Acon *hcg
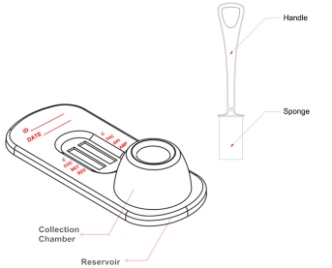
Ora-Check Complete 6 Drug Screen Device
Using an immunoassay cutoff level of 40 ng/mL, codeine can be detected in the oral fluid within 1 hour
following a single oral dose and can remain detectable for 7-21 hours after the dose2. Heroin metabolite
Package Insert for the AMP/MET/COC/OPI/THC/BZO
Materials Provided
6-monoacetylmorphine (6-MAM) is found more prevalently in excreted unmetabolized, and is also the
• Test devices
• Package insert
Test for Oral Fluids
major metabolic product of codeine and heroin.
A rapid, screening test for the simultaneous, qualitative detection of amphetamine, methamphetamine,
cocaine, opiates, THC ,BZO and their metabolites in human oral fluid.
For Forensic Use Only
The opiates assay contained within the Oral Fluid Drug Screen Device yields a positive result when the
DIRECTIONS FOR USE
opiates concentration in oral fluid exceeds 40 ng/mL.
Allow the test device, specimen, and/or controls to reach room temperature (15-30°C) prior to
testing. Instruct the donor to not place anything in the mouth including food, drink, gum or tobacco
products for at least 10 minutes prior to collection.
The Oral Fluid Drug Screen Device for AMP/MET/COC/OPI/THC/BZO is a lateral flow chromatographic
Tetrahydrocannabinol (THC), the active ingredient in the marijuana plant (cannabis sativa), is detectable
1. Specimen Collection
immunoassay for the qualitative detection of amphetamine, methamphetamine, cocaine, opiates, THC,
in oral fluid shortly after use. The detection of the drug is thought to be primarily due to the direct
2. Using the provided oral fluid swab, sweep the inside of the mouth for 3 minutes. The sponge will
BZO and their metabolites in oral fluids at the following cut-off concentrations:
exposure of the drug to the mouth (oral and smoking administrations) and the subsequent sequestering
gradually soften as oral fluid is absorbed, and should be completely saturated after 3 minutes.
of the drug in the buccal cavity.3 Historical studies have shown a window of detection for THC in oral fluid
3. Test Procedure
of up to 14 hours after drug use.3
4. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with
Amphetamine (MAMP)
patient or control identification. For best results, the assay should be performed within one hour.
The THC assay contained within the Oral Fluid Drug Screen Device yields a positive result when the
Methamphetamine (MET)
d-Methamphetamine
Insert the moistened swab firmly into the collection chamber. Press down firmly to release as much
Δ9-THC concentration in oral fluid exceeds 50 ng/mL.
liquid as possible.
6. Avoid trapping air bubbles in the specimen wells (S), and do not add any solution to the result areas.
Benzodiazepine (BZO)
Benzodiazepine (BZO):
7. As the test begins to work, color will migrate across the membrane.
Benzodiazepines are medications that are frequently prescribed for the symptomatic treatment of
8. Wait for the colored band(s) to appear. The result should be read at 5-10 minutes. Do not interpret
anxiety and sleep disorders. They produce their effects via specific receptors involving a neurochemical
the result after 20 minutes.
called gamma aminobutyric acid (GABA). Because they are safer and more effective, Benzodiazepines
This assay provides only a preliminary analytical test result. A more specific alternate chemical
have replaced Barbiturates in the treatment of both anxiety and insomnia. Benzodiazepines are also
method must be used in order to obtain a confirmed analytical result. Gas chromatography/mass
used as sedatives before some surgical and medical procedures, and for the treatment of seizure
spectrometry (GC/MS) and gas chromatography/tandem mass spectrometry (GC/MS/MS) are the
disorders and alcohol withdrawal.
preferred confirmatory methods. Professional judgment should be applied to any drug of abuse
test result, particularly when preliminary positive results are indicated.
The Oral Fluid Drug Screen Device for AMP/MET/COC/OPI/THC/BZO is an immunoassay based on the
The Oral Fluid Drug Screen Device for AMP/MET/COC/OPI/THC/BZO and their metabolites is a rapid, oral
principle of competitive binding. Drugs that may be present in the oral fluid specimen compete against
fluid screening test that can be performed without the use of an instrument. The test utilizes monoclonal
their respective drug conjugate for binding sites on their specific antibody.
antibodies to selectively detect elevated levels of specific drugs in human oral fluid.
During testing, a portion of the oral fluid specimen migrates upward by capillary action. A drug, if
Amphetamine (AMP)
present in the oral fluid specimen below its cut-off concentration, will not saturate the binding sites of its
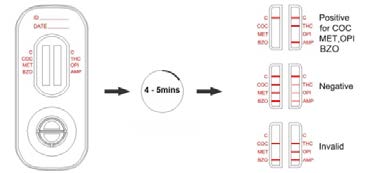
INTERPRETATION OF RESULTS
specific antibody. The antibody will then react with the drug-protein conjugate and a visible colored line
Amphetamine is a sympathomimetic amine with therapeutic indications. The drug is often self-
will show up in the test line region of the specific drug strip. The presence of drug above the cut-off
(Please refer to the previous illustration)
administered by nasal inhalation or oral ingestion. Depending on the route of administration,
concentration in the oral fluid specimen will saturate all the binding sites of the antibody. Therefore, the
NEGATIVE:* Two lines appear. One colored line should be in the control region (C), and another
amphetamine can be detected in oral fluid as early as 5-10 minutes following use1. Amphetamine can
colored line will not form in the test line region.
apparent colored line adjacent should be in the test region (Drug/T). This negative result indicates that
be detected in oral fluids for up to 72 hours after use1.
the drug concentration is below the detectable level.
A drug-positive oral fluid specimen will not generate a colored line in the specific test line region of the
*NOTE: The shade of color in the test line region (Drug/T) will vary, but it should be considered negative
The amphetamine assay contained within the Oral Fluid Drug Screen Device yields a positive result when
strip because of drug competition, while a drug-negative oral fluid specimen will generate a line in the
whenever there is even a faint line.
the amphetamine concentration in oral fluid exceeds 50 ng/mL.
test line region because of the absence of drug competition.
Methamphetamine (MAMP)
To serve as a procedural control, a colored line will always appear at the control line region, indicating
POSITIVE: One colored line appears in the control region (C). No line appears in the test region
that proper volume of specimen has been added and membrane wicking has occurred.
(Drug/T). This positive result indicates that the drug concentration is above the detectable level.
Methamphetamine is a potent stimulant chemically related to amphetamine but with greater CNS
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques
stimulation properties. The drug is often self-administered by nasal inhalation, smoking or oral
are the most likely reasons for control line failure. Review the procedure and repeat the test using a new
ingestion. Depending on the route of administration, methamphetamine can be detected in oral fluid as
The test contains membrane strips coated with drug-protein conjugates (purified bovine albumin) on the
test panel. If the problem persists, discontinue using the lot immediately and contact the manufacturer.
early as 5-10 minutes following use1. Methamphetamine can be detected in oral fluids for up to 72
test line, a goat polyclonal antibody against gold-protein conjugate at the control line, and a dye pad
hours after use1.
which contains colloidal gold particles coated with mouse monoclonal antibody specific to
Amphetamine, Methamphetamine, Benzoylecgonine, Morphine, Δ9- THC and BZO.
A procedural control is included in the test. A colored line appearing in the control region (C) is
The Methamphetamine assay contained within the Oral Fluid Drug Screen Device yields a positive result
considered an internal procedural control. It confirms sufficient specimen volume, adequate membrane
when the methamphetamine concentration in oral fluid exceeds 50 ng/mL.
wicking and correct procedural technique.
• For forensic use only. •
Do not use after the expiration date.
Cocaine is a potent central nervous system (CNS) stimulant and a local anesthetic derived from the coca
• The Oral Fluid test device should remain in the sealed pouch until use.
1. The Oral Fluid Drug Screen Device provides only a qualitative, preliminary analytical result. A
plant (erythroxylum coca). The drug is often self-administered by nasal inhalation, intravenous injection
• Saliva is not classified as biological hazard unless derived from a dental procedure.
secondary analytical method must be used to obtain a confirmed result. Gas chromatography/mass
and free-base smoking. Depending on the route of administration, cocaine and metabolites
• The used collector and device should be discarded according to federal, state and local regulations.
spectrometry (GC/MS) or gas chromatography/tandem mass spectrometry (GC/MS/MS) is preferred
benzoylecgonine and ecgonine methyl ester can be detected in oral fluid as early as 5-10 minutes
confirmatory methods.
following use1. Cocaine and benzoylecgonine can be detected in oral fluids for up to 24 hours after use1.
STORAGE AND STABILITY
2. A positive test result does not indicate the concentration of drug in the specimen or the route of
Store as packaged in the sealed pouch at 2-30°C. The test is stable through the expiration date printed
The cocaine assay contained within the Oral Fluid Drug Screen Device for cocaine and opiates yields a
on the sealed pouch. The test devices must remain in the sealed pouch until use. DO NOT FREEZE. Do
3. A negative result may not necessarily indicate a drug-free specimen. Drug may be present in the
positive result when the cocaine metabolite in oral fluid exceeds 20 ng/mL.
not use beyond the expiration date.
specimen below the cutoff level of the assay.
SPECIMEN COLLECTION AND PREPARATION
The drug class opiates refers to any drug that is derived from the opium poppy, including naturally
The oral fluid specimen should be collected using the collector provided with the kit. Follow the detailed
occurring compounds such as morphine and codeine and semi-synthetic drugs such as heroin. Opiates
Directions for Use below. No other collection devices should be used with this assay. Oral fluid collected
act to control pain by depressing the central nervous system. The drugs demonstrate addictive
at any time of the day may be used.
properties when used for sustained periods of time; symptoms of withdrawal may include sweating,
shaking, nausea and irritability. Opiates can be taken orally or by injection routes including intravenous, intramuscular and subcutaneous; illegal users may also take the intravenously or by nasal inhalation.

o-Hydroxyhippuric acid
PERFORMANCE CHARACTERISTICS
p-Hydroxytyramine
d/l-Isoproterenol
Analytical Sensitivity
A Phosphate-buffered saline (PBS) pool was spiked with drugs to target concentrations of ± 50% cut-off
and ± 25% cut-off and tested with the Oral Fluid Drug Screen Device. The results are summarized below.
Morphine 3-β-D-Glucuronide
d-Norpropoxyphene
Pentazocine hydrochloride
Diacetylmorphine (Heroin)
6-Monoacetylmorphine
Trans-2-phenylcyclopropylamine hydrochloride
Phenylpropanolamine
Benzodiazepine (BZO)
d-Pseudoephedrine
Chlordiazepoxide
Tetrahydrocortisone 3-acetate
Tetrahydrocortisone 3 (β-D-glucuronide)
Desalkyflurazepam
Analytical Specificity
The following table lists the concentration of compounds (ng/mL) above which the Oral Fluid Drug
Screen device for AMP/MET/COC/OPI/THC/PCP identified positive results at a read time of 10 minutes.
1. Moolchan, E., et al, "Saliva and Plasma Testing for Drugs of Abuse: Comparison of the Disposition and
Pharmacological Effects of Cocaine", Addiction Research Center, IRP, NIDA, NIH, Baltimore, MD. As
presented at the SOFT-TIAFT meeting October 1998.
2. Kim, I, et al, "Plasma and oral fluid pharmacokinetics and pharmacodynamics after oral codeine
administration", Clin Chem, 2002 Sept.; 48 (9), pp 1486-96.
3. Schramm, W. et al, "Drugs of Abuse in Saliva: A Review," J Anal Tox, 1992 Jan-Feb; 16 (1), pp 1-9
4. McCarron, MM, et al, "Detection of Phencyclidine Usage by Radioimmunoassay of Saliva," J Anal Tox.
Ecgonine methyl ester
1984 Sep-Oct.; 8 (5), pp 197-201.
AMPHETAMINE (AMP)
Cross-Reactivity
A study was conducted to determine the cross-reactivity of the test with compounds spiked into drug-
free PBS stock. The following compounds demonstrated no false positive results on the Oral Fluid Drug
ß-Phenylethylamine
Screen Device when tested with at concentrations up to 100 µg/mL.
p-Hydroxyamphetamine
(+)3,4-Methylenedioxyamphetamine (MDA)
N-Acetylprocainamide
Acetylsalicylic acid
METHAMPHETAMINE (MET)
d-Methamphetamine
Andatech Corporation Pty Ltd
d/l-Brompheniramine
Methoxyphenamine
Nunawading VIC 3131
3,4-Methylenedioxymethamphetamine (MDMA)
d/l-Chloropheniramine
(1R,2S) - (-) Ephedrine
MARIJUANA (THC)
Deoxycorticosterone
Dextromethorphan
[email protected]
11-nor-Δ9 - THC -9 COOH
Estrone-3-sulfate
Ethyl-p-aminobenzoate
l(–)-Epinephrine
Hydrochlorothiazide
Source: http://documents.andatech.com.au/manuals/ora-check-complete-6-1-saliva-drug-test-cassette.pdf
Contents lists available at Epilepsy & Behavior Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models Adriana Monserrath Orellana-Paucar , Ann-Sophie K. Serruys Tatiana Afrikanova , Jan Maes ,Wim De Borggraeve Jo Alen Fabián León-Tamariz Isabel María Wilches-Arizábala ,Alexander D. Crawford Peter A.M. de Witte , Camila V. Esguerra a Laboratory for Molecular Biodiscovery, Department of Pharmaceutical & Pharmacological Sciences, University of Leuven, Leuven, Belgiumb Escuela de Bioquímica y Farmacia, Facultad de Ciencias Químicas, Universidad de Cuenca, Cuenca, Ecuadorc Molecular Design and Synthesis, Department of Chemistry, University of Leuven, Leuven, Belgium
iPF670 shown with stand Designed for low-volume printing, these printers offer affordable AVAILABLE WORKFLOW SOLUTIONS solutions that can support a variety • Mobile printing app*• Direct Print & Share** of applications including: posters, • PosterArtist Lite presentations, technical documents, • Print Plug-in for Microsoft Office®, and more!






