Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Doi:10.1016/j.febslet.2008.04.014
FEBS Letters 582 (2008) 1950–1959
Centromeres: Old tales and new tools
P. Vagnarelli*, S.A. Ribeiro, W.C. Earnshaw*
Wellcome Trust Centre for Cell Biology, Institute of Cell and Molecular Biology, University of Edinburgh, Swann Building,
KingÕs Buildings, Mayfield Road, Edinburgh EH9 3JR, UK
Received 3 April 2008; accepted 11 April 2008
Available online 22 April 2008
Edited by Ulrike Kutay
point centromeres bind a single microtubule. (b) In regional
The centromere is a specialised region of the eukary-
centromeres (Shizosaccharomyces pombe, Drosophila melano-
otic chromosome that directs the equal segregation of sister chro-matids into two daughter cells during mitosis. In mitosis, the
gaster, Gallus gallus, Homo sapiens), the centromeric region
kinetochores mediate (1) microtubule capture and chromosome
is composed of much longer DNA arrays, and forms a larger
alignment at a metaphase plate; (2) the correction of improper
kinetochore that interacts with a number of microtubules. Re-
microtubule attachments; (3) the maintenance of an active check-
gional centromeres evolve naturally in association with charac-
point until bi-orientation is achieved by the whole complement of
teristic underlying DNA sequences, but are not absolutely
chromosomes; (4) the establishment of tension within the centro-
restricted to particular DNA sequences. In the diffuse centro-
mere which, in turn, contributes to silencing of the spindle check-
mere of holocentric chromosomes (e.g. Caenorhabditis ele-
point and triggers the onset of anaphase.
gans), microtubule-binding kinetochore determinants are
In this review, we will analyse how centromeres are organised
distributed along the length of the chromosome
with respect to chromatin types and arrangements.
Ó
In this review, we will analyse how centromeres are organ-
2008 Federation of European Biochemical Societies. Pub-
lished by Elsevier B.V. All rights reserved.
ised with respect to chromatin types and arrangements. Forother analyses of how the kinetochore is formed and organised
Keywords: Centromere; Kinetochore; CENP-A; Histone
we refer the reader to other reviews .
2. In the beginning there was DNA. .
Despite the high conservation of the segregation machinery
among eukaryotes, specific centromere-associated DNAs arehighly divergent and evolve rapidly during speciation. In S.
The centromere is a specialised region of the eukaryotic
cerevisiae, the centromeric DNA (CEN sequence) comprises
chromosome that directs the equal segregation of sister chro-
125 bp and consists of three elements: CDEI, CDEII and
matids into two daughter cells during mitosis. The centromere
CDEIII . In S. pombe, the centromere is composed of a
appears as a cytologically visible primary constriction on mito-
central core element (ctr) of 4–7 kb, and is flanked by imr se-
tic chromosomes of higher eukaryotes. The outer surface of
quences and otr outer repeats with an overall size range of 30–
the centromere is the site where the kinetochore assembles: this
110 kb, depending on the chromosome . In higher
represents the major machinery responsible for chromosome
eukaryotes, the centromeric DNA is normally characterised
segregation and is where interactions with microtubule plus
by highly repetitive tandem sequences. Because of this repeti-
ends occur. In mitosis, the kinetochores mediate (1) microtu-
tive organisation, the full sequence of a natural vertebrate cen-
bule capture and chromosome alignment at a metaphase plate;
tromere has yet to be achieved Nevertheless, several
(2) the correction of improper microtubule attachments; (3) the
studies have contributed to understanding the general rules
maintenance of an active checkpoint until bi-orientation is
for vertebrate centromeric DNA composition and organisa-
achieved by the whole complement of chromosomes; (4) the
establishment of tension within the centromere which, in turn,
In general, human centromeric DNA consists of arrays com-
contributes to silencing of the spindle checkpoint and triggers
prising 0.2–7 megabases of a 171 bp a-satellite motif repeated
the onset of anaphase
in tandem head-to-tail arrangement . This a-satellite
There are two different types of centromeres in eukaryotes:
DNA is present at all natural human centromeres .
localised centromeres and diffuse centromeres ()
a-Satellite can be subdivided in two types . Type I forms
The former can be subdivided into two variants. (a) In point
regular higher order repeat arrays (). Type II is mono-
centromeres (Saccharomyces cerevisiae), kinetochore for-
meric a-satellite and lacks a regular higher-order organisation.
mation on a discrete stretch of centromeric DNA is rigorously
Type I a-satellite DNA is associated with centromere function
specified by the underlying DNA sequence. Kinetochores of
and contains a 17 bp motif known as the CENP-B box whichrepresents the binding site for the centromeric protein CENP-B Type II a-satellite DNA usually flanks the type I
*Corresponding authors. Fax: +44 (0) 131 650 7100.
array and is often interrupted by LINE and SINE sequences
E-mail addresses: (P. Vagnarelli),
ed.ac.uk (W.C. Earnshaw).
0014-5793/$34.00 Ó 2008 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
doi:10.1016/j.febslet.2008.04.014
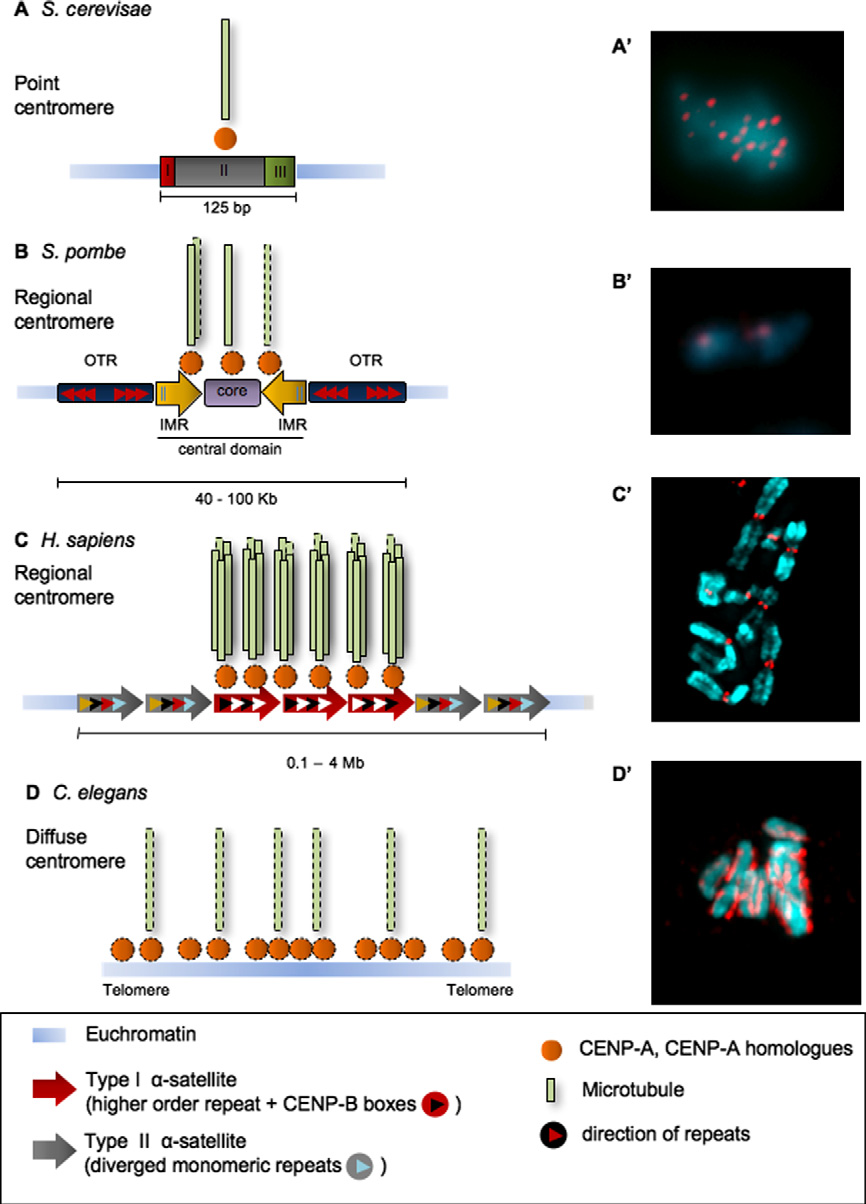
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
Fig. 1. Centromere organisation in eukaryotes. The DNA sequence of centromeric regions differs between species but the presence of CENP-A andits homologues (shown in orange) at the kinetochores is conserved. (A) S. cerevisiae has a point centromere that encompasses 125 bp and is composedof three conserved elements (CDEI, CDEII, CDEIII). This is wrapped around a single nucleosome containing the CENP-A homolog Cse4. Eachkinetochore in budding yeast makes only one stable microtubule attachment during metaphase. (A0) Meiotic chromosome spread of S. cerevisiae.
Red - Ndc10 kinetochore protein; Blue - DNA. Picture kindly provided by A. Marston, University of Edinburgh. (B) S. pombe has a regionalcentromere. The kinetochore assembles over the central domain, which contains the non-repetitive core (violet box) where Cnp1 localizes,surrounded by the innermost repeats (IMR; yellow box arrows). The outer repeats (OTR; blue box arrows) flank the central domain and are boundin heterochromatin. Each kinetochore interacts with 2–4 microtubules. (B0) Chromosomes of S. pombe. Red – Cnp1; Blue – DNA. Image kindlyprovided by A. Pidoux, University of Edinburgh. (C) H. sapiens has a more complex regional centromere. Two families of a-satellite DNA (red andgrey arrow boxes) are found only in the centromeric region. Type-I a-satellite (red box arrow) is composed of regular chromosome-specific higher-order repeating units, is characterized by the presence of CENP-B boxes (white triangles), and associates with CENP-A. Type-II a-satellite (grey boxarrows) consists of diverged alphoid monomer units (different coloured triangles). Between 20 and 30 microtubules attach to each humankinetochore. (C0) Partial metaphase spread of human chromosomes. Red – CENP-A; Blue – DNA. (D) C. elegans chromosomes have diffusecentromeres. CENP-A colocalizes with microtubule binding sites along the length of each chromosome. (D0) Metaphase chromosomes of C. elegans.
Red – Knl1; Blue – DNA. Image kindly provided by R. Gassmann, University of San Diego.
If the DNA sequence itself has not thus far given clues to the
This was first revealed by the analysis of stable dicentric
foundation for either the establishment or the maintenance of
chromosomes . These chromosomes are the products
a functional centromere, the analysis of ‘‘abnormal centro-
of duplications or more complex chromosome re-arrangements
meres'' identified by classical cytogenetics has opened the field
and contain two regions of sequence normally found within
to the concept that epigenetic factors are involved in the cen-
functional centromeres (they can be either from the same or
tromeric specification and propagation.
different chromosomes). In the case of dicentric chromosomes
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
containing two autosomal centromeres, both centromeric
Taken together these observations reveal that neither specific
DNA arrays bind the protein CENP-B, however one of the
DNA sequences (e.g. a-satellite) nor the DNA binding protein
two centromeres is subject to inactivation (centromere inacti-
CENP-B are essential or sufficient to dictate the assembly of a
vation) and therefore does not bind markers for the kineto-
chores, such as CENP-C and is no longer capable to
However, studies of de novo chromosome formation follow-
interact with the spindle microtubules. In the case of dicentric
ing the introduction of long stretches of cloned DNA into cells,
X chromosomes, the inactive centromere may fail to bind even
have shown that even if particular DNA sequences are not
the CENP-B protein. This led to the initial proposal that cen-
absolutely required, strong preferences govern experimental
tromere inactivation in these chromosomes might be caused by
centromere formation on naked DNA For example, de
modifications in chromatin structure (e.g. be epigenetic)
novo centromere assembly only occurs on type I a-satellite
The acquired chromatin modifications that lead to centromere
DNA . These studies also revealed that the CENP-B
inactivation are inherited from one cell division to the next,
box plays an essential role in the establishment but not in
therefore rendering this type of dicentric chromosome stable.
the maintenance of a functional centromere (see below)
Further evidence for epigenetic effects in centromere specifi-
We therefore conclude that in contrast to yeast point centro-
cation came from the analysis of neo-centromeric chromo-
meres, where DNA sequences specify sites of centromere
somes . These chromosomes are the products of
assembly, in regional centromeres some DNA characteristics,
complex re-arrangements in which an interstitial euchromatic
yet to be fully understood, specify a chromatin conformation,
chromosome region acquires the function of centromeric
which via epigenetic mechanisms controls the formation and
DNA and nucleates the formation of a kinetochore. An early
the maintenance of a functional centromere. Indeed, such epi-
and unpublished analysis of one such neocentromere is shown
genetic mechanisms can also operate on the regional centro-
in (see also These chromosomes lack a character-
mere of the fission yeast . Future studies aimed at
istic centromeric primary constriction and may, as in the case
elucidating the mechanisms of de novo chromosome formation
shown, have smaller kinetochores than the normal chromo-
and maintenance in vertebrates coupled with more extensive
somes. Because euchromatic chromosome arm sequences lack
sequence analyses of different neo-centromeric chromosomes
CENP-B boxes (as is the case for the a-satellite array found on
and evolutionary studies of centromere ‘‘drift'' during specia-
the Y chromosome both neocentromeres and the Y cen-
tion may eventually identify patterns of DNA sequence re-
tromere fail to bind CENP-B (). Once formed, neocen-
tromeres are fully functional and stably maintained through
approaches will be required to map out the epigenetic ‘‘land-
mitosis and meiosis .
scape'' that specifies centromere activity.
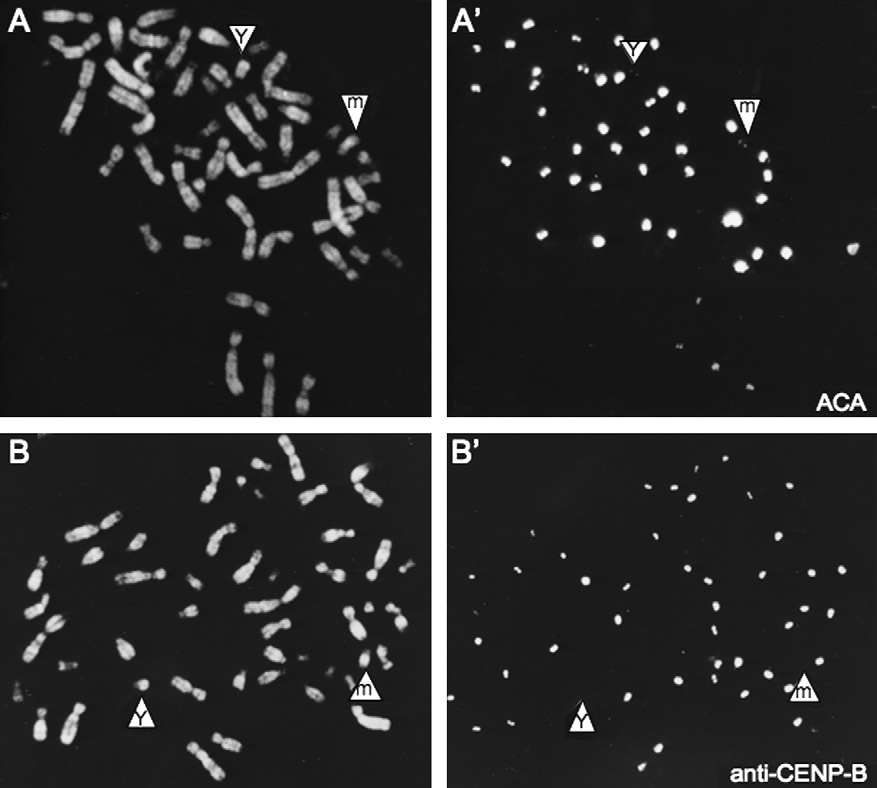
Fig. 2. A human chromosome in which a neocentromere has formed in euchromatin of a marker chromosome derived from chromosome 11. (A, A0)The marker chromosome (m) has a centromere that is labelled similar to that of the Y chromosome using anti-centromere autoantibodies that reactwith CENP-A, CENP-B and CENP-C. (B, B0) The centromere of both the marker and Y chromosomes fails to react with an antibody specific forCENP-B. This figure comes from a study performed in 1989, but not published until much later .
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
3. A special chromatin flavour
for centromeric chromatin assembly . In vivo studieshave shown that nucleosomes containing CENP-A generally
Despite the fact that common themes have yet to be identi-
lack histone H3. Since Cse4 interacts genetically with H2A
fied in the primary sequence of centromeric DNAs, important
and H4 and co-purifies with H2A, H2B and H4 it was gener-
similarities in chromatin organisation are shared by all eukary-
ally assumed that the centromeric-specific nucleosome is com-
otic centromeres. All centromeres contain a centromere-spe-
posed of two molecules each of Cse4, H2A, H2B and H4.
cific histone H3 variant, CENP-A (reviewed in
However, a recent study has reported that nucleosomes con-
This protein has subsequently been given other names, includ-
taining Cse4 possess an unusual composition, with H2A and
ing Cse4 (S. cerevisiae) , CID (D. melanogaster)
H2B replaced by one molecule of Scm3 , a protein first
ceHcp-3 (C. elegans) and HTR12 (Arabadopsis thali-
identified as dosage suppressor of a temperature-sensitive Cse4
allele in Saccharomyces cerevisiae. Smc3 can also displace
CENP-A is found not only at all natural centromeres in ver-
H2A and H2B from pre-assembled canonical octamers con-
tebrates but also at the variant functional centromeres we have
taining Cse4 in vitro. These results suggest that the budding
described above (e.g. neo-centromeres and de novo artificial
yeast centromeric nucleosome may be a hexamer containing
centromeres) . Studies of CENP-A knock-outs in mouse
two copies of Cse4, Scm3 and Histone H4. These exciting
DT40 C. elegans and by RNAi in human cells
new findings could represent a specialised situation that occurs
have clearly shown that CENP-A is essential for the estab-
only in the single nucleosome of the budding yeast point cen-
lishment and maintenance of centromeric function. All these
tromere. To date, orthologs of Scm3 have not been found in
studies therefore place CENP-A at or near the top of the hier-
other eukaryotes apart from fungi. However, these recent ad-
archical pathway that directs centromere/kinetochore forma-
vances in the understanding of budding yeast centromere
tion and activity.
structure suggest the possibility that the CENP-A-containing
CENP-A chromatin is localized in the inner kinetochore
chromatin could also be quite ‘‘special'' in higher eukaryotes.
plate (Carol A. Cooke and WCE, unpublished data), a part
Still on the quest for the special flavour of CENP-A contain-
of the electron-dense kinetochore structure visible at the pri-
ing chromatin, a recent study on Drosophila centromeric chro-
mary constriction of eukaryotic chromosomes. The outer kine-
matin organisation has produced provocative results. Based on
tochore does not contain detectable DNA In vitro studies
data obtained from cross-linking experiments and atomic force
suggest that nucleosomes containing CENP-A adopt a struc-
microscopy it was proposed that the nucleosomes containing
ture that exhibits greater conformational rigidity than conven-
Cid/CENP-A prepared from interphase cells are composed
tional chromatin, as judged by its deuterium exchange rate
of heterotypic tetramers termed ‘‘hemisomes'', and not canon-
relative to nucleosomes containing H3 How these in vitro
ical octamers These experiments are controversial, as the
findings relate to the in vivo organisation and behaviour of the
results could be influenced by the distribution of cross-linkable
CENP-A chromatin is still not clear. This special centromeric
residues in the Cid/CENP-A nucleosome However, an
chromatin may have a more compact structure which func-
octameric histone arrangement was detectable as a minor
tions as a platform for kinetochore assembly . This plat-
cross-linked species when CENP-A-containing nucleosomes
form must be structurally distinct and physically robust in
were prepared from mitotic cells.
order to maintain its architecture when tension within the spin-
In other experimental systems and using a range of analyses,
dle pulls the sister kinetochores towards opposite spindle poles
most results obtained to date are consistent with CENP-A
during metaphase.
occurring in canonical nucleosomes. In one study, CENP-A
In regional centromeres, 100–1000 kilobases of DNA (100–
chromatin was obtained from interphase nuclei by immuno-
300 kb estimated from ChIP studies on neocentromeres
precipitation using a monoclonal antibody In another
and �1 Mb from studies using fibre-FISH on artificial
study, CENP-A-containing nucleosomes were isolated by
chromosomes are organised in CENP-A containing chro-
means of a tag linked to an over-expressed form of CENP-
matin. These size discrepancies could either reflect the intrinsic
A. Therefore, the endogenous protein was still present and
technical limitations of the differing approaches used or the
data are generally lacking concerning the functionality of the
different substrates examined. Despite the differences, a com-
engineered protein apart from its localisation In some
mon theme seems to emerge – CENP-A nucleosomes are not
cases, only the tagged version of CENP-A was recovered in
arranged along a linear uninterrupted stretch of chromatin. In-
pulldowns together with H2A, H2B and H4. However, H3
stead, they appear to be clustered, with each cluster spaced
was also present in the purified samples although it was less
from the next by a region of CENP-A-free chromatin
abundant. From these experiments it was concluded that in
In at least some cases it appears that nucleosomes
human cells CENP-A nucleosomes are typically found in
containing H3K4Me2 (histone-H3 di-methylated on Lys 4)
homotypic octamers with only about 10% found in heterotypic
occupy the CENP-A-negative gaps within the CENP-A chro-
matin region This organisation for the centromeric chro-
Once CENP-A is incorporated into centromeric chromatin it
matin was unexpected since H3K4Me2 is a modification
remains stable across the whole cell cycle including during
characteristic of euchromatic regions In the future,
DNA replication. It has recently been demonstrated that load-
more extensive studies using different approaches will be re-
ing of new CENP-A into the replicated centromeres happens
quired to develop a general model for the organisation of the
only in late mitosis or early G1 and not in S or
CENP-A chromatin block at vertebrate centromeres.
G2 as originally proposed . Therefore, new incorporation
In budding yeast (the system in which centromeric chroma-
of CENP-A occurs only after cells pass through mitosis. The
tin organisation has been analysed in the greatest detail), a
nature of the priming event that happens during mitosis and
single nucleosome containing Cse4 forms the minimal unit
is required for new CENP-A assembly is still unknown – pro-
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
Fig. 3. Chromatin subdomains within the centromeres are characterised by different histone modifications and have different functions. (A, B)Chicken metaphase chromosomes stained for CENP-A (green) and H3K9Me3 (red) (A) or H3T3ph (red) (B). These histone modifications are presentonly in the inner centromeric chromatin of mitotic chromosomes. (C) Human metaphase chromosome stained for CENP-A (green) and HP1-a (red).
HP1-a is enriched in the centromeric and peri-centromeric heterochromatin. (D) Human mitotic chromosome stained for CENP-A (green) andH3K4Me2 (red). This histone modification is typical of euchromatin regions, however in rare instances it also appears to co-localise with CENP-Asignal (arrows) and inset. Picture kindly provided by S. Cardinale, University of Edinburgh. (E) Schematic representation of the primary constrictionof a regional centromere where the localisation of different histone modifications is indicated (left). Known functions, which are attributed to theinner centromeric chromatin are shown at the right.
teolysis of one or more centromere proteins is an obvious pos-
as a knockdown of CENP-A also compromised KLN2 locali-
At least two proteins are essential for CENP-A loading:
In summary, even though recent experiments have yielded
Mis18 and HsKNL2 hMis18 localises to centro-
novel information about the timely recruitment of CENP-A
meres in G2 but is removed as the cells enter mitosis. During
to centromeres and a few critical components have been identi-
mitotic exit it is recruited back to the centromeres at the same
fied for this process, further work is required to understand the
time when CENP-A is incorporated. Lack of Mis18 causes not
priming step that happens during mitosis and is required for
only loss of CENP-A from the centromeres but also an in-
new CENP-A assembly. Furthermore, these experiments raise
crease in acetylation of centromeric histones H3 and H4. This
the surprising possibility that (1) either kinetochores function
observation suggests that chromatin modifications are part of
in mitosis with half of their CENP-A binding sites unoccupied;
the priming event for CENP-A deposition. KLN2 (also known
or (2) CENP-A binding sites are created during mitotic exit
as Mis18BP1) has a myb motif that identifies it as a likely
either by chromatin modification (presumably acetylation) or
DNA-binding protein In mammalian cells, KNL2/
by proteolysis of a yet-to-be-identified substrate.
Mis18BP1 localises to the centromere during mitotic exit (lateanaphase–telophase and early G1) and its depletion abolishesthe localisation of CENP-A. Its function is conserved in C. ele-
4. What defines boundaries for the centromeric chromatin?
gans although its localisation at centromeres is not restricted toa specific window of mitosis. CENP-A and KLN2/Mis18BP1
Several different histone modifications have been discovered
are mutually interdependent for their centromere localisation,
as being limited, enriched or interspersed relative to the centro-
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
meric chromatin. One of these classical modifications is the
that co-operates together with the chromosomal passenger
tri-methylation of Histone H3 on Lysine 9 (H3K9Me3). In
protein TD60 to recruit the chromosomal passenger complex
vertebrates, H3K9Me3 is constrained to the inner centromeric
(CPC) to the centromere . However, the relative position-
chromatin and . In the fission yeast S. pombe, peri-
ing of H3K4Me2 and H3T3ph within the three-dimensional
centromeric heterochromatin is enriched in H3K9Me2 where
structure of the inner centromeric region remain to be deter-
it forms a binding site for Swi6 (the fission yeast homologue
of HP1) , which in its turn recruits the cohesin complex
Despite numerous outstanding questions, one picture clearly
HP1 and the cohesin complex localise in the inner centro-
emerges. The vertebrate centromere, although it contains
meric chromatin in vertebrates and . It is there-
rather homogeneous DNA sequence elements that form its
fore possible that H3K9Me3 in this case could have the
foundation, is built from distinct domains The inter-
same recruitment function as does H3K9Me2 in yeast. Yeasts
nal (inner centromere) domain is dedicated to the control of
also exhibit an enrichment of H3K9Me3 in pericentromeric
sister chromatid cohesion (factors include cohesin and PICH
chromatin but this modification does not seem to have a cru-
) and to the regulation of chromosome dynamics (chromo-
cial role in centromere formation or function.
somal passenger proteins), while the outer region containing
In humans and Drosophila, analysis of extended chromatin
CENP-A is dedicated to directing the assembly of the kineto-
fibres suggests that H3K4Me2 is interspersed with the
chore itself. These two sub-domains are apparently specified by
CENP-A-containing nucleosomes. However, this modification
different histone codes that can act as stable epigenetic markers
is detectable at centromeres in conventional mitotic chromo-
for the recruitment of critical components for centromeric
some preparations only in a minority of chromosome spreads
function and assembly ). We hypothesize that chromatin
and D. The function of such a euchromatic modifi-
folding within the outer centromere is also an important key
cation within the centromeric chromatin is not clear. It has
for the maintenance of a special topology. Data from a num-
been proposed that the alternating arrangement of CENP-A
ber of groups suggest that inner centromere chromatin and
and H3K4Me2-containing nucleosomes dictates a code speci-
CENP-A chromatin have different biophysical properties
fying centromeric chromatin .
that allow them to make different contributions to
Another distinct modification of inner centromeric chroma-
chromosome dynamics during mitosis
tin is the phosphorylation of histone H3 at threonine 3
In Drosophila, heterochromatin is thought to prevent the
This mitosis-specific modification is
spread of CENP-A onto noncentromeric DNA and balanced
generated by Haspin kinase and has recently been sug-
levels of euchromatic and heterochromatic domains are
gested to have an important role in chromosome segregation
achieved by different histone-modifying enzymes and chroma-
H3 phosphorylation on T3 appears to be a priming event
tin components In S. pombe, barriers to heterochromatin
Fig. 4. Centromeric chromatin organisation: possible models. (A) Model 1. Kinetochore chromatin is organised in a discontinuous array of DNAregions enriched for CENP-A and regions devoid of CENP-A. ChIP on CHIP analysis from the lab of Peter Warburton suggests that 2–3nucleosomes containing CENP-A are clustered next to a nucleosome which is CENP-A negative (B) This arrangement would produce a 30 nmchromatin fibre in which each turn contains CENP-A unless the periodicity differs from region to region. (C) Model 2. Based on fiber-FISH analysis,CENP-A containing chromatin is interspersed with chromatin containing H3K4Me2. This array is then flanked by a stretch of chromatin enriched inH3K9Me3 . Based on the limitations of the technique, which produces fibres of unknown axial compaction that not homogeneous in size, it isdifficult to determine the exact level of chromatin organisation to which these observations relate. (D) Model 3. CENP-A containing chromatin maypreferentially fold on itself, generating a distinct domain at the surface of the centromere. Stabilisation of the CENP-A domain could be either theresult of the unusual biophysical properties of CENP-A nucleosomes , or the presence of ‘‘stabilising factors'' recruited by CENP-A to the innerkinetochore.
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
are defined by tRNA genes and require bound transcrip-
CENP-A assembly . The balance between these antagonis-
tion factors and RNA polymerase III to function In
tic activities of CENP-B: CENP-A deposition and heterochro-
humans, centromere chromatin and constitutive heterochro-
matin formation, appears to be critical in determining whether
matin also apparently exist as distinct domains that are sepa-
exogenous a-satellite DNA will form a de novo kinetochore or
a block of silent heterochromatin. This leads to the interesting,
H3K9Me2. CENP-A mild over-expression causes a decrease
though difficult to test, hypothesis that when de novo centro-
in the amount of H3K9Me2 immediately flanking the centric
mere activation or inactivation occurs using endogenous se-
chromatin but does not change the level of H3K4Me2
quences, CENP-B should have a critical role in determining
It therefore appears that CENP-A can spread along a a-satel-
the outcome. For example, chromosome rearrangements pro-
lite array, displacing H3K9Me2 but not H3K9Me3.
ducing dicentric chromosomes, might not undergo centromere
To date, detailed studies of the functional relevance of cen-
inactivation in the absence of CENP-B.
tromeric-specific histone modifications in vertebrates have
All of the above leave us with a further puzzle to solve. How
been mainly restricted to descriptive approaches. These are
do cells activate a neo-centromere on a DNA region other than
limited by the fact that drastic changes to the centromeric
a-satellite where CENP-B boxes are lacking? Indeed, neocen-
chromatin status would cause cell death. Only recently has a
tromeres, like the human Y chromosome, lack associated
novel approach yielded a HAC with a synthetic kinetochore
CENP-B ), and thus their initial formation is almost cer-
whose chromatin can be successfully manipulated and specifi-
tainly CENP-B independent. However, neocentromere DNA
cally inactivated in vivo making it the first conditional
lacking CENP-B boxes is unable to nucleate de novo kineto-
centromere outside of budding yeast This new synthetic
chore assembly in human cells .
kinetochore contains regular repeats of a synthetic dimeric a-satellite DNA repeat, with one monomer having a tetracyclinerepressor (tetR) binding site (tetO) in place of the CENP-B
6. Rigid, plastic or elastic?
box. Using tetR fusion proteins it is possible to target any pro-tein of interest into the synthetic kinetochore in vivo and assess
Once the centromere is established and has directed the
the subsequent chromatin changes and their consequence for
assembly of a kinetochore, it exerts its main functions during
kinetochore function Using this system, it was found that
cell division. In mitosis, centromeres have essential signalling
either ‘‘opening'' or ‘‘closing'' of the centromeric chromatin
functions (we refer the reader to other reviews on this subject
(by recruiting the tTA transcriptional activator or the tTS
– Centromeres also have important mechanical/bio-
transcriptional silencer, respectively) results in kinetochore
physical properties which play a key role in the generation of
inactivation. The binding of a transcriptional silencer caused
an equilibrium of forces during chromosome alignment and
the loss of CENP-A and H3K4Me2 accompanied by accumu-
lation of histone H3K9Me3 and recruitment of HP1. These re-
During chromosome bi-orientation at prometaphase, sister
sults indicate that a balance between the different subtypes of
kinetochores are attached to spindle fibres emanating from
centromeric chromatin is essential for vertebrate kinetochore
opposite poles. The pulling forces generated on the paired
opposing sister kinetochores are counterbalanced by the resis-
This new experimental system should allow a detailed anal-
tance provided by the centromere. Thus, it is within the inner
ysis of the molecular events which are temporally and spatially
centromere chromatin that spindle tension exerts its effect and
required for the maintenance of centromeric function.
is sensed by an as-yet unknown mechanism. What are the bio-physical properties of the centromeric chromatin and what isthe role (if any) of the different domains of the centromeric
5. How to make a centromere de novo
The stretching of inner centromere chromatin is manifested
From the early studies of human artificial chromosomes
as sister kinetochore ‘‘breathing'' when chromosomes are
(HACs), it emerged that de novo centromere formation from
aligned and bi-oriented at metaphase Microtubule-depen-
cloned alphoid DNA required the presence of CENP-B boxes
dent stretching of chromatin in bi-oriented chromatids has
. However, this was difficult to reconcile with the surpris-
been known for many years but the compliant element that
ing news that mice lacking CENP-B were viable and fertile
regulates this intrinsic chromatid response is still a mystery
. In order to rationalise this apparent paradox it was
. Existing data reveal that the centromeric chromatin
tempting to speculate that CENP-B is important for the estab-
can be deformed. Are these deformations plastic or elastic and
lishment of a centromere but becomes dispensable for its main-
which kind of centromeric chromatin is involved?
tenance in normal circumstances. In fact, it is only recently
Recent studies in budding yeast confirm that the centromeric
(almost 10 years after the initial studies) that it has been pos-
chromatin is elastic. Upon depletion of H3 and H4 the com-
sible to show that cloned a-satellite DNA arrays with wild-
paction of pericentric chromatin decreased, so that sister kine-
type CENP-B boxes are not competent for de novo centromere
tochores were stretched further apart when chromosomes were
formation and CENP-A assembly in cells lacking CENP-B
bi-oriented. Surprisingly, this did not change the amplitude of
(CENP-B�/�) What does CENP-B do?
the typical mitotic oscillations . Therefore, the nature of the
The recent studies reveal that CENP-B has a dual role, on
determinants of the elastic behaviour of centromeric chroma-
the one hand recruiting CENP-A to the chromatin during de
tin remains elusive.
novo centromere formation, and on the other actively enhanc-
Centromere elasticity has the curious property of being vec-
ing the H3K9Me3 modification of chromatin containing chro-
torial. When sister kinetochores are pulled in opposite orienta-
mosomally-integrated a-satellite DNA without stimulating
tions, the chromatin between them is clearly elastic, as
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
described above. However, if the chromatin linking sister kine-
[18] Mitchell, A.R., Gosden, J.R. and Miller, D.A. (1985) A cloned
tochores is bent into a U-shape so that both kinetochores face
sequence, p82H, of the alphoid repeated DNA family found atthe centromeres of all human chromosomes. Chromosoma 92,
the same spindle pole, it is unable to straighten itself by elastic
recoil unless pulled by spindle forces . Thus, kinetochores
[19] Ikeno, M., Masumoto, H. and Okazaki, T. (1994) Distribution
are axially elastic but radially plastic. How the chromatin
of CENP-B boxes reflected in CREST centromere antigenic sites
fibers pack within and between kinetochores to give the centro-
on long-range alpha-satellite DNA arrays of human chromo-
mere these remarkable properties is only one of many fascinat-
some 21. Hum. Mol. Genet. 3, 1245–1257.
[20] Alexandrov, I., Kazakov, A., Tumeneva, I., Shepelev, V. and
ing questions that the centromere/kinetochore holds for future
Yurov, Y. (2001) Alpha-satellite DNA of primates: old and new
investigators to answer.
families. Chromosoma 110, 253–266.
[21] Earnshaw, W.C. and Rothfield, N. (1985) Identification of a
Acknowledgements: S.R. is supported by a Ph.D. studentship from The
family of human centromere proteins using autoimmune sera
Darwin Trust of Edinburgh. The experiments in the WCE Lab are sup-
from patients with scleroderma. Chromosoma (Berl.) 91, 313–
ported by The Wellcome Trust, of which WCE is a Principal Research
[22] Earnshaw, W.C., Sullivan, K.F., Machlin, P.S., Cooke, C.A.,
Kaiser, D.A., Pollard, T.D., Rothfield, N.F. and Cleveland,D.W. (1987) Molecular cloning of cDNA for CENP-B, themajor human centromere autoantigen. J. Cell Biol. 104, 817–829.
[23] Masumoto, H., Masukata, H., Muro, Y., Nozaki, N. and
Okazaki, T. (1989) A human centromere antigen (CENP-B)
[1] Maiato, H., Deluca, J., Salmon, E.D. and Earnshaw, W.C.
interacts with a short specific sequence in alphoid DNA, a
(2004) The dynamic kinetochore–microtubule interface. J. Cell
human centromeric satellite. J. Cell Biol. 109, 1963–1973.
Sci. 117, 5461–5477.
[24] Prades, C., Laurent, A.M., Puechberty, J., Yurov, Y. and
[2] Nakagawa, H., Lee, J.K., Hurwitz, J., Allshire, R.C., Nakay-
Roizes, G. (1996) SINE and LINE within human centromeres. J.
ama, J., Grewal, S.I., Tanaka, K. and Murakami, Y. (2002)
Mol. Evol. 42, 37–43.
Fission yeast CENP-B homologs nucleate centromeric hetero-
[25] Earnshaw, W.C. and Migeon, B. (1985) A family of centromere
chromatin by promoting heterochromatin-specific histone tail
proteins is absent from the latent centromere of a stable
modifications. Genes Dev. 16, 1766–1778.
isodicentric chromosome. Chromosoma (Berl.) 92, 290–296.
[3] Ekwall, K. (2007) Epigenetic control of centromere behavior.
[26] Merry, D.W., Pathak, S., Hsu, T.C. and Brinkley, B.R. (1985)
Annu. Rev. Genet. 41, 63–81.
Anti-kinetochore antibodies: use as probes for inactive centro-
[4] Pluta, A.F., Mackay, A.M., Ainsztein, A.M., Goldberg, I.G. and
meres. Am. J. Hum. Genet. 37, 425–430.
Earnshaw, W.C. (1995) The centromere: hub of chromosomal
[27] Earnshaw, W.C., Ratrie, H. and Stetten, G. (1989) Visualization
activities. Science 270, 1591–1594.
of centromere proteins CENP-B and CENP-C on a stable
[5] Maddox, P.S., Oegema, K., Desai, A. and Cheeseman, I.M.
dicentric chromosome in cytological spreads. Chromosoma
‘‘Holo''er than thou: chromosome segregation and
(Berl.) 98, 1–12.
kinetochore function in C. elegans. Chromosome Res. 12, 641–
[28] Qin, N., Bartley, J., Wang, J.C. and Warburton, P.E. (2007) A
neocentromere derived from a supernumerary marker deleted
[6] McAinsh, A.D., Tytell, J.D. and Sorger, P.K. (2003) Structure,
from the long arm of chromosome 6. Cytogenet. Genome Res.
function, and regulation of budding yeast kinetochores. Annu.
119, 154–157.
Rev. Cell Dev. Biol. 19, 519–539.
[29] Choo, K.H. (1997) Centromere DNA dynamics: latent centro-
[7] Cheeseman, I.M. and Desai, A. (2008) Molecular architecture of
meres and neocentromere formation. Am. J. Hum. Genet. 61,
the kinetochore–microtubule interface. Nat. Rev. Mol. Cell Biol.
[30] Williams, B.C., Murphy, T.D., Goldberg, M.L. and Karpen,
[8] Tanaka, T.U. and Desai, A. (2008) Kinetochore–microtubule
G.H. (1998) Neocentromere activity of structurally acentric
interactions: the means to the end. Curr. Opin. Cell Biol. 20, 53–
mini-chromosomes in Drosophila. Nature Genet. 18, 30–37.
[31] Wandall, A., Tranebjaerg, L. and Tommerup, N. (1998) A
[9] Clarke, L. and Carbon, J. (1980) Isolation of a yeast centromere
neocentromere on human chromosome 3 without detectable a-
and construction of functional small circular chromosomes.
satellite DNA forms morphologically normal kinetochores.
Nature 287, 504–509.
Chromosoma (Berl.) 107, 359–365.
[10] Clarke, L. and Carbon, J. (1985) The structure and function of
[32] Barry, A.E., Bateman, M., Howman, E.V., Cancilla, M.R.,
yeast centromeres. Ann. Rev. Genet. 19, 29–56.
Tainton, K.M., Irvine, D.V., Saffery, R. and Choo, K.H. (2000)
[11] Clarke, L., Amstutz, H., Fishel, B. and Carbon, J. (1986)
The 10q25 neocentromere and its inactive progenitor have
Analysis of centromeric DNA in the fission yeast Schizo-
identical primary nucleotide sequence: further evidence for
saccharomyces pombe. Proc. Nat.Acad. Sci. (USA) 83, 8253–
epigenetic modification. Genome Res. 10, 832–838.
[33] Floridia, G., Gimelli, G., Zuffardi, O., Earnshaw, W.C., War-
[12] Nakaseko, Y., Adachi, Y., Funahashi, S., Niwa, O. and
burton, P.E. and Tyler-Smith, C. (2000) A neocentromere in the
Yanagida, M. (1986) Chromosome walking shows a highly
DAZ region of the human Y chromosome. Chromosoma 109,
homologous repetitive sequence present in all the centromere
regions of fission yeast. The EMBO J. 5, 1011–1021.
[34] Tyler-Smith, C. et al. (1999) Transmission of a fully functional
[13] Clarke, L. (1990) Centromeres of budding and fission yeasts.
human neocentromere through three generations. Am. J. Hum.
Trends Genet. 6, 150–154.
Genet. 64, 1440–1444.
[14] Schueler, M.G., Higgins, A.W., Rudd, M.K., Gustashaw, K.
[35] Voullaire, L. et al. (2001) Mosaic inv dup(8p) marker chromo-
and Willard, H.F. (2001) Genomic and genetic definition of a
some with stable neocentromere suggests neocentromerization is
functional human centromere. Science 294, 109–115.
a post-zygotic event. Am. J. Med. Genet. 102, 86–94.
[15] Rudd, M.K. and Willard, H.F. (2004) Analysis of the centro-
[36] Alonso, A., Mahmood, R., Li, S., Cheung, F., Yoda, K. and
meric regions of the human genome assembly. Trends Genet. 20,
Warburton, P.E. (2003) Genomic microarray analysis reveals
distinct locations for the CENP-A binding domains in three
[16] Maio, J.J. (1971) DNA strand reassociation and polyribonucle-
human chromosome 13q32 neocentromeres. Hum. Mol. Genet.
otide binding in the African green monkey, Cercopithecus
12, 2711–2721.
aethiops. J. Mol. Biol. 56, 579–595.
[37] Saffery, R., Irvine, D.V., Griffiths, B., Kalitsis, P., Wordeman, L.
[17] Choo, K.H., Vissel, B., Nagy, A., Earle, E. and Kalitsis, P.
and Choo, K.H. (2000) Human centromeres and neocentromeres
(1991) A survey of the genomic distribution of alpha satellite
show identical distribution patterns of >20 functionally impor-
DNA on all human chromosomes, and derivation of a new
tant kinetochore-associated proteins. Hum. Mol. Genet. 9, 175–
consensus sequence. Nucleic Acids Res. 19, 1179–1182.
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
[38] Voullaire, L.E., Slater, H.R., Petrovic, V. and Choo, K.H. (1993)
[59] Oegema, K., Desai, A., Rybina, S., Kirkham, M. and Hyman,
A functional marker centromere with no detectible alpha-
A.A. (2001) Functional analysis of kinetochore assembly in
satellite, satellite III, or CENP-B protein: activation of a latent
Caenorhabditis elegans. J. Cell Biol. 153, 1209–1226.
centromere? Am. J. Hum. Genet. 52, 1153–1163.
[60] Goshima, G., Kiyomitsu, T., Yoda, K. and Yanagida, M. (2003)
[39] du Sart, D. et al. (1997) A functional neo-centromere formed
Human centromere chromatin protein hMis12, essential for
through activation of a latent human centromere and consisting
equal segregation, is independent of CENP-A loading pathway.
of non-alpha-satellite DNA. Nat. Genet. 16, 144–153.
J. Cell Biol. 160, 25–39.
[40] Depinet, T.W. et al. (1997) Characterization of neo-centromeres
[61] Cooke, C.A., Bazett-Jones, D.P., Earnshaw, W.C. and Rattner,
in marker chromosomes lacking detectable alpha-satellite DNA.
J.B. (1993) Mapping DNA within the mammalian kinetochore.
Hum. Mol. Genet. 6, 1195–1204.
J. Cell Biol. 120, 1083–1091.
[41] Earnshaw, W.C., Bernat, R.L., Cooke, C.A. and Rothfield, N.F.
[62] Black, B.E., Foltz, D.R., Chakravarthy, S., Luger, K., Woods
(1991) Role of the centromere/kinetochore in cell cycle control.
Jr., V.L. and Cleveland, D.W. (2004) Structural determinants for
Cold Spring Harbor Symp. Quant. Biol. 56, 675–685.
generating centromeric chromatin. Nature 430, 578–882.
[42] Masumoto, H., Nakano, M. and Ohzeki, J. (2004) The role of
[63] Lo, A.W., Craig, J.M., Saffery, R., Kalitsis, P., Irvine, D.V.,
CENP-B and alpha-satellite DNA: de novo assembly and
Earle, E., Magliano, D.J. and Choo, K.H. (2001) A 330 kb
epigenetic maintenance of human centromeres. Chromosome
CENP-A binding domain and altered replication timing at a
Res. 12, 543–556.
human neocentromere. Embo J. 20, 2087–2096.
[43] Ikeno, M. et al. (1998) Construction of YAC-based mammalian
[64] Lam, A.L., Boivin, C.D., Bonney, C.F., Rudd, M.K. and
artificial chromosomes. Nat. Biotechnol. 16, 431–439.
Sullivan, B.A. (2006) Human centromeric chromatin is a
[44] Grimes, B.R., Rhoades, A.A. and Willard, H.F. (2002) Alpha-
dynamic chromosomal domain that can spread over noncentro-
satellite DNA and vector composition influence rates of human
meric DNA. Proc. Natl. Acad. Sci. USA 103, 4186–4191.
artificial chromosome formation. Mol. Ther. 5, 798–805.
[65] Blower, M.D., Sullivan, B.A. and Karpen, G.H. (2002) Con-
[45] Mejia, J.E., Alazami, A., Willmott, A., Marschall, P., Levy, E.,
served organization of centromeric chromatin in flies and
Earnshaw, W.C. and Larin, Z. (2002) Efficiency of de novo
humans. Dev. Cell 2, 319–330.
centromere formation in human artificial chromosomes. Genom-
[66] Alonso, A. et al. (2007) Co-localization of CENP-C and CENP-
ics 79, 297–304.
H to discontinuous domains of CENP-A chromatin at human
[46] Kouprina, N. et al. (2003) Cloning of human centromeres by
neocentromeres. Genome Biol. 8, R148.
transformation-associated recombination in yeast and genera-
[67] Sullivan, B.A. and Karpen, G.H. (2004) Centromeric chromatin
tion of functional human artificial chromosomes. Nucleic Acids
exhibits a histone modification pattern that is distinct from both
Res. 31, 922–934.
euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 11,
[47] Okada, T., Ohzeki, J., Nakano, M., Yoda, K., Brinkley, W.R.,
Larionov, V. and Masumoto, H. (2007) CENP-B controls
[68] Jenuwein, T. and Allis, C.D. (2001) Translating the histone code.
centromere formation depending on the chromatin context. Cell
Science 293, 1074–1080.
131, 1287–1300.
[69] Kouzarides, T. (2007) Chromatin modifications and their
[48] Steiner, N. and Clarke, L. (1994) A novel epigenetic effect can
function. Cell 128, 693–705.
alter centromere function in fission yeast. Cell 79, 865–874.
[70] Bloom, K., Hill, A., Kenna, M. and Saunders, M. (1989) The
[49] Henikoff, S., Ahmad, K. and Malik, H.S. (2001) The centromere
structure of a primitive kinetochore. TIBS 14, 223–227.
paradox: stable inheritance with rapidly evolving DNA. Science
[71] Furuyama, S. and Biggins, S. (2007) Centromere identity is
293, 1098–1102.
specified by a single centromeric nucleosome in budding yeast.
[50] Black, B.E. and Bassett, E.A. (2008) The histone variant CENP-
Proc. Natl. Acad. Sci. USA 104, 14706–14711.
A and centromere specification. Curr. Opin. Cell Biol. 20, 91–
[72] Camahort, R., Li, B., Florens, L., Swanson, S.K., Washburn,
M.P. and Gerton, J.L. (2007) Scm3 is essential to recruit the
[51] Stoler, S., Keith, K.C., Curnick, K.E. and Fitzgerald-Hayes, M.
histone h3 variant cse4 to centromeres and to maintain a
(1995) A mutation in CSE4, an essential gene encoding a novel
functional kinetochore. Mol. Cell 26, 853–865.
chromatin-associated protein in yeast, causes chromosome
[73] Mizuguchi, G., Xiao, H., Wisniewski, J., Smith, M.M. and Wu,
nondisjunction and cell cycle arrest at mitosis. Genes Dev. 9,
C. (2007) Nonhistone Scm3 and histones CenH3-H4 assemble
the core of centromere-specific nucleosomes. Cell 129, 1153–
[52] Henikoff, S., Ahmad, K., Platero, J.S. and van Steensel, B.
(2000) Heterochromatic deposition of centromeric histone H3-
[74] Stoler, S., Rogers, K., Weitze, S., Morey, L., Fitzgerald-Hayes,
like proteins. Proc. Natl. Acad. Sci. USA 97, 716–721.
M. and Baker, R.E. (2007) Scm3, an essential Saccharomyces
[53] Buchwitz, B.J., Ahmad, K., Moore, L.L., Roth, M.B. and
cerevisiae centromere protein required for G2/M progression and
Henikoff, S. (1999) A histone-H3-like protein in C. elegans.
Cse4 localization. Proc. Natl. Acad. Sci. USA 104, 10571–10576.
Nature 401, 547–548.
[75] Dalal, Y., Furuyama, T., Vermaak, D. and Henikoff, S. (2007)
[54] Moore, L.L., Morrison, M. and Roth, M.B. (1999) HCP-1, a
Structure, dynamics, and evolution of centromeric nucleosomes.
protein involved in chromosome segregation, is localized to the
Proc. Natl. Acad. Sci. USA 104, 15974–15981.
centromere of mitotic chromosomes in Caenorhabditis elegans. J.
[76] Obuse, C., Yang, H., Nozaki, N., Goto, S., Okazaki, T. and
Cell Biol. 147, 471–480.
Yoda, K. (2004) Proteomics analysis of the centromere complex
[55] Talbert, P.B., Masuelli, R., Tyagi, A.P., Comai, L. and Henikoff,
from HeLa interphase cells: UV-damaged DNA binding protein
S. (2002) Centromeric localization and adaptive evolution of an
1 (DDB-1) is a component of the CEN-complex, while BMI-1 is
Arabidopsis histone H3 variant. Plant Cell 14, 1053–1066.
transiently co-localized with the centromeric region in inter-
[56] Harrington, J.J., Van Bokkelen, G., Mays, R.W., Gustashaw, K.
phase. Genes Cells 9, 105–120.
and Willard, H.F. (1997) Formation of de novo centromeres and
[77] Foltz, D.R., Jansen, L.E., Black, B.E., Bailey, A.O., Yates, J.R.
construction of first-generation human artificial microchromo-
and Cleveland, D.W. (2006) The human CENP-A centromeric
somes. Nat. Genet. 15, 345–355.
nucleosome-associated complex. Nat. Cell Biol. 8, 458–469.
[57] Howman, E.V., Fowler, K.J., Newson, A.J., Redward, S.,
[78] Schuh, M., Lehner, C.F. and Heidmann, S. (2007) Incorporation
MacDonald, A.C., Kalitsis, P. and Choo, K.H. (2000) Early
of Drosophila CID/CENP-A and CENP-C into centromeres
disruption of centromeric chromatin organization in centromere
during early embryonic anaphase. Curr. Biol. 17, 237–243.
protein A (Cenpa) null mice. Proc. Natl. Acad. Sci. USA 97,
[79] Jansen, L.E., Black, B.E., Foltz, D.R. and Cleveland, D.W.
(2007) Propagation of centromeric chromatin requires exit from
[58] Regnier, V., Vagnarelli, P., Fukagawa, T., Zerjal, T., Burns, E.,
mitosis. J. Cell Biol. 176, 795–805.
Trouche, D., Earnshaw, W. and Brown, W. (2005) CENP-A is
[80] Maddox, P.S., Hyndman, F., Monen, J., Oegema, K. and Desai,
required for accurate chromosome segregation and sustained
A. (2007) Functional genomics identifies a Myb domain-
kinetochore association of BubR1. Mol. Cell Biol. 25, 3967–
containing protein family required for assembly of CENP-A
chromatin. J. Cell Biol. 176, 757–763.
P. Vagnarelli et al. / FEBS Letters 582 (2008) 1950–1959
[81] Shelby, R.D., Monier, K. and Sullivan, K.F. (2000) Chromatin
requires TD-60, microtubules, and substrate priming phosphor-
assembly at kinetochores is uncoupled from DNA replication. J.
ylation. Science 319, 469–472.
Cell Biol. 151, 1113–1118.
[95] Baumann, C., Korner, R., Hofmann, K. and Nigg, E.A. (2007)
[82] Hayashi, T., Fujita, Y., Iwasaki, O., Adachi, Y., Takahashi, K.
PICH, a centromere-associated SNF2 family ATPase, is regu-
and Yanagida, M. (2004) Mis16 and Mis18 are required for
lated by Plk1 and required for the spindle checkpoint. Cell 128,
CENP-A loading and histone deacetylation at centromeres. Cell
118, 715–729.
[96] Skibbens, R.V. and Salmon, E.D. (1997) Micromanipulation of
[83] Fujita, Y., Hayashi, T., Kiyomitsu, T., Toyoda, Y., Kokubu, A.,
chromosomes in mitotic vertebrate tissue cells: tension controls
Obuse, C. and Yanagida, M. (2007) Priming of centromere for
the state of kinetochore movement. Exp Cell Res. 235, 314–324.
CENP-A recruitment by human hMis18alpha, hMis18beta, and
[97] Bouck, D.C. and Bloom, K. (2007) Pericentric chromatin is an
M18BP1. Dev. Cell 12, 17–30.
elastic component of the mitotic spindle. Curr. Biol. 17, 741–748.
[84] Bannister, A.J., Zegerman, P., Partridge, J.F., Miska, E.A.,
[98] Scott, K.C., Merrett, S.L. and Willard, H.F. (2006) A hetero-
Thomas, J.O., Allshire, R.C. and Kouzarides, T. (2001) Selective
chromatin barrier partitions the fission yeast centromere into
recognition of methylated lysine 9 on histone H3 by the HP1
discrete chromatin domains. Curr. Biol. 16, 119–129.
chromo domain. Nature 410, 120–124.
[99] Scott, K.C., White, C.V. and Willard, H.F. (2007) An RNA
[85] Nakayama, J., Rice, J.C., Strahl, B.D., Allis, C.D. and Grewal,
polymerase III-dependent heterochromatin barrier at
S.I. (2001) Role of histone H3 lysine 9 methylation in epigenetic
yeast centromere 1. PLoS ONE 2, e1099.
control of heterochromatin assembly. Science 292, 110–113.
[100] Nakano, M. et al. (2008) Inactivation of a human kinetochore
[86] Bernard, P., Maure, J.F., Partridge, J.F., Genier, S., Javerzat, J.P.
by specific targeting of chromatin modifiers. Dev. Cell 14, 507–
and Allshire, R.C. (2001) Requirement of heterochromatin for
cohesion at centromeres. Science 11, 11.
[101] Hill, A. and Bloom, K. (1987) Genetic manipulation of
[87] Sumara, I., Vorlaufer, E., Gieffers, C., Peters, B.H. and Peters,
centromere function. Mol. Cell. Biol. 7, 2397–2405.
J.M. (2000) Characterization of vertebrate cohesin complexes
[102] Ohzeki, J., Nakano, M., Okada, T. and Masumoto, H. (2002)
and their regulation in prophase. J. Cell Biol. 151, 749–
CENP-B box is required for de novo centromere chromatin
assembly on human alphoid DNA. J. Cell Biol. 159, 765–775.
[88] Waizenegger, I.C., Hauf, S., Meinke, A. and Peters, J.M. (2000)
[103] Hudson, D. et al. (1998) Centromere protein B null mice are
Two distinct pathways remove mammalian cohesin from chro-
mitotically and meiotically normal but have lower body and
mosome arms in prophase and from centromeres in anaphase.
testis weights. J. Cell Biol. 141, 309–319.
Cell 103, 399–410.
[104] Saffery, R. et al. (2001) Construction of neocentromere-based
[89] Chevillard, C., Reik, W., McDermott, M., Fontes, M., Mattei,
human minichromosomes by telomere-associated chromosomal
M.G. and Singh, P.B. (1993) Chromosomal localization of
truncation. Proc. Natl. Acad. Sci. USA 98, 5705–5710.
human homologs of the Drosophila heterochromatin protein 1
[105] Musacchio, A. and Salmon, E.D. (2007) The spindle-assembly
(HP1) gene. Mamm. Genome 4, 124–126.
checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–
[90] Vagnarelli, P.B. and Earnshaw, W.C. (2001) INCENP loss from
an inactive centromere correlates with the loss of sister chroma-
[106] DeLuca, J.G. (2007) Spindle microtubules: getting attached at
tid cohesion. Chromosoma 110, 393–401.
both ends. Curr. Biol. 17, 966–969.
[91] Polioudaki, H., Markaki, Y., Kourmouli, N., Dialynas, G.,
[107] Pearson, C.G., Yeh, E., Gardner, M., Odde, D., Salmon, E.D.
Theodoropoulos, P.A., Singh, P.B. and Georgatos, S.D. (2004)
and Bloom, K. (2004) Stable kinetochore–microtubule attach-
Mitotic phosphorylation of histone H3 at threonine 3. FEBS
ment constrains centromere positioning in metaphase. Curr.
Lett. 560, 39–44.
Biol. 14, 1962–1967.
[92] Dai, J., Sultan, S., Taylor, S.S. and Higgins, J.M. (2005) The
[108] Rieder, C.L., Davison, E.A., Jensen, L.W.C., Cassimeris, L. and
kinase haspin is required for mitotic histone H3 Thr 3
Salmon, E.D. (1986) Oscillatory movements of monoroiented
phosphorylation and normal metaphase chromosome alignment.
chromosomes and their position relative to the spindle pole
Genes Dev. 19, 472–488.
result from the ejection properties of the aster and half-spindle.
[93] Dai, J., Sullivan, B.A. and Higgins, J.M. (2006) Regulation of
J. Cell Biol. 103, 581–591.
mitotic chromosome cohesion by Haspin and Aurora B. Dev.
[109] Loncarek, J., Kisurina-Evgenieva, O., Vinogradova, T., Hergert,
Cell 11, 741–750.
P., La Terra, S., Kapoor, T.M. and Khodjakov, A. (2007) The
[94] Rosasco-Nitcher, S.E., Lan, W., Khorasanizadeh, S. and
centromere geometry essential for keeping mitosis error free is
Stukenberg, P.T. (2008) Centromeric
aurora-B activation
controlled by spindle forces. Nature 450, 745–749.
Source: http://files.jhoandriveras.webnode.com.ve/200000028-ac736ae685/Centromeres%20Old%20tales%20and%20new%20tools.pdf
Guatemala, viernes 25 de septiembre de 2015 El universo es fuego eterno que crece y decrece incesantemente, dejó sugerido Heráclito, el huraño oráculo de Éfeso (hoy Turquía) al norte de Mileto, en la Grecia antigua. De esta percepción diríase dialéctica, se ha derivado buena parte de las escuelas filosóficas que han nutrido el pensamiento occidental contemporáneo.
DICTAMEN Nº. 122/2010, de 7 de julio.* Expediente relativo a reclamación de responsabilidad patrimonial de la Administra-ción Sanitaria a instancia de D. K, D.ª C, D.ª T, D. Z, D.ª J y D.ª Q, como consecuencia de la asistencia sanitaria recibida por su hijo y hermano respectivamente, D. X, en el Área de Salud Mental del Servicio de Salud de Castilla-La Mancha (SESCAM).





