Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Ijcpr.org
International Journal of Current Pharmaceutical Research
Academic Sciences
Vol 5, Issue 4, 2013
Research Article
POTENTIOMETRIC CARBON PASTE ISEs FOR DETERMINATION OF FLUOXETINE
HYDROCHLORIDE IN PHARMACEUTICAL PREPARATIONS
EMAD M. HUSSIEN, NAHLA S. ISMAIL AND FATMA M. ABDEL-GAWAD
National Organization for Drug Control and Research, Egypt. Email: [email protected]
Received: 01 July 2013, Revised and Accepted: 28 July 2013
ABSTRACT
Carbon paste ion selective electrodes for fluoxetine hydrochloride (FXCl) were prepared and characterized in terms of composition, response time and usable pH range. The electrodes were applied to assay of FXCl in the drug substance and pharmaceutical product. The electrodes are based on fluoxetinium-phosphomolebdate (FX-PM) or fluoxetinium-tetraphenylborate (FX-TPB) as an ion exchanger dissolved in dioctylphthalate (DOP) as pasting liquid. The electrodes showed a sub-Nernestian slope of 52.0 mV/decade over the concentration ranges from 3 x 10-5 to 1 x 10-2 mol/L and a near-Nernestian slope of 56.5 mV/decade over the concentration range from 4 x 10-5 to 10-2 mol/L for (FX-PM) and (FX-TPB) based electrodes, respectively. The electrodes exhibited good selectivity for fluoxetine cations with respect to a large number of inorganic cations, organic cations, sugars and amino acids. The proposed electrodes offer the advantages of simplicity, accuracy and applicability to turbid solution
Keywords: Fluoxetine hydrochloride; Carbon paste ion selective electrodes; Potentiometric determination, Prozac capsule.
Preparation of the ion exchangers
Fluoxetine hydrochloride (FXCl) ((±)-N-methyl-3-phenyl-3-[(α,α,α-
The FX-TPB ion pair was prepared by mixing 100 ml of 10-2 mol/L
trifluoro-p-tolyl)-oxy]propyl-amine hydrochloride) (Scheme 1) is a
solution of FXCl with 100 ml of 10-2 mol/L solution of NaTPB. The
selective serotonin reuptake inhibitor (SSRI) antidepressant drug,
precipitate was filtered, washed thoroughly with bidistelled water,
which has been widely prescribed for treatment of depression [1]
and dried at room temperature
and some other important disorders [2]. Fluoxetine overdoses may lead to seizures, rapid heartbeat and in worst cases to suicidal
The FX-PM ion-associate was prepared by the addition of one
volume of 10-2 mol/L phosphomolybdic acid to three volumes of 10-2 mol/L fluoxetine hydrochloride solution. The precipitate was filtered, washed with bidistelled water and allowed to dry at room temperature.
Preparation of the electrodes
A Teflon holder (12 cm length) with a hole at one end (7 mm diameter, 3.5 mm deep) was as the electrode body. Electrical
contact with carbon paste was made with a stainless-steel rod through the center of the holder. This rod can move up and down
by screw movement. Modified carbon paste was prepared by mixing FX-PM or FX-TPB with DOP using 5 ml of THF. THF was
Several analytical methods for the determination of fluoxetine in
allowed to evaporate at room temperature. Carbon powder was
pharmaceutical preparation have been developed such as
then added and intimate homogenization was achieved by
chromatography [3-6], spectrophotometry [7-9], voltammetry [10,
careful mixing in a mortar. The paste was packed into the hole of
11]. These method are either expensive or use undesirable solvent
the electrode body. The carbon paste was smoothed onto paper
until it had a shiny appearance and was used directly for
Previously, we reported on the construction of plastic membrane ion
selective electrodes for fluoxetine [12]. A favorable characteristic of
ion selective electrodes is the speed, electivity and ease of
Electrochemical system
performing the assay. Herein, we extend this approach by the construction, potentiometric characterization, and analytical
Potentiometric measurements were carried out with HI 9321
application of fluoxetine-selective carbon paste electrodes. The
microprocessor pH meter. A saturated Ag/AgCl electrode was used
proposed electrodes have the advantage of easy preparation and
as an external reference electrode. The internal reference electrode
generation of new active surface. The electrodes are based on the
was a coated wire Ag/AgCl electrode.
phosphomolebdate as ion-exchangers and dioctylphthalate (DOP) as
The electrochemical system is represented as follows:
Stainless steel/modified carbon paste/test solution
//KCl salt
MATERIALS AND METHODS
Materials and reagents
Construction of calibration graph
Fluoxetine hydrochloride drug substance was obtained from Amriya
The calibration graph for each electrode was constructed using
Company (Alexandria, Egypt). Prozac capsules were from Eli-Lilly
solutions of different concentrations of fluoxetine hydrochloride
Company (Lilly France S.A.S., France). Sodium tetraphenylborate
covering the concentration range from 10-6 to 10-2 mol/L. The cell
(Na-TPB) and phosphomolybdic acid (PMA) were obtained from
potential was recorded for each solution at constant stirring at room
Fluka. Carbon powder and dioctylphthalate (DOP) were purchased
temperature and plotted against log [FXCl]. The slope of the
from Aldrich. Tetrahydrofurane (THF) was purchased from Lab-
calibration graph was calculated using Nernestain equation
.
Scan Analytical Science. All reagents were of chemically pure grades and bidistilled water was used throughout.
Hussien et al.
Int J Curr Pharm Res, Vol 5, Issue 4, 18-22
Where: R is the gas constant, F is the Faraday equivalent and z is the
Stock sample solution of Prozac capsules was prepared into 100-
charge of the analyte. The term E°ISE is a constant which is the sum of
mL measuring flask by dissolving an amount of the capsule
all invariants in the system.
powder (taken from average of 20 capsules), equivalent to 345.0 mg fluoxetine hydrochloride, in 50.0 ml bidistilled water. The
Selectivity
solution was sonicated for 5 min and completed to mark with
Potentiometric selectivity coefficient Kpot
bidistilled water. The filtrate and washings were collected into a
i,j for different inorganic and
organic cations were evaluated using the separate solution method
100-ml standard volumetric flask and diluted to mark with
(SSM) [13] and matched potential method (MPM) [14]. In the SSM
bidistilled water.
the EMF value (Ei and Ej) of the electrode in pure solution of each of
An Aliquot of analyte solution containing 6.90, 10.35, and 17.25 mg
the primary and the interfering ion, of equal concentration, are used for calculating the selectivity coefficient. The selectivity coefficient
of drug was pipette into a 100-mL beaker, and the solution was
diluted to 50 mL with bidistilled water. The solution was titrated
i,j is calculated using Nickolsky-Eisenman equation.
with 10-2 mol/L Na-TPB solution using the proposed electrodes. The volume of the titrant at the end point was obtained using the differential method.
In the matched potential method the concentration of fluoxetine
RESULTS AND DISCUSSION
hydrochloride solution was increased from ai = 1 × 10−6 mol/L
Composition and characteristics of the electrodes
(reference solution) to a'i = 1 × 10−3 mol/L, and the change in potential (∆E) was recorded. Then, small amounts of a solution of an interfering
Preliminary experiments showed that carbon paste electrodes,
ion of concentration aj ( from 1 × 10−2 to 1 × 10−3 mol/L) was added to a
which are free from ion-associate modifier have no response toward
new 1 × 10−6 mol/L reference fluoxetine hydrochloride solution until the
fluoxetine. Consequently, the ion associates fluoxetinium-
same potential change (∆E) is achieved. The selectivity coefficient was
phsphomolebdate (FX-PM), fluoxetinium-phosphotungestate (FX-
calculated using the following equation:
fluoxetinium-renicate
tetraphenylborate (FX-TPB) were prepared and investigated as modifiers for the paste. FX-PTA was practically insoluble in the
pasting liquid (DOP), and FX-RC was soluble in water, therefore,
these ion-associates were not useful as modifiers and were excluded. The ion-associates, FX-TPB and FX-PM, were used for the
preparation of modified carbon paste electrodes. The effect of varying the composition of the paste on the response of the
Potentiometric determination of fluoxetine hydrochloride in
electrodes toward fluoxetine was investigated in terms of linear
the drug substance and in prozac capsules
range, slope and detection limit. It was observed that the sensitivity
Stock solution of fluoxetine hydrochloride drug substance was
and linearity depend significantly on the amount (w/w %) of the
prepared into 100.0 mL measuring flask by dissolving 345.0 mg
modifier in the paste. Table 1 summarizes the effect of varying the
fluoxetine hydrochloride drug substance in 50 mL bidistilled water
composition of the paste on the response of FX- PMA and FX-TPB
and diluting to mark with bidistilled water.
based electrodes.
Table 1: Effect of changing the composition on the response of the electrodes at room temperature
Electrode
Composition % (w/w)
Slope (mV/decade)
Detection
Ion-pair
range (mol/L)
limit (mol/L)
3.0 x 10-5 - 1.0 x 10-2
3.0 x 10-5 - 1.0 x 10-2
3.0 x 10-5 - 1.0 x 10-2
8.0 x 10-4 - 1.0 x 10-2
1.0 x 10-4 - 1.0 x 10-2
1.0 x 10-5 - 1.0 x 10-2
4.0 x 10-5 - 1.0 x 10-2
1.0 x 10-4 - 1.0 x 10-2
1.0 x 10-4 - 1.0 x 10-2
a Relative standard deviation (four preparations)
A carbon paste electrode containing 5.0% FX-PM, 47.5% carbon
Response time
powder and 47.5% DOP, showed a sub-Nernestian slope of 52.0±0.68 mV/decade with a linear response in the concentration
The dynamic response time of the proposed electrodes was
range from 3.0 x 10-5 to 1.0 x 10-2 mol/L and a detection limit of 3.0 x
studied under stirring by measuring the time required to achieve a
10-5 mol/L. Whereas, a carbon paste modified with FX-TPB (5.0%
steady state potential (within ± 1 mV) after successive immersion
FX-TPB, 47.5% carbon powder and 47.5% DOP), exhibited a near
of the electrode in a series of fluoxetine hydrochloride solutions
Nernestian response of 56.5±0.36 mV/decade. The linear range and
from 1.0 x 10-6 to 1.0 x 10-2 mol/L, each having a 10-fold increase
the lower detection limit were 4.0 x 10-5 to 1.0 x 10-2 mol/L and 1.0 x
in concentration. A small potential drift (2 mV/min.) was observed
10-5, respectively. The calibration curves of FX-PM and FX-TPB
for the FX-PM based carbon paste electrode at lower
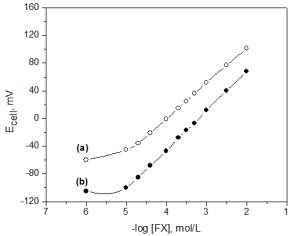
modified carbon paste electrodes are shown in Figure 1.
concentrations of fluoxetine hydrochloride, 10-6 and 10-5 mol/L. The cell potential remained constant (within ± 1 mV) for about 5
Calibration of the electrodes at different time intervals over four
minutes at 10-4 and 10-3 mol/L. A drift in the cell potential of 1.4
weeks showed that the electrodes retained their sensitivity to
mV/min was observed again when the electrode was immersed in
fluoxetine for 10 days and 16 days for FX-PM and FX-TPB electrodes,
10-2 mol/L. Contrary to the FX-PM electrode, the FX-TPB electrode
respectively. Afterwards, the slopes were decrease gradually to
showed a small potential drift of 0.6 mV/min when the electrode
reach 42 mV and 48 mV/decade for FX-PM and FX-TPB electrodes,
was immersed in 10-6 mol/L. The cell potential was steady within
±1 mV at 10-5 mol/L and up to 10-2 mol/L fluoxetine hydrochloride
Hussien et al.
Int J Curr Pharm Res, Vol 5, Issue 4, 18-22
solution. Figure 2 shows the potential time response of FX-PM and
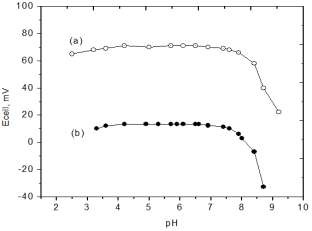
independent of the pH in the range from 4 to 7 for 10-3 mol/L
FX-TPB based carbon paste electrodes.
fluoxetine hydrochloride solution. At higher pH (pH >7), the potential reading changes slightly due to the conversion of
Effect of pH
fluoxetine hydrochloride (pKa = 8.7) to the fluoxetine base. Further
In order to check the dependence of the potential of the electrodes
addition of NaOH (at pH >8.7) lead to a dramatic change in the
on the pH of the solution, potential-pH curves were constructed. The
potential of the electrodes due to further depletion of fluoxetine
pH of 10-3 mol/L fluoxetine hydrochloride solution was altered by
hydrochloride and diffusion of OH- into the surface of the electrode.
the addition of small volumes of 0.1 mol/L NaOH or 0.1 mol/L HCl.
Interference from H+ at lower pH (pH <4) was observed for both
shows that the potential of the electrodes is practically
Fig. 1: Calibration curve of a) FX-PM and b) FX-TPB carbon paste electrodes.
Fig. 1: Typical potential-time plot for the response of a) FX-PM and b) FX-TPB carbon paste electrodes.
Fig. 2: Effect of pH change of 10-3 mol/L FXCl solution using a) FX-PM and b) Na-TPB based carbon paste electrodes.
Hussien et al.
Int J Curr Pharm Res, Vol 5, Issue 4, 18-22
Effect of interference
presence of high concentration of the interfering ions without fear of interference.
The interference of some common inorganic cations, sugars and
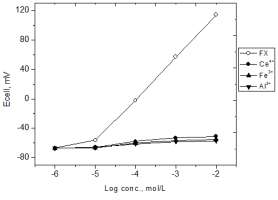
amino acids was investigated using the separate and matched potential method [13, 14]; the two methods were recommended by the IUPAC in 1976 and 1995, respectively. The MPM was recommended as a method that gives analytically relevant practical selectivity coefficient values and overcome the limitation of the SSM. In case of MPM, the selectivity coefficient was calculated using the concentration of the interfering ion that induces a cell potential change of ≥10 mV. The selectivity coefficient were not calculated for interfering ions which induced a cell potential change of <10 mV. The selectivity coefficients are summarized in Table 2. Due to the very small values of kMPMi,j they are tabulated as the negative logarithm –log kMPMi,j Practical calibration curves showed that there is small interference from Al3+, Fe3+ and Ce4+ at high concentration (figure 4). The selectivity coefficient values obtained by the MPM confirms a small interference from Al3+, Fe3+ and Ce4+ at high concentration whereas the SSM showed that the electrodes are extremely selective to FX ions for Al3+, Fe3+ and Ce4+, which is
practically not correct.
Fig. 4: Ecell-logc curves for determing the MPM selectivity
coefficient of FX-PM carbon paste electrodes for FXCl against
The selectivity coefficient values recorded in Table 2 indicate that
Al3+, Fe3+ and Cr4+. The initial reference solution of FXCl was 1 x
the electrode can be used for determination of fluoxetine in
10-6 mol/L.
Table 2: Selectivity factor values
for FX- selective PVC membrane.
Interferent
Analytical application
recovery obtained was within ± 2% for the drug substance and Prozac capsule, respectively. The relative standard deviation was ≤2.0%,
The proposed electrodes were proved useful for the assay of
indicate reasonable repeatability and reproducibility of the proposed
fluoxetine in the drug substance and pharmaceutical product by
methods. The accuracy and precision of the assay of fluoxetine
potentiometric titration using tetraphenylborate as a titrant. The end
hydrochloride in the drug substance and in Prozac capsule using the
point was determined by the first derivative method. The accuracy and
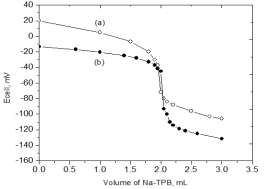
proposed electrodes are summarized in Table 3. Figure 5 shows the
precision were tested at three different concentration levels (6.90-
potentiometric titration curves of FXCl with Na-TPB using the FX-PM
17.25 mg/50 mL), five samples were used at each level. The mean
and FX-TPB electrodes.
Table 3: Accuracy and precision for quantification of fluoxetine hydrochloride in the drug substance and Prozac capsule using FX-PM and
FX-TPB carbon paste electrodes.
Fluoxetine
Found ± SD
Recovery
Found ± SD
Recovery
Reported
(mg/50 ml)
(mg/50 ml)
(mg/50 ml)
method [8]
Mean ± standard deviation of five determinations.
Hussien et al.
Int J Curr Pharm Res, Vol 5, Issue 4, 18-22
To compare the proposed methods to a reference method,
stability and applicability over a wide pH range. The carbon paste
fluoxetine in capsules was assayed by spectrophotometry using
electrode has the advantage of being easy to prepare and
2,3-dichloro-5,6-dicyano-p-penzoquinone (DDQ) [8]. Statistical
regeneration of the active surface.
comparison using Student's t- and F-ratio tests at 95% confidence level, the calculated t- and F- values did not exceed the critical
REFERENCES
values, indicating that there is no significant difference between
M. Asberg, P. Thoren, L. Traskman, L. Bertilsson, V. Ringberger,
the proposed and the spectrophotometric methods with regard to
Science, 191 (1976) 478.
accuracy and precision.
S. H. Y. Wong, S. S. Dellafera, R. Fernandes, H. Kranzler, J. Chromatogr., 499 (1990) 601.
United States Pharmacopeia and National Formulary USP 34 NF 29 (2011), Fluoxetine Hydrochloride, p 2877
M. A. Raggi, F. Bugamelli, G. Casamenti, R. Mandrioli, D. De Ronchi, V. Volterra, J. Pharmaceut. Biomed. Anal., 18 (1998) 699.
M. A. El-Dawy, M. M. Mabrouk, F. A. El-Barbary, J. Pharmaceut. Biomed. Anal., 30 (2002) 561.
J. J. Berzas, C. Guiberteau, A. M. Contento, V. Rodriguez, Chromatographia 56 (2002) 545.
A. H. Prabhakar, V. B. Patel, R. Giridhar, J. Pharmaceut. Biomed. Anal., 20 (1999) 427.
L. I. Bebawy, N. El-Kousy, J. K. Suddik, M. Shokry, J. Pharmaceut. Biomed. Anal., 21 (1999) 133.
B. Starczewska, K. Mielech, J. Pharmaceut. Biomed. Anal., 23
Fig. 5: Potentiometric titration of 6.90 mg FXCl against 10−2
10. A. da Silva, J. C. Lima, M. T. O. Teles, A. M. O. Brett, Talanta 49
mol/L Na-TPB using a) FX-PM and b) FX-TPB carbon paste
electrodes.
11. H. P. A. Nouws, C. Delerue-Matos, A. A. Barros, J. A. Rodrigues, A.
Santos-Silva, F. Borges, Analyt. Lett., 40 (2007) 1131.
CONCLUSION
12. E. M. Hussien, F. M. Abdel-Gawad, Y. M. Issa, Biochem. Eng. J., 53
The proposed carbon paste electrodes based on fluoxetinium-
phosphomolebdate or fluoxetinium-tetraphenylborate as the
13. G. G. Guilbault, R. A. Durst, M. S. Frant, H. Freiser, E. H. Hansen,
electroactive compounds might be a useful analytical tool and an
T. S. Light, E. Pungór, G. Rechnitz, N. M. Rice, T. J. Rohm, W.
interesting alternative in the determination of FX the drug substance
Simon, and J. D. R. Thomas, Pure & Appl. Chem., 48 (1976) 127.
and pharmaceutical product. The present electrodes show high
14. Y. Umezawa, K. Umezawa, H. Sato, Pure & Appl. Chem., 67
sensitivity, reasonable selectivity, fast static response, long-term
Source: http://www.ijcpr.org/Issues/Vol5Issue4/722.pdf
Huerta, Schade, Granell (Eds): Connecting a Digital Europe through Location and Place. Proceedings of the AGILE'2014 International Conference on Geographic Information Science, Castellón, June, 3-6, 2014. ISBN: 978-90-816960-4-3 Analysing spatiotemporal patterns of antibiotics prescriptions Luise Hutka and Lars Bernard Technische Universität Dresden Professorship of Geoinformation Systems
United States Court of Appeals For the Seventh Circuit CHRISTINE BJORNSON, MICHAEL J. ASTRUE, Commissioner of Social Security, Appeal from the United States District Court for the Northern District of Illinois, Eastern Division. No. 10 C 5835—Elaine E. Bucklo, Judge. ARGUED DECEMBER 14, 2011—DECIDED JANUARY 31, 2012 Before POSNER, MANION, and WOOD, Circuit Judges.







