308. Taub AF. Treatment of rosacea with intense pulsed light.
J Drugs Dermatol 2003; 3:254–9.
333. Ross BS, Levine VJ, Nehal K et al. Pulsed dye laser treat-
309. Civatte A. Poikilodermie reticulae pigmentaire du visage et
ment of warts: an update. Dermatol Surg 1999; 25:377–
du col. Ann Derm Syph 1923; 6:605.
310. Batta K, Hindson C, Cotterill JA, Foulds IS. Treatment of poik-
334. Stern P, Levine N. Controlled localized heat therapy in cuta-
iloderma of Civatte with the potassium titanyl phosphate
neous warts. Arch Dermatol 1992; 128:945.
(KTP) laser. Br J Dermatol 1999; 140:1191–2.
335. Diakuzono N, Joffe SN, Tajiri H et al. Laserthermia: a com-
311. Wheeland RG, Applebaum J. Flashlamp-pumped pulsed dye
puter controlled contact Nd:YAG system for interstitial local
laser therapy for poikiloderma of Civatte. J Dermatol Surg
hyperthermia. Med Instrum 1987; 21:275.
Oncol 1990; 16:12.
336. Pfau A, Abd-El-Raheem TA, Baumler W et al. Nd:YAG laser
312. Haywood RM, Monk BE. Treatment of poikiloderma of
hyperthermia in the treatment of recalcitrant verrucae vul-
Civatte with the pulsed dye laser: a series of seven cases.
gares (Regensburg's technique). Acta Derm Venereol (Stockh)
J Cutan Laser Ther 1999; 1:45–8.
1994; 74:212.
313. Goldman MP, Weiss RA. Treatment of poikiloderma of
337. Pfau A, Abd-El-Raheem TA, Baumler W et al. Treatment of
Civatte on the neck with an intense pulsed light source. Plast
recalcitrant verrucae vulgares with Nd:Yag laser hyperther-
Reconstr Surg 2001; 107:1376–81.
mia (Regensburg's technique): preliminary results in 31
314. Weiss RA, Goldman MP, Weiss MA. Treatment of poikilo-
cases. J Dermatol Treat 1995; 6:39.
derma of Civatte with an intense pulsed light source. Der-
338. Li Y, Yang K. Treatment of recalcitrant flat warts using
matol Surg 2000; 26:823–8.
frequency-doubled Q-switched Nd-YAG laser. Lasers Surg
315. Zheng P, Lavker RM, Kligman AM. Anatomy of striae. Brit J
Med 2001; 29:244–247.
Dermatol 1985; 112:185.
339. Tuncel A, Gorgu M, Ayhan M, et al. Treatment of anogeni-
316. Arem AJ, Kischer CW. Analysis of striae. Plast Recontr Surg
tal warts by pulsed dye laser. Dermatol Surg 2002; 28:350–
1980; 65:22.
Source: http://www.khotanpub.ir/UploadedFiles/PFiles/19c40234b6bf430.pdf
Natural Support for Sexual Performance and Libido For Men and Women James Meschino DC, MS, ND In the normal aging process the decline in sex hormone levels and the declining number of functioning nerve endings in the genitalia region often contribute to a reduction in sex drive, arousal capabilities, climax and intensity of pleasure-full sensations. This can become a source of frustration and disappointment that is a common problem, which affects the quality of life for many men and women over the age of 50. As a result many people turn to hormone replacement therapy and erectile dysfunction medications to help overcome these problems. Unfortunately, hormone replacement therapy can increase risk of breast cancer, endometrial cancer, prostate cancer as well as heart attack and stroke. In addition, erectile dysfunction medication is not suitable for all men, carries risk for sudden death and does not affect libido, only performance. In my view, before resorting to pharmaceutical drugs, individuals should first turn to natural libido and sexual performance-enhancing herbs, which have been shown to be safe and effective in human clinical trials. The following herbal compounds have been used individually for many years by health practitioners in various countries as therapies for low libido and sexual dysfunction. When combined into a single formulation, the synergistic effects of these herbs can exert a potent influence, helping to maximize, restore and prolong sexual desire, arousal and performance in both men and women.
Oktober - November - Dezember 2013 Belgien - Belgique 88 P.P. - P.B.B 5595 Eupen Mail Erscheint dreimonatlich Abs.: Freie Krankenkasse, Postfach 2, 4760 Büllingen des Lebens Wechseljahre - der Körper im Umbruch Rauchfrei durchs Leben Kostenlose Behandlung für SIS-Karte ade! Kinder und Jugendliche











