Fisio.fmed.edu.uy
Neuroscience 141 (2006) 559 –568
OXYTOCIN RECEPTORS IN THE NUCLEUS ACCUMBENS FACILITATE
"SPONTANEOUS" MATERNAL BEHAVIOR IN ADULT FEMALE
PRAIRIE VOLES
D. E. OLAZÁBAL* AND L. J. YOUNG
Prairie voles (
Microtus ochrogaster) display biparental and
Department of Psychiatry and Behavioral Sciences, Center for Behav-
high levels of affiliative behavior
ioral Neuroscience, Yerkes National Primate Research Center, Emory
University, 954 Gatewood Road NE, Atlanta, GA 30322, USA
Interestingly, approximately half (⬃55%) of naïveadult female prairie voles show "spontaneous" maternal
Abstract—Oxytocin and the nucleus accumbens have been
extensively implicated in the regulation of maternal behavior,
behavior when first exposed to pups
and the processing of pup-related stimuli relevant for this
These females lick, groom
behavior. Oxytocin receptor density in the nucleus accum-
and hover over the pups immediately after the first expo-
bens is highly variable in virgin female prairie voles, as is
sure. However, ⬃45% of adult female prairie voles either
their behavioral response to pups, ranging from neglecting
ignore/neglect the pups or display infanticidal behavior
and infanticidal to full maternal behavior. We hypothesized
that oxytocin receptor in the nucleus accumbens facilitates
the expression of "spontaneous" maternal behavior in prairie
Several studies show that oxytocin facilitates positive
voles. Forty sexually-naive adult females were exposed to
social interactions, including maternal behavior, in several
pups for the first time and tested for maternal behavior.
Oxytocin receptor binding in the nucleus accumbens and
other brain regions was later determined using autoradiog-
raphy. Females that showed maternal behavior (lick and
In fact, oxytocin has been
groom the pups and hover over them for at least 30 s, nⴝ
24)
proposed to facilitate the process of bringing conspecifics
had higher oxytocin receptor density in the nucleus accum-
into close proximity for the formation of a social bond
bens (shell subregion) (P<0.05) than females that did not
If this is the case, oxytocin may
show maternal behavior or attacked the pups (nⴝ
16). No
also be critical in the initiation of contact between naïve
differences were found in other brain regions (medial preop-
female prairie voles and pups, resulting in the expression
tic area, septum, prelimbic cortex).
of maternal behavior.
In a second experiment, we tested whether infusions of
Although the role of the nucleus accumbens (NA) in
the oxytocin receptor antagonist (d(CH ) 1,Tyr(Me)2,Orn8)-
regulating maternal behavior is still unclear, as is the case
AVT into the nucleus accumbens would block "spontaneous"
maternal behavior. As a control region, oxytocin receptor
for the rest of the behaviors it modulates
antagonist was also infused into the caudate putamen. Ten
females were infused bilaterally into the nucleus accum-
bens or caudate putamen with either 2 ng/0.5
l of oxytocin
receptor antagonist or CSF (vehicle). While five of 10 nu-
there is evidence that the NA is involved in
cleus accumbens CSF-infused animals showed maternal
promoting maternal responses and the processing of pup-
behavior, none of the nucleus accumbens oxytocin recep-
tor antagonist-infused subjects did (0/10;
2, P<0.01).
Nucleus accumbens oxytocin receptor antagonist-infused
females recovered the next day and were not different from
controls. Animals infused with CSF or oxytocin receptor
antagonist into the caudate putamen did not differ (four/10,
Previous studies suggest that differences in oxytocin
four/10). This is the first study to show that the nucleus
receptor (OTR) distribution may be related to the species-
accumbens is involved in the regulation of "spontaneous"
typical pattern of social behavior, in particular affiliative
maternal behavior and that oxytocin receptor in this brain
behavior We have recently
region facilitates maternal responses. 2006 IBRO. Pub-
found that OTR distribution may also be related to the
lished by Elsevier Ltd. All rights reserved.
presence or absence of juvenile maternal behavior (alsocalled alloparental behavior) across species
Key words: aversion, crouching, pups, Microtus ochrogaster.
Juvenile female meadow voles and mice donot show positive responses to pups at the age ⬃20 days
*Corresponding author. Tel: ⫹1-404-727-8269; fax: ⫹1-404-727-8070.
while juvenile female rats display maternal responses only
E-mail address:
[email protected] (D. E. Olazábal).
after 2–3 days of pup exposure. These three species have
Abbreviations: CP, caudate putamen; LS, lateral septum; MPOA, me-
very low levels of OTR in the NA. In contrast, juvenile
dial preoptic area; NA, nucleus accumbens; OTA, oxytocin antagonist;OTR, oxytocin receptor; PLC, prelimbic cortex.
prairie voles show spontaneous alloparental behavior and
0306-4522/06$30.00⫹0.00 2006 IBRO. Published by Elsevier Ltd. All rights reserved.
doi:10.1016/j.neuroscience.2006.04.017
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
have significantly higher density of OTR in the NA. In
behavior nor attacked the pups during the 15 min test period were
addition, prairie voles show significant individual variability
categorized as females that "ignored" the pups. Pups were re-
in OTR binding in the NA and this binding
moved from the cage at the end of the test, or immediately afterbeing attacked by the subject, in order to avoid injury. Pups that
is positively correlated with the quality of alloparental be-
received serious injuries, despite our precautions, were killed.
havior displayed by juvenile females
Subjects that performed the attack were categorized as females
that "attack" pups.
Given this relationship between OTR in the NA and
juvenile maternal behavior across species and within juve-
Experiment I
nile prairie voles, we hypothesized that OTR in the NA
Brain tissue collection and radioligand receptor autoradiography.
plays a role in regulating adult female "spontaneous" ma-
Subjects were 40 adults (60 –90 days of age) exposed to pups as
ternal behavior. In the present study we investigated
described above. Four days later, all animals were deeply anes-
whether OTR binding in the NA of adult, naïve female
thetized with Isofluorane (NovaplusTM; Abbott Laboratories, IL,
prairie voles is associated with the response to pups during
USA) and decapitated. Brains were removed from the skulls,frozen immediately on dry ice and stored at ⫺80 °C until sec-
a 15 min interaction test. Other brain regions with high
tioned. A cryostat was used to obtain five serial sets of 20-m-
density in OTR or implicated in maternal or social behavior,
thick frozen sections of the brains. Sections collected extended
such as lateral septum (LS, me-
from the olfactory nuclei to the caudal region of the basolateral
dial preoptic area (MPOA, and pre-
amygdala and were mounted in separate Superfrost plus slides
limbic cortex (PLC, were also investi-
(Fisher, Pittsburgh, PA, USA) and stored at ⫺80 °C until used for
gated. In a second experiment we directly tested the role of
Slides were processed for receptor autoradiography using
NA OTR in the regulation of spontaneous maternal behav-
125I labeled OTR antagonist, [125I]-ornithine vasotocin analog
ior by infusing OTR antagonist into the NA and a control
(NEN/PerkinElmer, 125IOVTA, 2200 Ci/mmol) using standard pro-
site (caudate putamen, CP).
cedures in our laboratory On the day of receptorautoradiography, the sections were removed from the freezer andallowed to dry and equilibrate to room temperature for 1 h before
the binding started. Sections were immersed in 0.1% paraformal-dehyde in phosphate-buffered saline (pH 7.4) for 2 min at room
temperature. Slides were then rinsed twice in Tris–HCl buffer (pH
All subjects were naïve female prairie voles from our colony
7.4) and later incubated for 60 min in 50 pM 125I-OVTA in Tris with
maintained at the Yerkes Laboratory Animal Facility at Emory
10 mM MgCl , 0.1% bovine serum albumin (RIA grade, fraction V,
University. This facility is accredited by the Association for Assess-
Sigma) and 0.05% bacitracin. Unbound ligand was removed by
ment and Accreditation of Laboratory Animal Care (AAALAC). Prai-
four washes in 50 mM Tris pH 7.4, 10 mM MgCl . The slides were
rie voles are regularly weaned in our animal facility at age 19 –21
finally quickly dipped in dH O and rapidly dried and exposed to
days, and maintained in same-sex groups of two to three in cages
BioMax MR film (Kodak, Rochester, NY, USA) along with 125I
28⫻17⫻13 cm with transparent Plexiglas walls under a 12-h
autoradiographic microscale standards (Amersham Biosciences)
dark/light cycle and a stable environmental temperature of
for 48 h. To control for variability all slides were processed at the
22 °C with access to food (LabDiet® rabbit, Purina, Richmond,
same time and sufficient amount of the solutions were prepared to
IN, USA) and water
ad libitum. Bed-ócobs® Laboratory Animal
use the same solutions for all slides. All further details are de-
Bedding (OH, USA) was used as bedding material.
scribed elsewhere
All procedures used in this study have been conducted in
The analysis of the autoradiography
accordance with the National Institutes of Health Guide for the
was performed by applying our standard previously published
Care and Use of Laboratory Animals (NIH Publications No. 80-23)
methods Optical density readings
and approved by the Institutional Animal Care and Use Committee
were measured and converted to decompositions per minute
of Emory University (IACUC). Every effort was made to minimize
(d.p.m./milligram tissue equivalent) based on I125 autoradio-
the number of animals used and their suffering. Additional adult
graphic standards (Amersham Biosciences) by using our auto-
lactating females, not included in the experiment, served as do-
mated computer-based image analysis system and AISTM soft-
nors of pups for the maternal behavior test.
ware version 6.0 (Imaging Research Inc.).
OTR binding for the CP, LS, MPOA, NA (shell and core
Maternal behavior test
subregion), and PLC was measured using ⬃four (CP, LS, NA, andPLC) or two (MPOA) brain sections for each brain region and the
Subjects were individually housed in a clean cage and allowed to
average reading recorded. Background reading, taken from an
habituate for 45–90 min before the maternal behavior test began.
adjacent area with no OTR binding, from two sections of each
Maternal behavior test has been described elsewhere
brain was also recorded and averaged. Specific binding was
and will be briefly summarized here. Two pups
calculated subtracting the average background reading for each
(2–5 days old) were placed into the cage opposite to where the
brain from the readings for all brain regions taken from that spe-
subject was located. The behaviors scored for 15 min included:
cific animal. All further details of our quantitative methods were
number of animals that attacked pups, time spent licking and
carried out as previously specified in
grooming, time hovering immobile over at least one pup (quies-cence crouching) or doing other activities (active crouching). Our
criteria for considering an animal maternal were that it licked thepups ⬎5 s, spent ⬎30 s adopting crouching postures over the
Oxytocin antagonist infusions into the NA and CP.
pups, and never attacked the pups. Most of the maternal animals
were 40 adults (60 –90 days of age). Animals were anesthetized
spend more than 8 min adopting crouching posture. However,
with isofluorane (3%), placed in a Kopf stereotaxic apparatus and
those animals that licked and crouched at least 30 s over the pups
implanted with a 26-gauge double guide cannula (C235G, Plastics
did not ignore or avoid the pups, so they were also considered
One Inc.) aimed at the NA (nosebar at ⫺2 mm; 1.6 mm rostral,
maternal. Animals that neither reached the criteria for maternal
0.85 mm lateral, and ⫺4.5 mm ventral to the bregma) or CP
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
(1.4 mm rostral, 1.5 mm lateral, and ⫺.3 mm ventral to thebregma). These coordinates were chosen based on pilot studies.
The axis was moved down and the guide cannula inserted into twoholes drilled in the skull following the NA or CP coordinates. The
guide cannula was fixed using a drop of instant adhesive (LoctiteR454 PrismR) attached to part of the skull and the lateral part of the
guide cannula. After the instant adhesive dried, the lower part ofthe cannula was covered by cranioplastic powder (Plastics OneInc.) mixed with fast curing acrylic liquid (Ortho-Jet Liquid; Lang
Dental Manufacturing Co. Inc., IL, USA). The dental cement wasallowed to dry for 10 min and then the guide cannula was sepa-
rated from the holder. Immediately after surgery, females wereinjected with buprenorphine (0.01 g/30 g; Reckitt Benckiser
Pharmaceuticals Inc., Richmond, VA, USA) as an analgesic. An-imals were allowed to recover for one week and then anesthetized
again using isofluorane (3%) to place the 33-gauge needle(C235I, Plastics One Inc.) into the guide cannula. The needle
extended 0.5 mm beyond the guide cannula into the brain and was
connected to Hamilton syringes with PE-50 tubes (Plastics OneInc.). Ten animals were injected bilaterally either with the OTR
125I-OVTA binding (dpm/mg)
antagonist d(CH ) ,[Tyr(Me)2,Thr4,Orn8,Tyr9-NH ]-vasotocin (OTA)
2 ng/0.5 l in CSF (ALZETR artificial CSF) or vehicle (CSF). Twohours later these animals were exposed to pups for 15 min and
tested for maternal behavior as described above. A previous studyexamining the role of the NA OTR in pair bondingfound that a similar dose (1 ng/side) was effective when injected
into the NA, but not the adjacent caudate region. This OTA has
very high affinity for OTR and occupies the receptor for more than10 h After the behavioral test, animals were
maintained in the same cages until next day when were tested
again for maternal responses to pup exposure. Finally, all subjectswere deeply anesthetized with isofluorane, killed, and the brains
removed and sectioned to verify proper cannula placement. Verifica-
tion of cannula placement was done by visualizing the location of thetrack left by the cannula using high magnification of fresh mounted
sections. The most ventral portion of the track left by the cannula was
drawn on photocopies of frontal plane diagrams of the brain.
For experiment I, all animals were categorized as being maternal
or non-maternal (ignore or attack). OTR binding in the CP, LS,
MPOA, NA (shell and core), and PLC was analyzed by
t-test.
I-OVTA binding (dpm/mg)
Spearman rank correlation was applied to investigate whether arelationship existed between OTR binding and time spent in qui-
Fig. 1. Frequency distribution of OTR binding in adult female prairie
escence crouching posture in maternal females. Data from exper-
voles. Histograms show the density of OTR binding in the shell sub-
iment II were analyzed by chi-square. Statistical significance was
division of the nucleus accumbens (NAs) and the LS of the animals
P⬍0.05. Data are expressed as means⫾S.E.
used in experiment I.
non-maternal females (1057⫾141; 219⫾25; 551⫾59;1927⫾73 respectively). The time spent adopting crouching
Experiment I
posture also varied among the maternal females
Relationship between OTR density in the brain and ma-
OTR binding in the shell and core subregions of the NA
ternal response.
Twenty-four of the 40 females reached
was positively correlated to time spent adopting quies-
our criteria for maternal behavior while the rest (16) did not
cence crouching posture in maternal prairie voles
show any maternal response. There was significant vari-
(rho⫽.53,
P⬍0.01; and rho⫽.60,
P⬍0.005 respectively).
ability in the OTR binding mainly in the NA, and LS
There was a positive correlation between OTR binding in
When maternal and non-maternal animals were com-
the CP and time adopting crouching posture in maternal
pared, maternal females showed higher OTR density in the
prairie voles (rho⫽.56,
P⬍0.01). There was no correlation
NA (shell subregion) than animals that did not show any
between NA OTR density and licking and grooming (shell
maternal response (
P⬍0.05) OTR density in
rho⫽⫺.029
P⬎0.89; core rho⫽.01
P⬎0.96) or time spent
the core subregion of the NA did not reach statistical
in active crouching (shell rho⫽⫺.25
P⬎0.20; core
significance (
P⫽0.08) but was higher in maternal than
rho⫽⫺.18
P⬎0.38). No significant correlation was found
non-maternal females OTR density in the CP,
between OTR binding in the MPOA, LS or PLC and quies-
MPOA, LS, and PLC was not different in maternal
cence crouching posture in maternal animals (rho⫽⫺.01,
(1207⫾167; 222⫾30; 586⫾54; 1986⫾79 respectively) or
P⫽0.95; rho⫽⫺.05,
P⫽0.80; rho⫽.3;
P⫽0.12 respectively).
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
binding ( 1000
Fig. 2. OTR binding in maternal and non-maternal animals. OTR binding in the shell and core subdivisions of the NA was higher in maternal (white
bars) than in non-maternal females (black bars). Data are expressed as means⫾S.E. (* P⬍0.05).
males infused with OTA into the NA showed no maternal
Oxytocin antagonist infusion into the NA, but not the
response while control CSF-infused females showed the
CP, blocks spontaneous maternal behavior.
previously described variability in maternal response; five
placement analysis showed that all animals had cannula
maternal and five non-maternal (2, P⬍0.05; There
properly placed in the NA area as shown in Cannula
was no difference between the groups in the latency to
placement extended along the rostral and caudal NA. Fe-
approach pups, number of approaches to pups, and time
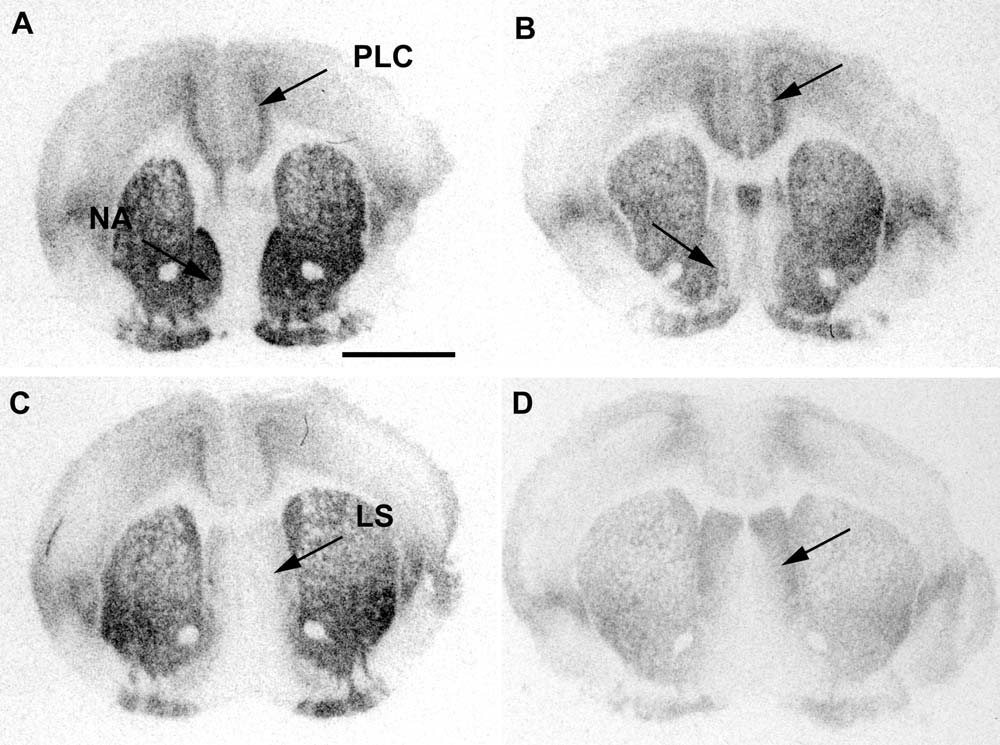
Fig. 3. Pictures of sample brain sections showing the autoradiographic signal for I125 OTA for two animals representative of the group of maternal
(A, C) and non-maternal females (B, D). Note that while OTR binding is clearly higher in the NA of the maternal animal, no differences are seen in
the PLC between maternal and non-maternal females. Note also that in these sections, the LS binding is higher in non-maternal females,
demonstrating that the difference is not due to overall decrease in OTR binding or technical artifacts (C, D). Scale bar⫽2 mm.
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
them showed all the components of maternal behavior,licking and grooming the pups and crouching over them
more than 5 min.
Appropriate cannula placement in the CP was also con-
firmed Two CP- and CSF-infused animals were
excluded because cannula placement was too rostral and out
of the striatum. The rest of the cannulae were distributed in
the medial and lateral CP. Females infused with OTA or CSF
into the CP did not differ in their response to pups, except for
their latency to approach to them Both groups
showed the normal variability in maternal behavior (four/10
maternal in CSF and four/10 maternal in OTA;
Maternal animals infused with CSF and OTA only differedin their latency to approach to pups (10⫾6 and 83⫾43
Total time crouching over pups
respectively, P⬍0.05). No significant differences were foundin the latency to retrieve all pups (464⫾134; 420⫾104); time
Fig. 4. Frequency distribution of time spent crouching over the pups.
licking and grooming (113⫾61; 104⫾86) or total time adopt-
Histogram shows the time that maternal females from exp 1 spent
ing crouching postures (123⫾59; 85⫾58). When OTA-in-
crouching over the pups.
fused animals were tested again on the next day, only one
sniffing pups When the animals were tested on
more OTA-infused animal showed maternal care (five/10).
the next day, no difference was found between control andOTA infused groups, four in nine OTA-infused females
licked and groomed the pups and adopted crouching pos-ture for more than 5 min. One animal was not retested
In the present study we found for the first time that the NA
because its cannula was removed overnight. Five addi-
is critical for the expression of "spontaneous" maternal
tional naïve pre-screened maternal females were also in-
responses and that OT in this brain region, extensively
fused with a lower dose of OTA (0.5 ng/.5 l) and all of
associated with the reward pathway, and the processing of
Fig. 5. Cannula placement. location of cannulae across the rostral and caudal NA and CP for each control CSF- (left) and OTA- (right) infused animal
is represented by black and gray bars respectively.
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
Fig. 6. OTA into the NA, but not the CP, blocks maternal response. Bars show percentage of maternal (white) and non-maternal (black) animals. A
higher number of CSF-NA-infused controls showed maternal responses than OTA-NA-infused animals (P⬍0.05). There was no difference between
OTA-CP- and CSF-CP-infused animals.
novelty and salient stimuli, facilitates maternal responses.
effect of cannula implant or the brief period of anesthesia
This and previous studies
on the maternal response. Previous studies show that a
from our laboratory suggest that OTR in the NA play a
minor stress does not affect maternal response in females
critical role across species and within species in facilitating
All animals were healthy and OTA
the initial interaction of naïve subjects with pups. We have
infusion effects were transient as shown by the recovery of
shown recently that the binding of OTR in the NA is cor-
the maternal response on the next day. The lack of effect
related to the quality of maternal behavior displayed by
of similar dose of OTA infused into the CP (brain region
juvenile prairie voles In the
with high OTR density and near the NA), suggests that the
present study we also found that naïve adult female prairie
disruption of maternal behavior after NA OTA infusions is
voles that behave maternally when exposed to pups had
specific, and not consequence of the diffusion of OTA to
higher OTR binding in the NA than females that attacked or
other brain regions.
ignored them. In addition we confirmed the role of these
It is important to point out that prairie voles are very
receptors in facilitating maternal behavior by transiently
unique in their interaction with pups. They are highly re-
disrupting maternal responses with oxytocin antagonist
sponsive to pups both as juveniles and adults, and they
infusions into the NA.
spend most of the 15 min maternal test licking/grooming
In these experiments, pups were a novel stimulus for
and hovering over the pups. Neither rats, meadow voles
our female subjects. Maternal responses are known to be
nor mice show this highly intense spontaneous maternal
affected by novelty
behavior. Interestingly, in contrast to other species, adult
female prairie voles have a very high density of OTR in the
and previous selection of maternal females for this exper-
NA (shell and core). This increased binding of OTR in the
iment would remove this important aspect of female–pup
NA suggests several interesting possibilities on oxytocin
interaction. In experiment 2, the normal response to pups
and dopamine interactions in this brain region, as sug-
shown by control animals demonstrates that there was no
gested previously by other authors For example, oxytocin may interact
Table 1. Initial approach to pups displayed by OTA and CSF infused
with the dopaminergic inputs to the NA to mediate the
female prairie voles
attractive value of pup related stimuli or facilitating mater-nal response by other mechanisms. Oxytocin may also act
in the NA to disinhibit the approach to the novel stimuli
(pups), to reduce the reactivity to pup-related stimuli, in-
crease the reinforcement properties of pups, and/or to
CSF-NA, n⫽10
reduce locomotor activity facilitating the contact with pups
OTA-NA, n⫽10
and pup stimulation. The time females spent interacting
positively with pups, and specifically adopting crouching
CSF-CP, n⫽10
postures may then be increased by all these mechanisms.
OTA-CP, n⫽10
Maternal responses of naïve females from several spe-
cies are known to be affected by high reactivity to pups as
Data are expressed as mean⫾S.E.M.
novel stimuli, generally called neophobic response (Mayer
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
propose that ventral stimulation and suckling-induced in-
Then, it is possible that oxytocin in prairie voles may be
hibition of dopamine may be required for kyphosis and
implicated in this process. However, maternal behavior
quiescent nursing. The NA (shell subregion) send projec-
may also be facilitated by the reduction of the reactivity to
tions to the ventrolateral part of the periaqueductal gray
the new testing environment. Previous studies in rats show
region that has been impli-
that oxytocin facilitation of maternal behavior may be de-
cated in the mediation of quiescence crouching posture in
pendent on the exposure to a novel environment
rats. Whether a similar mechanism can be triggered, for
Oxytocin has also
example, through ventral stimulation of naïve female prai-
been implicated in the mediation of stress and corticoste-
rie voles by the very active prairie vole pups needs to be
further investigated. Other studies also suggest that oxy-
tocin interacts with dopamine action in the NA and pre-
If as proposed by
vents the ability of cocaine to induce an increase in spon-
those studies, oxytocin reduces corticosterone and the
taneous locomotor activity
response to stress, then in experiment I, the differences in
OTR density in the CP was also positively correlated
the response to pups found in animals with high or low
with time spent adopting quiescence crouching posture.
OTR density in the NA might be partially due to differences
However, note that we have previously reported that OTR
in the response to the mild stressful or challenging condi-
density in the NA and CP is highly correlated (R⫽.89;
tion of the maternal test. In experiment II, our NA OTA-
In contrast to OTA infusions
infused animals may have also shown a transient deficit in
into the NA, OTA infusions into the CP delayed the ap-
their capability to cope with the novelty of pup's related
proach to pups. However, that delayed response did not
stimuli or the challenging conditions of the maternal test.
block the emergence of full maternal behavior in OTA-
suggested that oxytocin may
infused animals. This finding cannot be explained as a
also be necessary to activate circuits in the mesolimbic
consequence of diffusion of OTA to the NA because OTA-
system that motivates rat dams to direct maternal behavior
NA- and CSF-NA-infused animals did not differ in their
toward pups. Previous studies found that centrally infused
latency to approach to pups. In addition, the latency to
OT facilitated maternal behavior
approach to pups in OTA-CP-infused females was differ-
and i.c.v. administration of OT antagonist or
ent to controls but in the range of the latency to approach
to pups shown by animals with CSF and OTA infusions into
disrupted or delayed maternal
behavior in rats. The site of action of oxytocin to facilitate
OTR binding variability in the brain has previously been
maternal behavior in rats has been unclear. However, one
associated with individual differences in maternal re-
of the places where oxytocin infusions have facilitated
maternal behavior in rats is the ventral tegmental area.
For example, increased OTR binding in the MPOA,
found that antagonists in the ventral
the LS, the central nucleus of the amygdala, paraventricu-
tegmental area blocked retrieval and nursing postures,
lar nuclei of the hypothalamus, and the bed nucleus of the
while other studies found some facilitation after oxytocin
stria terminalis were associated with increased levels of
infusions into this brain region
licking and grooming in lactating females
The ventral tegmental area sends
In the present study we found no relationship
dense dopaminergic terminals to the ventral striatum and,
between spontaneous maternal behavior and OTR density
in prairie voles, may interact with oxytocin in the NA to
in the MPOA, LS and PLC. Previous studies in prairie voles
increase the attractive value of pups.
also failed to find a positive relationship between OTR
A third alternative explanation may be that oxytocin
binding in the LS and maternal behavior
interacts with dopamine in the NA to reduce locomotor
activity during female–pup interaction. The NA has been
Comparisons across species and within juvenile prairie
previously implicated in the mediation of crouching posture
voles also showed that OTR binding in the MPOA does not
contribute to our understanding of variability in pup-in-
Blockage of the dopaminergic activity in this brain region
duced maternal behavior
facilitates passive component of maternal behavior in-
Although both and
creasing the time dams spend nursing over pups. In par-
found changes in OTR density in the MPOA asso-
ticular, found that that the D1/D2
ciated with lactating maternal behavior, note that in non-
antagonist cis-flupenthixol infused into the NA enhanced
lactating females (similar to our present paradigm)
kyphotic nursing and low dosages of haloperidol resulted
did not find any difference in OTR density in
in more rapid onset and longer duration of nursing
the MPOA. However, the difference between previous find-
In addition, another study showed that
ings in rats and ours in prairie
this facilitation of nursing postures occurred both in female
voles may be also due to the species-specific function of
and male prairie voles In the present
oxytocin in accordance with the species behavioral and
study, we found that OTR binding in the NA was positively
reproductive strategy
correlated to the time maternal females spent adopting
Our finding on spontaneous maternal behavior in prai-
quiescence crouching posture.
rie voles agrees with sug-
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
gestion that oxytocin participates in the mediation of "non-
mechanisms underlying NA OTR facilitation of "spontane-
hormonal" or pup-induced maternal behavior. There is ex-
ous" maternal responses and the effect of OTR overex-
tensive evidence showing that the presence or not of the
pression in the NA in species that show no or minimal
ovaries does not affect pup-induced maternal behavior in
"spontaneous" positive interaction with pups.
virgin rats On the other hand, there is no evidence that
Acknowledgments—The authors want to thank Lorra Miller for her
fluctuations in the gonadal hormones of virgin animals can
excellent job managing our vole colony and Katherine Sharer for
influence maternal responses or OTR density. OTR den-
her assistance with autoradiography. This study was supported by
sity in the NA of prairie voles reaches mature levels around
MH56538 and MH064692 to L.J.Y., and RR00165 to Yerkes
20 days of age and there is no further developmental
National Primates Research Center.
change after that (D. E. Olazábal and L. J. Young, unpub-lished observations). In addition, no sexual dimorphismhas been reported for OTR in the NA. However, chronic
absence of gonadal hormones or drastic changes in pre-
Amico JA, Mantella RC, Vollmer RR, Li X (2004) Anxiety and stress
natal or early postnatal exposure to gonadal hormones
responses in female oxytocin deficient mice. J Neuroendocrinol
might influence spontaneous maternal behavior. In adult
16:319 –324.
prairie voles, OTR density is extremely resistant to hor-
Bales KL, Kramer KM, Lewis-Reese AD, Carter CS (2006) Effects of
stress on parental care are sexually dimorphic in prairie voles.
monal or other environmental changes. However, whether
Physiol Behav 87:424 – 429.
a brief interaction with pups can modify NA OTR binding in
Berridge KC, Robinson TE (1998) What is the role of dopamine in
maternal animals has never been investigated before.
reward: hedonic impact, reward learning, or incentive salience?
Oxytocin has multiple functions as described in the
Brain Res Brain Res Rev 28(3):309 –369.
introduction and OTR distribution and expression may af-
Berridge KC (2003) Pleasure of the brain. Brain Cogn 52:106 –128.
fect other behaviors or physiological functions. We must
Carter CS (2003) Developmental consequences of oxytocin. Physiol
Behav 79:383–397.
point out that the OTR receptor in the NA also facilitates
Champagne F, Diorio J, Sharma SH, Meaney MJ (2001) Naturally
pair bonding in prairie voles and perhaps also same-sex
occurring variations in maternal behavior in the rat are associated
interactions. Central infusions of OT have been shown to
with differences in estrogen-inducible central oxytocin receptors.
enhance social interactions in male and female prairie
Proc Natl Acad Sci U S A 98(22):12736 –12741.
voles while oxytocin
Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A,
antagonists (i.c.v. or in the NA) reduced the time a female
Meaney MJ (2004) Variations in nucleus accumbens dopamine
prairie voles spent with her male partner
associated with individual differences in maternal behavior in therat. J Neurosci 24(17):4113– 4123.
Then, it is possible that oxytocin in prairie
Cho MM, DeVries AC, Williams JR, Carter CS (1999) The effects of
voles increases positive social interactions, including inter-
oxytocin and vasopressin on partner preferences in male and
action with pups, either disinhibiting the animal to ap-
female prairie voles (Microtus ochrogaster). Behav Neurosci 113:
proach to conspecifics, reducing social avoidance or/and
increasing the attractive value of social stimuli. Whether
DeVries AC, Johnson CL, Carter CS (1997) Familiarity and gender
OT infusions into the NA also influence male spontaneous
influence social preferences in prairie voles (Microtus ochro-gaster). Can J Zool 75:295–301.
parental responses, same-sex social interaction, or affect
Fahrbach SE, Morrell JI, Pfaff DW (1985) Role of oxytocin in the onset
the response to novelty or exploratory behavior needs to
of estrogen-facilitated maternal behavior. In: Oxytocin: Clinical and
be further investigated.
laboratory studies (Amico JA, Robinson AG, ed), pp 372–387. New
It must be clearly stated that oxytocin may facilitate
York: Elsevier.
maternal responses in brain regions that do not show any
Fahrbach SE, Morrell JI, Pfaff DW (1986) Effect of varying the duration
difference in OTR binding across species or through de-
of pre-test cage habituation on oxytocin induction of short-latency
velopment, as is apparently the case for the MPOA
maternal behavior. Physiol Behav 37:135–139.
Fleming AS, Anderson V (1987) Affect and nurturance: mechanisms
However, we have found that compar-
mediating maternal behavior in two female mammals. Prog Neu-
ative and developmental studies can be very useful to
ropsychopharmacol Biol Psychiatry 11(2–3):121–127.
understand individual differences in behavior. Based on
Francis DD, Champagne FC, Meaney MJ (2000) Variations in mater-
the evidence that OTR binding in the NA of rats declines
nal behavior are associated with differences in oxytocin receptor
through development as maternal response to pups does
levels in the rat. J Neuroendocrinol 12:1145–1148.
Francis DD, Young LJ, Meaney MJ, Insel TR (2002) Naturally occur-
ring differences in maternal care are associated with the expres-
and OTR binding in the NA is higher in prairie voles, that
sion of oxytocin and vasopressin (V1a) receptors: Gender differ-
show very intense spontaneous maternal responses, com-
ences. J Neuroendocrinol 14:349 –353.
pared with other three rodent species
Getz LL, Carter CS, Gavish L (1981) The mating system of the prairie
we hypothesized and found evidence that
vole, Microtus ochrogaster : Field and laboratory evidence for pair-
OTR binding variability in the NA of juvenile
bonding. Behav Ecol Sociobiol 8:189 –194.
and adult prairie voles was associated with
Groenewegen HJ, Wright CI, Beijer AV (1996) The nucleus accumbens:
gateway for limbic structures to reach the motor system? Prog Brain
the quality of their maternal responses. We finally further
Res 107:485–511.
tested this relationship with oxytocin antagonist infusions
Hansen S, Bergvall AH, Nyiredi S (1993) Interaction with pups en-
in the NA and confirmed OT facilitation of "spontaneous"
hances dopamine release in the ventral striatum of maternal rats:
maternal responses. Future studies will investigate the
a microdialysis study. Pharmacol Biochem Behav 45(3):673– 676.
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine
Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD
in motivated behavior: a unifying interpretation with special reference
(2005) Medial preoptic area interactions with nucleus accumbens-
to reward-seeking. Brain Res Brain Res Rev 31(1):6 – 41.
ventral pallidum circuit and maternal behavior in rats. Behav Brain
Insel TR, Shapiro LE (1992) Oxytocin receptor distribution reflects
Res 158:53– 68.
social organization in monogamous and polygamous voles. Proc
Olazábal DE, Morrell JI (2005) Juvenile rats show immature neuronal
Natl Acad Sci U S A 89:5981–5985.
patterns of c-Fos expression to first pup exposure. Behav Neurosci
Insel TR (1992) Oxytocin-A neuropeptide for affiliation: Evidence from
behavioral, receptor autoradiographic, and comparative studies.
Olazábal DE, Young LJ (2005) Variability in "spontaneous" maternal
behavior is associated with anxiety-like behavior and affiliation in
Insel TR, Winslow JT, Wang ZX, Young L, Hulihan TJ (1995) Oxytocin
naïve juvenile and adult female prairie voles (Microtus ochro-
and the molecular basis of monogamy. Adv Exp Med Biol
gaster). Dev Psychobiol 47(2):166 –178.
Olazábal DE, Young LJ (2006) Species and individual differences in
Keer SE, Stern JM (1999) Dopamine receptor blockade in the nucleus
juvenile female alloparental care are associated with oxytocin re-
accumbens inhibits maternal retrieval and licking, but enhances
ceptor density in the striatum and the lateral septum. Horm Behav
nursing behavior in lactating rats. Physiol Behav 67(5):659 – 669.
49(5):681– 687.
Kelley AE (2004) Memory and addiction: Shared neural circuitry and
Pedersen CA, Prange AJ (1979) Induction of maternal behavior in
molecular mechanisms. Neuron 44:161–179.
virgin rats after intracerebroventricular administration of oxytocin.
Keverne EB, Kendrick KM (1994) Maternal behaviour in sheep and its
Proc Natl Acad Sci U S A 76(12):6661– 6665.
neuroendocrine regulation. Acta Paediatr Suppl 397:47–56.
Pedersen CA, Prange AJ (1985) Oxytocin and mothering behavior in
Kovacs GL, Sarnyai Z, Babarczi E, Szabo G, Telegdy G (1990) The
the rat. Pharmacol Ther 28:287–302.
role of oxytocin-dopamine interactions in cocaine-induced locomo-
Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ (1985)
tor hyperactivity. Neuropharmacology 29(4):365–368.
Oxytocin antiserum delays onset of ovarian steroid-induced mater-
Li M, Fleming AS (2003a) The nucleus accumbens shell is critical for
nal behavior. Neuropeptides 6(2):175–182.
normal expression of pup-retrieval in postpartum female rats. Be-
Pedersen CA, Caldwell JD, Walker Ch, Ayers G, Mason GA (1994)
Oxytocin activates the postpartum onset of rat maternal behavior in
hav Brain Res 145:99 –111.
the ventral tegmental and medial preoptic areas. Behav Neurosci
Li M, Fleming AS (2003b) Differential involvement of nucleus accum-
bens shell and core subregions in maternal memory in postpartum
Petersson M, Eklund M, Uvnas-Moberg K (2005) Oxytocin decreases
female rats. Behav Neurosci 117(3):426 – 445.
corticosterone and nociception and increases motor activity in
Lim MM, Murphy AZ, Young LJ (2004) Ventral striatopallidal oxytocin
OVX rats. Maturitas 51:426 – 433.
and vasopressin V1a receptors in the monogamous prairie vole
Phelps SM, Young LJ (2003) Extraordinary diversity in vasopressin
(Microtus ochrogaster). J Comp Neurol 468:555–570.
(V1a) receptor distributions among wild prairie voles (Microtus
Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine
ochrogaster): patterns of variation and covariation. J Comp Neurol
interact to regulate pair bond formation in female prairie voles.
466(4):564 –576.
Robbins TW, Everitt BJ (2002) Limbic-striatal memory systems and
Lonstein JS, Simmons DA, Swann JM, Stern JM (1998) Forebrain
drug addiction. Neurobiol Learn Mem 78(3):625– 636.
expression of c-fos due to active maternal behaviour in lactating
Roberts RL, Miller AK, Taymans SE, Carter CS (1998) Role of social
rats. Neuroscience 82(1):267–281.
and endocrine factors in alloparental behavior of prairie voles
Lonstein JS, DeVries GJ (2000) Sex differences in the parental be-
(Microtus ochrogaster). Can J Zool 76:1862–1868.
havior of rodents. Neurosci Biobehav Rev 24:669 – 686.
Rosenblatt JS (1967) Nonhormonal basis of maternal behavior in the
Lonstein JS, DeVries GJ (2001) Social influences on parental and
rat. Science 156:(781):1512–1514.
nonparental responses toward pups in virgin female prairie voles
Salamone JD, Correa M (2002) Motivational views of reinforcement:
(Microtus ochrogaster). J Comp Psychol 115(1):53– 61.
implications for understanding the behavioral functions of nucleus
Lonstein JS (2002) Effects of dopamine receptor antagonism with
accumbens dopamine. Behav Brain Res 137:3–25.
haloperidol on nurturing behavior in the biparental prairie vole.
Salo AL, Shapiro LE, Dewsbury DA (1993) Comparisons of nipple
Pharmacol Biochem Behav 74:11–19.
attachment and incisor growth among four species of voles (Mi-
Mantella RC, Vollmer RR, Amico JA (2005) Corticosterone release is
crotus). Dev Psychobiol 27(5):317–330.
heightened in food or water deprived oxytocin deficient male mice.
Shapiro LE, Insel TR (1989) Ontogeny of oxytocin receptors in rat
Brain Res 56 – 61.
forebrain: A quantitative study. Synapse 4:259 –266.
Mayer AD, Rosenblatt JS (1975) Olfactory basis for the delayed onset
Stern JM (1987) Pubertal decline in maternal responsiveness in Long-
of maternal behavior in virgin female rats: experimental effects.
Evans rats: Maturational influences. Physiol Behav 41:93–98.
J Comp Physiol Psychol 89(7):701–710.
Stern JM, Taylor LA (1991) Haloperidol inhibits maternal retrieval and
Mayer AB, Rosenblatt JS (1979) Ontogeny of maternal behavior in the
licking, but enhances nursing behavior and litter weight gains in
laboratory rat: Early origins in 18- to 27-day-old young. Dev Psy-
lactating rats. J Neuroendocrinol 3:591–596.
chobiol 12:407– 424.
Stern JM, Keer SE (1999) Maternal motivation of lactating rats is
McCarthy MM, Kow LM, Pfaff DW (1992) Speculations concerning the
disrupted by low dosages of haloperidol. Behav Brain Res 99(2):
physiological significance of central oxytocin in maternal behavior.
Ann N Y Acad Sci 652:70 – 82.
Stern JM, Lonstein JS (2001) Neural mediation of nursing and related
McGuire B, Novak M (1984) A comparison of maternal behaviour in
maternal behaviors. Prog Brain Res 133:263–278.
the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochro-
Thomas JA, Birney EC (1979) Parental care and mating system of
gaster) and pine voles (M. pinetorum). Anim Behav 32:1132–1141.
the prairie vole, Microtus ochrogaster. Behav Ecol Sociobiol 5:
Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R (2000) Brain
oxytocin inhibits basal and stress-induced activity of the hypo-
Uvnas-Moberg K (1998) Oxytocin may mediate the benefits of positive
thalamo-pituitary-adrenal axis in male and female rats: Partial
social interaction and emotions. Psychoneuroendocrinology 23(8):
action within the paraventricular nucleus. J Neuroendocrinol 12:
819 – 835.
Uvnas-Moberg K, Eklund M, Hillegaart V, Ahlenius S (2000) Improved
Numan M, Insel TR (2003) The neurobiology of parental behavior.
conditioned avoidance learning by oxytocin administration in high-
New York: Springer-Verlag.
emotional male Sprague-Dawley rats. Regul Pept 88:27–32.
D. E. Olazábal and L. J. Young / Neuroscience 141 (2006) 559 –568
van Leengoed E, Kerker E, Swanson HH (1987) Inhibition of post-
Witt DM, Carter CS, Walton D (1990) Central and peripheral effects of
partum maternal behaviour in the rat by injecting an oxytocin
oxytocin administration in prairie voles (Microtus ochrogaster).
antagonist into the cerebral ventricles. J Endocr 112:275–282.
Pharmacol Biochem Behav 37:63– 69.
Wang ZX, Liu Y, Young LJ, Insel TR (2000) Hypothalamic vasopressin
Witt DM, Insel TR (1991) A selective oxytocin antagonist attenuates
gene expression increases in both males and females postpartum
progesterone facilitation of female sexual behavior. Endocrinology
in a biparental rodent. J Neuroendocrinol 12(2):111–120.
Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD
Young LJ (1999) Oxytocin and vasopressin receptors and species-
(2004) Oxytocin attenuates stress-induced c-fos mRNA expression in
typical social behaviors. Horm Behav 36(3):212–221.
specific forebrain regions associated with modulation of hypothala-
Young LJ, Lim MM, Gingrich B, Insel TR (2001) Cellular mechanisms
mus-pituitary-adrenal activity. J Neurosci 24(12):2974 –2982.
of social attachment. Horm Behav 40(2):133–138.
(Accepted 14 April 2006)
(Available online 24 May 2006)
Source: http://www.fisio.fmed.edu.uy/Olazabal/neuroscienceDEO.pdf
Published OnlineFirst July 15, 2014; DOI: 10.1158/1535-7163.MCT-14-0013 Cancer Biology and Signal Transduction Quinacrine Overcomes Resistance to Erlotinib by InhibitingFACT, NF-kB, and Cell-Cycle Progression in Non–Small CellLung Cancer Josephine Kam Tai Dermawan1, Katerina Gurova2, John Pink3, Afshin Dowlati4, Sarmishtha De1,Goutham Narla5, Neelesh Sharma4, and George R. Stark1
Date d'application 1er Février 2013 Version en vigueur Prise en charge médico-chirurgicale des infections osseuses Référence(s) Ce protocole décrit la prise en charge des infections osseuses SPILF 2009, CRIOGO 2011, HAS. Références 8.g « Maitrise du risque infectieux » et Chap II. « Prise en charge du patient » Médecin, Chirurgien




