Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Anticonvulsant activity of bisabolene sesquiterpenoids of curcuma longa in zebrafish and mouse seizure models

Contents lists available at
Epilepsy & Behavior
Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish
and mouse seizure models
Adriana Monserrath Orellana-Paucar , Ann-Sophie K. Serruys Tatiana Afrikanova , Jan Maes ,Wim De Borggraeve Jo Alen Fabián León-Tamariz Isabel María Wilches-Arizábala ,Alexander D. Crawford Peter A.M. de Witte , Camila V. Esguerra a Laboratory for Molecular Biodiscovery, Department of Pharmaceutical & Pharmacological Sciences, University of Leuven, Leuven, Belgiumb Escuela de Bioquímica y Farmacia, Facultad de Ciencias Químicas, Universidad de Cuenca, Cuenca, Ecuadorc Molecular Design and Synthesis, Department of Chemistry, University of Leuven, Leuven, Belgium
Turmeric, obtained from the rhizomes of Curcuma longa, is used in South Asia as a traditional medicine for the
Received 20 December 2011
treatment of epilepsy. To date, in vivo studies on the anticonvulsant activity of turmeric have focused on its
Revised 16 February 2012
principal curcuminoid, curcumin. However, poor absorption and rapid metabolism have limited the thera-
Accepted 21 February 2012
peutic application of curcumin in humans. To explore the therapeutic potential of turmeric for epilepsy fur-
Available online 5 April 2012
ther, we analyzed its anticonvulsant activity in a larval zebrafish seizure assay. Initial experiments revealedthat the anticonvulsant activity of turmeric in zebrafish larvae cannot be explained solely by the effects of
Keywords:Curcuma longa
curcumin. Zebrafish bioassay-guided fractionation of turmeric identified bisabolene sesquiterpenoids as ad-
ditional anticonvulsants that inhibit PTZ-induced seizures in both zebrafish and mice. Here, we present the
first report of the anticonvulsant properties of bisabolene sesquiterpenoids and provide evidence which war-
rants further investigation toward the mechanistic understanding of their neuromodulatory activity.
2012 Elsevier Inc. All rights reserved.
Ar-turmeroneα-AtlantonePentylenetetrazoleZebrafish PTZ modelMouse PTZ model
depression, cognitive impairment and the fact that seizures inabout one third of patients suffering from epilepsy remain resistant
Epilepsy is a widespread neurological disorder that affects approx-
to these existing treatments Hence, there is a clear need to con-
imately 50 million people worldwide . According to the World
tinue to identify novel AEDs that effectively control pharmaco-
Health Organization (WHO), about 1% of the total burden of disease
resistant seizures with minimal or no adverse effects.
corresponds to different forms of epilepsy. Its pharmacological treat-
Numerous studies point to medicinal plants as an interesting
ment comprises a number of currently available antiepileptic drugs
source of novel AEDs . One interesting example in this regard is
(AEDs). The main problems concerning AEDs are the high incidence
losigamone derived from the kava kava plant and originally used by
of side effects ranging from gastrointestinal distress, hepatotoxicity,
traditional healers in the South Pacific as an anxiolytic, which isnow in early clinical development as a novel AED . Likewise,Curcuma longa, a perennial herb of the Zingiberaceae family native
Abbreviations: AEDs, antiepileptic drugs; DAD, diode array detection; DMSO, di-
to South Asia, has been used not only as a condiment and color addi-
methyl sulfoxide; dpf, days post-fertilization; ddH2O, double-distilled water; ESI-MS,
tive in food but also in traditional medicine against seizures . The
electrospray ionization mass spectrometry; i.v., intravenous; i.p., intraperitoneal; LC–
major active chemical constituents of turmeric (C. longa rhizome
MS, liquid chromatography–mass spectroscopy; MES, maximal electroshock seizure;
powder) are the curcuminoids (3–5%) and turmeric oil (2–7%). Tur-
MTC, maximum tolerated concentration; PEG, polyethylene glycol; p.o., "per os", orally;PTZ, pentylenetetrazole; RP-HPLC, reverse phase-high performance liquid chromatog-
meric oil is mainly composed of bisabolene sesquiterpenoids: ar-,
raphy; s.c., subcutaneous; TLE, temporal lobe epilepsy.
α,β-turmerone and α-atlantone, whereas the curcuminoids include
⁎ Corresponding author at: Laboratory for Molecular Biodiscovery, Department of
curcumin, monodemethoxycurcumin and bisdemethoxycurcumin
Pharmaceutical and Pharmacological Sciences, University of Leuven, Gasthuisberg
Nearly all investigations on the medicinal properties of turmeric
Campus, Building O&N2, Room 08.4226, Herestraat 49, P.O. Box 824, BE-3000 Leuven,
have been focused on curcumin, and its anticonvulsant activity has
Belgium. Fax: + 32 16 323460.
E-mail address: (P.A.M. de Witte).
been demonstrated in several rodent models such as the iron-
1525-5050/$ – see front matter 2012 Elsevier Inc. All rights reserved.
doi:
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
induced epileptogenesis maximal electroshock kainic acid-
A "voucher specimen" serves as a reliable reference for the identi-
induced and pentylenetetrazole-kindling models. Although
ty of botanical samples for further investigations. For this reason, a
its anticonvulsant properties have been demonstrated, phase I clinical
voucher specimen of turmeric (no. 7850) was deposited in the
trials have revealed important pharmacokinetic limitations for curcu-
Herbarium Azuay (HA), Universidad del Azuay, Cuenca, Ecuador.
min. When administrated p.o., curcumin is poorly absorbed throughthe gut. Therefore, the amount of curcumin in the circulation is very
2.3. Experimental animals
low. Thus, pharmacokinetic issues have limited its therapeutic appli-cations. For this reason, formulation studies have been performed to
All procedures for animal experiments were performed in
enhance curcumin bioavailability The fact that nearly all the
accordance with European and National Regulations and ap-
studies have been conducted on curcumin has led to the assumption
proved by the Animal Care and Use Committee of the University of
that the anticonvulsant properties of turmeric are due solely to its ac-
Leuven (Belgium).
tivity. However, turmeric oil could also be accountable for its anticon-vulsant activity as other essential oils have been shown to be effective
2.3.1. Zebrafish (D. rerio)
in controlling seizure generation Intriguingly, a few studies
Adult zebrafish of the Tg (fli 1a: EGFP)y1 strain were reared at
have reported on the neuroprotective activity of turmeric oil
28.5 °C on a 14/10-hour light/dark cycle. Eggs were collected from
In this paper, we report on the anticonvulsant activity of turmeric
natural breeding and fostered in embryo medium (17 mM NaCl,
methanolic extract and its constituents as assessed in a zebrafish PTZ-
2 mM KCl, 1.8 mM Ca(NO3)2, 0.12 mM MgSO4, 1.5 mM HEPES buffer
induced seizure model. Further confirmation of this activity was car-
pH 7.1–7.3 and 0.6 μM methylene blue) in an incubator at 28.5 °C.
ried out in the mouse PTZ model.
Zebrafish are considered ‘embryos' between 0 and 72 hours post-
The zebrafish (Danio rerio) has emerged over the last decade as a
fertilization (hpf). From 72 hpf to 14 days post-fertilization (dpf),
valuable model for genetic studies and drug screening. The strength
they are referred to as ‘early larvae' or ‘larvae' Sorting of
of this in vivo model relies on its high genetic, physiologic and phar-
zebrafish embryos and larvae and medium refreshment were
macologic homology to humans. Their high fecundity and small size
performed daily until 7 dpf. After completing the experiments, all
allow for the performance of tests in a medium- to high-throughput
larvae were sacrificed through administration of an overdose of
fashion using minute (microgram scale) quantities of compound
. The zebrafish also holds promise as an in vivo model for iden-tifying novel neuroactive compounds since the dopaminergic, seroto-
2.3.2. Mice (Mus musculus)
nergic, and GABAergic systems develop early during embryogenesis
Male C57Bl/6 mice (20–30 g) at 8 weeks of age were acquired from
and are already functional in larvae . Additionally, their rapid de-
Charles River Laboratories. The mice were housed in poly-acrylic cages
velopment ex utero and optical transparency make it possible to easily
under 12/12-hour light/dark cycle at 28 °C in a quiet room. The
detect morphological and behavioral effects of test compounds on liv-
animals were fed ad libitum with a pellet diet and water until they
ing embryos and larvae . More recently, zebrafish have also prov-
were 10 weeks old (the age when all assays were carried out).
en useful for the primary screening of potential novel anticonvulsantsbased on the proconvulsant pentylenetetrazole (PTZ) . Like-
2.4. Preparation of methanolic extract of turmeric
wise, PTZ has been used as a chemoconvulsant in rodent models,and the timed PTZ infusion test has proven competence in enabling
Turmeric (5 g) was extracted through maceration in methanol
the identification of AEDs with different mechanisms of action
(50 ml). The extract was concentrated using a rotary evaporator
In this study, we confirmed the reported anticonvulsant properties
(Büchi Rotavapor R-114, Germany) to obtain a yield of 0.20 g.
of turmeric and curcumin. Further testing of turmeric oil and its chro-matographic fractions revealed additional constituents capable of
2.5. Preparation of turmeric volatile oil and isolation of major
suppressing PTZ-induced seizure behaviors in larval zebrafish. Mass
spectrometry and NMR analyses of these active purified fractionsrevealed them to belong to the class of bisabolene sesquiterpenoids:
Volatile oil from turmeric was obtained by subjecting 4 × 100 g
ar-turmerone, α,β-turmerone and α-atlantone. The anticonvulsant
of turmeric to hydro-distillation using a Clevenger-type apparatus
activity of turmeric oil, ar-turmerone and α,β-turmerone, identified
for 3 h. A pale yellowish and odiferous oil was obtained (8.56 g).
using the zebrafish PTZ assay, was then confirmed in the equivalent
Turmeric oil was dried over anhydrous sodium sulfate and stored at
mouse PTZ-induced seizure model.
4 °C until used.
The isolation of some major compounds present in the essential
2. Materials and methods
oil was carried out by semi-preparative RP-HPLC, as adapted fromthe work of He and colleagues A LaChrom Elite HPLC System
2.1. Chemicals and reagents
(VWR Hitachi) equipped with diode array detection (DAD) and anEconosphere 10-μm C18 (250×10 mm) reversed phase column
Dimethyl sulfoxide (99.9%, spectroscopy grade) and the curcumi-
(Grace Davison Discovery Sciences, Belgium) attached to an
noid mixture from turmeric (98%; curcumin, demethoxycurcumin
Econosphere 10 μm C18 (33×7 mm) guard column (Grace Davison
and bisdemethoxycurcumin) were procured from Acros Organics.
Discovery Sciences, Belgium) were used. Amounts of 10-mg volatile
Diethyl ether (99.9%, spectroscopy grade), deuterated chloroform
oil were repeatedly processed. The column was operated at a flow
(99.8% D, contains 0.1% (v/v) TMS) and PTZ were obtained from
rate of 5 ml/min at room temperature. The profile of the gradient elu-
Sigma-Aldrich, methanol (99.8%, reagent grade) and acetonitrile
tion was: double-distilled water (ddH2O) (A) and acetonitrile (B);
(100%, HPLC grade) from Fisher Scientific. Sodium valproate was
0–15 min, 40–60% B; 15–20 min, 60–100% B. The analytes were moni-
obtained from Sanofi-Synthelabo.
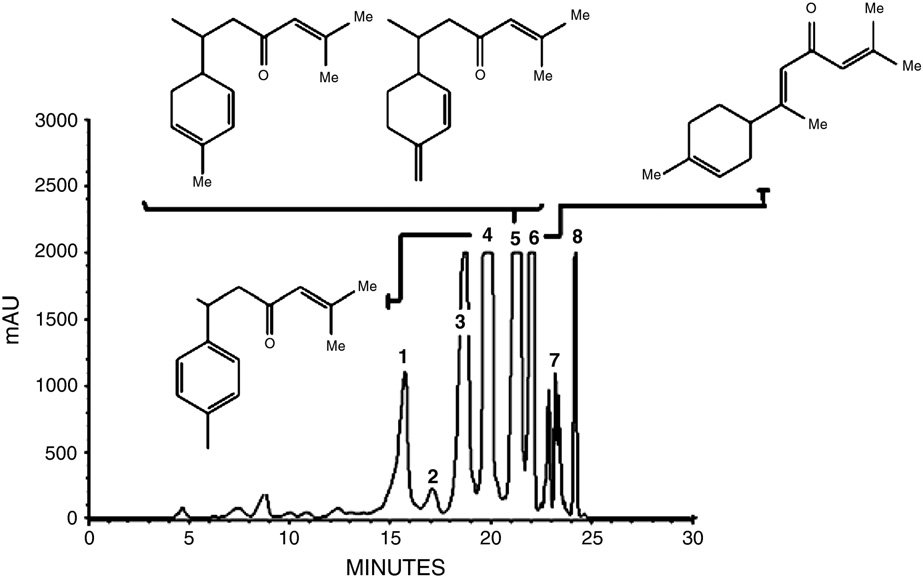
tored with DAD at 260 nm. Eight fractions from turmeric oil were indi-vidually collected (). Solvents from the collected fractions were
2.2. Plant material
removed by separation between diethyl ether and ddH2O. The etherphase was collected and dried over anhydrous sodium sulfate. Ether
Dried rhizome powder of C. longa (turmeric) was acquired from a
was removed by passing a slow stream of nitrogen over the sample at
local supplier in Belgium with India as the source of origin. Micro-
room temperature. The concentrated samples were stored at 4 °C until
scopic authentication according to was completed by R. Ansaloni.
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
larva per well. Each stock solution of sample (dissolved in 100%
Fractions collected from the RP-HPLC analysis of
DMSO) was diluted in embryo medium to achieve a final DMSO con-
turmeric oil. The collected fractions are referred
centration of 1%. In cases where sample had to be diluted further (i.e.
as percentage (%) of content in turmeric oil.
more than 1:100), DMSO was added so as to maintain a final concen-
Fraction no.
tration of 1%. Each sample was loaded in duplicate adjacent rows. The
larvae were then incubated at room temperature under dark and
quiet conditions for 1 h. Equal volumes (100 μl) of embryo medium
(vehicle) and 40-mM PTZ were then added respectively to the first
and second rows of each sample group (resulting in a doubling of
the total incubation volume and halving of the final DMSO concentra-
tion to 0.5% and PTZ to 20 mM). The total locomotor activity of each
larva was video-tracked and assessed in the presence of either vehicle
Fraction 4: ar-turmerone; fraction 5: α,β-turmerone; fraction 6: α-atlantone.
2.6. Chemical structure elucidation of bisabolene sesquiterpenoids
2.6.1. Nuclear magnetic resonance (NMR) analysis
1H and 13C NMR spectra of fractions 4, 5 and 6 (see below) were
obtained from Bruker 300 Avance and Bruker 600 Avance II+ equip-
ment using deuterated chloroform as solvent and tetramethylsilane
(TMS) as internal standard.
2.6.2. Mass spectroscopy (MS) analysis
Total movement in 30 min
The LC–MS analysis was performed on an Agilent 1100 system
equipped with degasser, quaternary pump, auto-sampler, UV-DAD
detector and thermostated column module coupled to Agilent 6110
3.1 µg/mL
6.2 µg/mL 12.5 µg/mL
single–quadrupole MS. Data acquisition and quantification wereobtained from Agilent LC/MSD Chemstation software. Fractions 4, 5
3.1 µg/mL + PTZ
6.2 µg/mL + PTZ
12.5 µg/mL + PTZ
and 6 (see further) were analyzed on a Grace Prevail RP-C18 column
3 μm (150 mm × 2.1 mm) at a flow rate of 0.2 ml/min. The LC gradient
comprised two solvents: double-distilled water (ddH2O) + 0.1% for-mic acid (A) and acetonitrile (B); 0–17 min, 40–60% B; 17–32 min,60–100% B.
The ESI-MS analysis was completed in a Thermo Electron LCQ Ad-
vantage apparatus with Agilent 1100 pump and injection system
coupled to Xcalibur data analysis software.
2.7. Determination of maximum tolerated concentration in zebrafish
Total movement in 30 min
The aim of this assay was to determine the range of appropriate
2.5 µg/mL
10 µg/mL
concentrations to be tested in zebrafish for the evaluation of anticon-vulsant activity. Seven-dpf larvae were placed into a 24-well plate
5 µg/mL + PTZ
2.5 µg/mL + PTZ
10 µg/mL + PTZ
(tissue culture plate, flat bottom, FALCON®, USA), six larvae per
well. They were incubated with different concentrations of sample
dissolved in 1 ml of embryo medium (at a final DMSO concentrationof 1%). The larvae were examined during a period of 24 h in sampleand compared to control group to detect the following signs of toxic-
ity: absence of startle response to plate taps, changes in heart rate orcirculation, presence of edema, loss of posture, paralysis and death.
Thus, the maximum tolerated concentration (MTC) was defined as
the highest concentration at which no signs of toxicity were observedin 6 out of 6 zebrafish larvae within 24 h of exposure to sample.
Total movement in 30 min
2.8. Evaluation of anticonvulsant activity in the zebrafish PTZ model
Zebrafish larvae from 7 dpf were monitored using the ViewPoint
2.5 µg/mL
10 µg/mL
VideoTrack System for Zebrafish™ (Version 2.3.1.0, ViewPoint,
5 µg/mL + PTZ
2.5 µg/mL + PTZ
10 µg/mL + PTZ
France). The system consists of an infrared light source, a high-resolution digital video camera to capture larval movements within
Fig. 1. Evaluation of the anticonvulsant activity of turmeric in the zebrafish PTZ seizure
a defined time period (30 min in our experimental set-up) and the
assay. (A) Turmeric methanolic extract; (B) curcuminoids and (C) turmeric oil. Tested
software to analyze larval locomotor activity.
concentrations are indicated along the x-axis, and the total gross locomotor activityexhibited by zebrafish larvae within 30 min is displayed along the y-axis. Data are
The highest concentrations of the samples tested correspond to
expressed as the mean ± SD (n = 10–12). Statistically significant differences between
the previously determined MTC values. Zebrafish larvae were placed
vehicle-treated and sample-treated (white bars) or PTZ-treated and sample plus PTZ-
in a tissue culture, flat bottom 96-well plate (FALCON®, USA); one
treated groups (gray bars) are labeled as * for p b0.05 and ** for p b 0.01.
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
only, vehicle with sample, PTZ only, or PTZ and sample (see Supple-
glass syringes containing: a) sample; heparin 2 UI/ml and b) PTZ
mentary Fig. S1). Video-tracking of larval movements commenced
(7.5 mg/ml ddH2O; heparin 2 UI/ml). These syringes were mounted
exactly 5 min after addition of embryo medium or PTZ to the wells
on a motor-driven infusion double pump (ALADOIN-1000 11VDC,
and was recorded for 30 min. A total of 8 wells in each plate were
0.75 Å, World Precision Instruments). Thus, 100 μl of control vehicle
left without larvae (medium only) as a negative control, so that
(PEG200:DMSO 1:1; heparin 2 UI/ml) or sample dissolved in vehicle
each experimental parameter consisted of 10 to 12 larvae. Results
were i.v. infused at a rate of 50 μl/min for 2 min. Ten minutes later,
were registered as the average value of the total time of larval move-
mice were released from the restrainer and placed in a transparent
ment during 30 min.
poly-acrylic cage (32 × 14 × 12.5 cm) for observation. Pentylenetetra-zole was then infused at a rate of 150 μl/min. Seizure manifestation
2.9. EEG recordings
stages in mice were scored according to the time between the startof PTZ infusion and the following behavioral events: ear, tail and
Zebrafish larvae were allowed to swim in 400 μl of either 10 μg/ml
myoclonic twitch, forelimb clonus, falling, tonic hindlimb extension
turmeric oil or vehicle for 1 h in a well of a 24-well plate. An equal
and death Under these conditions, i.v. infused PTZ triggered
volume of 40-mM PTZ was then added to the well. After a 15-
all aforementioned behavioral parameters, culminating in death of
minute exposure, the larvae were embedded in a 2% low-melting-
mice treated with vehicle, approximately 3 min after the start of infu-
point agarose. A glass electrode filled with artificial cerebrospinal
sion. For this reason, behavior was observed up to a maximum of
fluid composed of: 124 mM NaCl, 2 mM KCl, 2 mM MgSO4, 2 mM
5 min of PTZ infusion. Any surviving mice were then sacrificed. Penty-
CaCl2, 1.25 mM KH2PO4, 26 mM NaHCO3 and 10 mM glucose (resis-
lenetetrazole doses were calculated according to the formula: PTZ
tance 1–5 MΩ), was placed into the optic tectum. Recordings were
dose [mg/kg] =(PTZ concentration [mg/ml] × infusion rate [ml/
performed in current clamp mode, low-pass filtered at 1 kHz, high-pass
s] × infusion duration [s] × 1000)/mouse weight [g].
filtered at 0.1 Hz, digital gain 10, and sampling interval 10 μs (MultiClamp700B amplifier, Digidata 1440A digitizer, both Axon Instruments, USA).
2.11. Statistical analysis
The recordings started each time exactly 5 min after removal from theproconvulsant bath and were continued for 10 min.
All statistical analyses were performed using GraphPad Prism 5
A spike was defined as interictal-like if its amplitude exceeded
software (GraphPad Software, Inc.). Values were presented as means
three times the background and lasted for less than 3 s. Longer
± standard deviation (SD). The locomotor activity of zebrafish larvae
discharges were counted as ictal-like ones (Fig. S2). We quantified
was evaluated using one-way ANOVA followed by Dunnett's multiple
the number and average duration of either type of electrographic
comparison test. Statistically significant differences (pb0.05) between
activity as well as the total cumulative duration of all forms of
a treatment group and the equivalent control groups (vehicle or PTZ)
epileptiform discharges during the ten-minute recordings using
were considered indicative of decrease or increase in locomotor ac-
Clampfit 10.2 software.
tivity of zebrafish larvae. For evaluation of the EEG data and the mouseexperiments, significant differences (pb 0.05) between estimated time in-
2.10. Generation of PTZ-induced seizures in mice: timed i.v. PTZ infusion
tervals prior to above-mentioned seizure stages were calculated using the
unpaired Student's t-test.
Mice were randomly divided into groups of five animals (vehicle
and sample). The animals were isolated into individual cages andpre-warmed in front of an infrared lamp for 10 min to dilate the tail
3.1. Evaluation of the anticonvulsant activity of turmeric
veins. They were then placed in a restrainer, and the lateral tail veinwas catheterized with a 1-cm long, 29-gauge needle. After confirming
The analysis of the methanolic extract of turmeric (C. longa rhi-
correct placement, the needle was secured to the tail with surgical
zome powder) revealed anticonvulsant activity in the zebrafish larval
tape and kept in place until the end of the test. The needle attached
PTZ assay (In order to identify the active constituents present
to a 0.7-m long polyethylene catheter was connected to two 2.5-ml
in the methanolic extract of turmeric, the anticonvulsant properties
Fig. 2. HPLC chromatogram of turmeric oil and its major constituents. Peak 4 corresponds to ar-turmerone; peak 5 to α,β-turmerone and peak 6 to α-atlantone.
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
of curcuminoids and turmeric oil were also assessed through video-
tracking analysis (Curcuminoids showed anticonvulsant activ-
ity at 2.5 μg/ml (p b0.05) and at 5 and 10 μg/ml (p b 0.01) in our larvalPTZ assay. Further analysis uncovered an additional anticonvulsant
activity for turmeric oil. The larvae showed a decrease (p b 0.01) ofPTZ-induced convulsions after exposure to turmeric oil (10 μg/ml).
Notably, exposure of zebrafish larvae to curcuminoids or turmeric
oil alone (i.e. in the absence of proconvulsant) also resulted in a slightincrease in locomotor activity compared to vehicle-treated controls(B,C). However, no obvious signs of toxicity (as measured by
change in heart rate, loss of posture, lack or delay in response to tac-
tile stimuli, or death (see Methods, were observed in
these larvae.
3.2. Isolation and evaluation of the anticonvulsant activity of bisabolene
sesquiterpenoids from turmeric oil
To purify the active constituents in turmeric oil, we performed
semi-preparative RP-HPLC analysis. Eight peaks were identified(and individually collected. From the eight peaks collected,fractions 2 and 7 were not tested in the zebrafish PTZ assay since
the collected amounts were not sufficient for carrying out the assay
(). Fractions 1, 3 and 8 did not display any anticonvulsant ac-tivity in the zebrafish PTZ assay (data not shown). A significant de-
crease in the convulsions triggered by PTZ was observed for
fractions 4, 5 and 6. Fraction 4 showed anticonvulsant activity at
46 μM (p b 0.05), fraction 5 at 23 μM (pb 0.01) and fraction 6 at con-
centrations of 23 μM (p b 0.05) and 46 μM (pb 0.01) Sodiumvalproate, a well-known AED, was included as a positive control for
the zebrafish PTZ assay . Sodium valproate showed significantactivity at 250 μM (p b0.05) and 500 μM and 1 mM (p b 0.01) (Supple-mentary Fig. S4A).
Fractions 4, 5 and 6 that showed seizure inhibitory activity in
the larval zebrafish PTZ assay were further analyzed for chemical
structure elucidation. 1H and 13C NMR spectra of fraction 4 are in
agreement with reported values for ar-turmerone Nuclearmagnetic resonance analysis indicated fraction 5 as a mixture (1:1)of two isomeric structures identified as α,β-turmerone by 1D- and
2D-NMR analysis Fraction 6 was identified as α-atlantone
(probably the E-isomer) based on 1H and 13C NMR spectra
(data not shown).
Once again, as observed for the curcuminoid and turmeric oil-
treated larvae, exposure of zebrafish larvae to α,β-turmerone, ar-
Fig. 3. Evaluation of the anticonvulsant activity of bisabolene sesquiterpenoids in thezebrafish PTZ seizure assay. (A) Ar-turmerone; (B) α,β-turmerone and (C) α-
turmerone or α-atlantone alone also resulted in a slight increase in
atlantone. The x-axis represents the tested concentration for each one of the sesquiter-
locomotor activity compared to vehicle-treated controls ().
penoids. The y-axis indicates the total gross locomotor activity exhibited by zebrafish
However, no obvious signs of toxicity were observed. This raised the
larvae within 30 min. Data are expressed as the mean ± SD (n = 10–12). Statistically
question as to whether turmeric oil or its individual constituents
significant differences between vehicle-treated and sample-treated (white bars) or
could perhaps exhibit mild proconvulsant activity.
PTZ-treated and sample plus PTZ-treated groups (gray bars) are labeled as * forp b 0.05 and ** for p b 0.01.
To determine whether turmeric oil can act as a proconvulsant on
zebrafish larvae and to confirm whether the decrease in convulsion-like movements observed in larvae co-treated with turmeric oil andPTZ was truly an inhibition of seizure activity, we carried out open-field recordings from larval brains. The analysis of electrographic ac-
3.3. Evaluation of the anticonvulsant activity of turmeric, turmeric oil
tivity of larvae showed that exposure to 10 μg/ml of turmeric oil
and bisabolene sesquiterpenoids in the mouse PTZ-induced seizure model
partly protects the larvae from PTZ-induced seizures. Turmeric oilproduced no effect on the number and duration of interictal-like
The anticonvulsant activity of curcumin has been described previ-
spikes compared to controls ,B,H,I; Supplementary Figs.
ously in mice but no assay for turmeric oil has evaluated its
S3C,D) but significantly reduced both the number and the duration
anticonvulsant properties before. Thus, further confirmation of the
of ictal-like discharges (,H,I). Moreover, the latter were ab-
positive results obtained in zebrafish with turmeric oil and the bisa-
sent in 3 out of five recordings (Supplementary Fig. S3D). In addition,
bolene sesquiterpenoids was performed in the mouse assay. Mice
the cumulative duration of all forms of epileptiform discharges
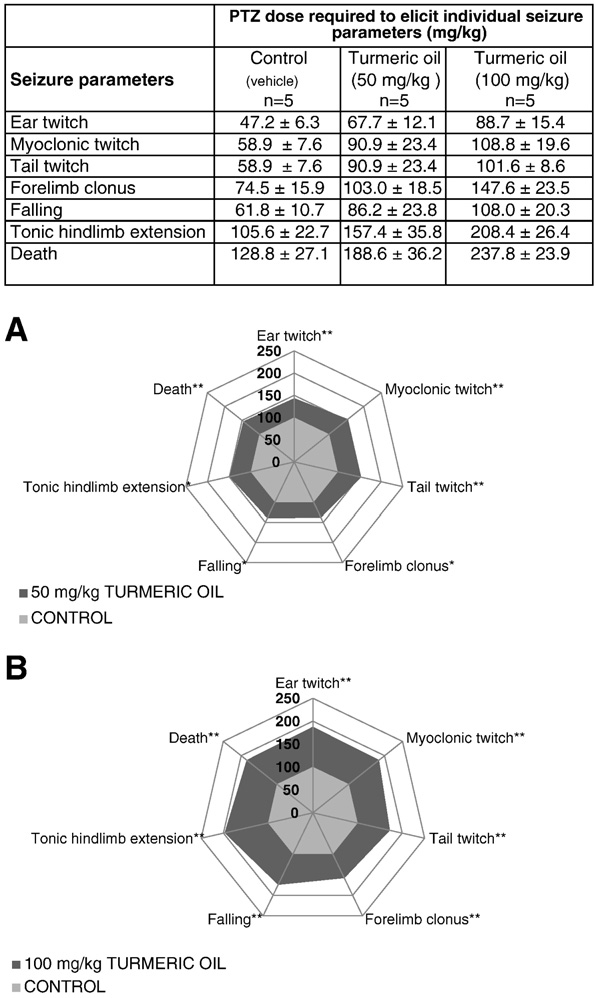
treated with turmeric oil (50 mg/kg) showed a significant increase
quantified was shortened after turmeric oil exposure E). Im-
in PTZ doses required to trigger all behavioral endpoints: forelimb
portantly, turmeric oil on its own did not induce epileptiform dis-
clonus, falling and tonic hindlimb extension (p b 0.05) and ear, myo-
charges in our experimental set-up (F,G; Supplementary
clonic, tail twitch, and death (p b0.01), compared to control group
Figs. S3A,B).
(). Moreover, a dose of 100-mg/kg turmeric oil in the mouse
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
PTZ assay exhibited significant activity in delaying seizure generation
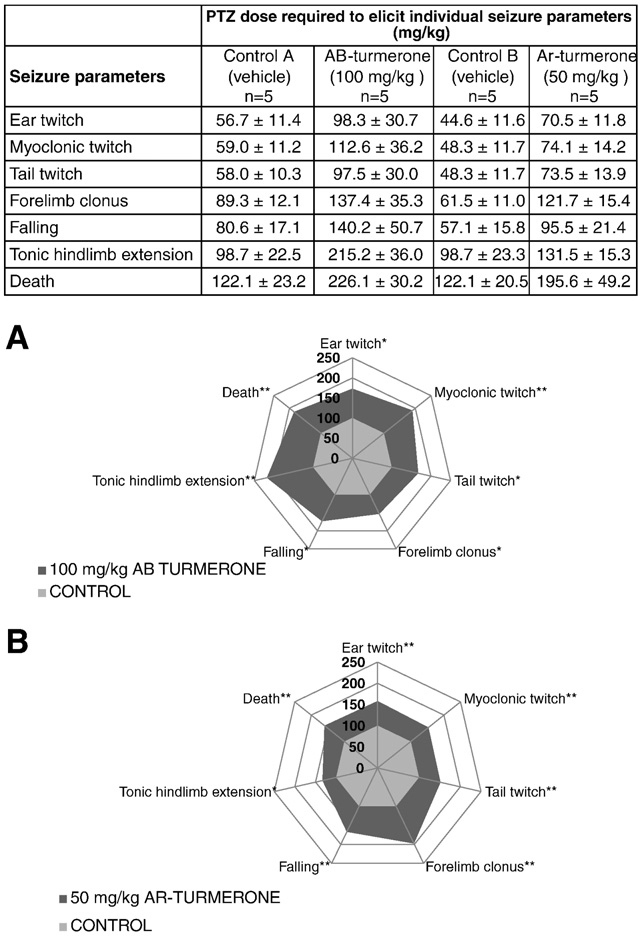
for all seizure parameters and death as compared to control (p b 0.01)Regarding the active bisabolene sesquiterpenoids, ar-
Turmeric, the powdered rhizomes of C. longa, has been used for
turmerone and α,β-turmerone were assessed using the mouse PTZ
centuries as a food condiment but also as an ethnomedical treatment
seizure model ). Mice infused with a dose of 50 mg/kg of ar-
against seizures. We evaluated the anticonvulsant activity of turmeric
turmerone exhibited significant resistance to the generation of sei-
methanolic extract in the zebrafish PTZ-induced seizure model
zures leading to an increase in the required dose of PTZ to trigger all
with positive results. This finding is in line with the anticonvulsant
assessed events: tonic hindlimb extension (p b 0.05) and ear, myo-
properties of turmeric described in previous studies. In these studies,
clonic and tail twitch, forelimb clonus, falling and death (p b0.01).
curcumin has often been cited as the principal substance responsible
Likewise, the anticonvulsant activity of α,β-turmerone was evaluat-
for the activity of turmeric Nevertheless, bioavailability
ed, and positive results were also found with a dose of 100 mg/kg
analysis of curcumin evidenced poor absorption, rapid metabolism
for all seizure parameters: forelimb clonus, falling, ear and tail twitch
and excretion impeding its ability to reach the brain in order to
(p b 0.05) and myoclonic twitch, tonic hindlimb extension and death
exert any potential therapeutic action. Conversely, the other main
(p b 0.01). α-Atlantone was not tested in the mouse model since the
constituent of turmeric, turmeric oil, has not been evaluated before
collected amount was not sufficient to carry out the assay.
for potential anticonvulsant properties.
Sodium valproate was included as positive control in our PTZ
The compounds isolated from turmeric oil through semi-
tail infusion method for AED screening in mice (Supplementary
preparative RP-HPLC were individually evaluated in the zebrafish
Fig. S4B). Using this assay, sodium valproate (50 mg/kg) was
PTZ assay. This assay revealed significant activity for turmeric oil
capable of delaying tonic hindlimb extension (p b0.01) and
and the major bisabolene sesquiterpenoids: ar-, α,β-turmerone and
death (p b 0.05).
α-atlantone. All of the aforementioned constituents exhibited
number in 10 min
average duration, s
number in 10 min
average duration, s
cumulative duration, s
Fig. 4. Electrographic evaluation of the anticonvulsant activity of turmeric oil in the larval zebrafish PTZ seizure model. (A–E) Graphical representation of the changes in seizureparameters analyzed. VHC, vehicle-treated, n = 3; TO, turmeric oil-treated, n = 3; VHC + PTZ, vehicle-treated larvae after PTZ exposure, n = 5; TO + PTZ, turmeric oil-treated larvaeafter PTZ exposure, n = 5. (A) Number of interictal-like spikes; (B) average duration of interictal-like spikes; (C) number of ictal-like discharges; (D) average duration of ictal-likedischarges; (E) cumulative duration of all forms of epileptiform activity. (F–I) Representative 2-minute fragments of electrographic activity recordings. (F) Spontaneous electricalactivity in vehicle-treated control; (G) basal activity after exposure to 10-μg/ml turmeric oil; (H) PTZ-induced spiking in vehicle-treated larva; (I) reduced amount of PTZ-inducedepileptiform activity after exposure to 10-μg/ml turmeric oil.
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
Fig. 4 (continued).
anticonvulsant properties at lower concentrations compared to sodi-
The anticonvulsant properties of turmeric oil in the zebrafish
um valproate. One possibility for the more potent activity of bisabo-
model were successfully corroborated in the mouse PTZ model
lene sesquiterpenoids in the zebrafish PTZ assay compared to
(50 mg/kg, 100 mg/kg). Further analysis leads to the identification
sodium valproate could be related to their higher lipophilicity
of ar-turmerone and α,β-turmerone as the putative compounds re-
(logP). Empirical evidence suggests that small molecules with higher
sponsible for the anticonvulsant activity found for turmeric oil in
lipophilicity enter zebrafish embryos and larvae more readily than
mice. These findings validate the zebrafish PTZ-induced seizure
hydrophilic ones (our own unpublished observations). The bisabo-
model as a primary screening tool for identifying novel potential
lene sesquiterpenoids display around one hundred times higher lipo-
AEDs and reveal the major bisabolene sesquiterpenoids ar-turmerone
philicity than sodium valproate. Thus, these results are also in line
and α,β-turmerone as anticonvulsant drug candidates to be investigated
with the neuroprotection studies in rodent models which have
further. At effective doses, neither turmeric oil nor the tested bisabolene
shown that turmeric oil and its main bisabolene sesquiterpenoids
sesquiterpenoids induced sedation, motor impairment or any other sign
easily cross the blood-brain barrier, likely due to their lipophilic na-
of toxicity in mice.
ture which allows them to easily pass through cell membranes
Interestingly, anticonvulsant properties have been reported also for
Turmeric oil and its constituents present better bio-
other essential oils such as Laurus nobilis and Cymbopogon winterianus
availability and cross biomembranes with less difficulty when com-
. Although their active constituents have not been isolated to
pared to curcumin.
identify the responsible compound/s for the anticonvulsant activity, it
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
Fig. 6. Evaluation of the anticonvulsant activities of α,β-turmerone and ar-turmeronein the mouse PTZ seizure model. Top panel: table listing PTZ dose/s required to elicitthe indicated seizure behaviors after treatment with bisabolene sesquiterpenoid or ve-
Fig. 5. Evaluation of the anticonvulsant activity of turmeric oil in the mouse PTZ seizure
hicle only. Graphical depiction of tabulated results from (A) α,β-turmerone at a dose of
model. Top panel: table listing PTZ dose/s required to elicit the indicated seizure behaviors
100 mg/kg and (B) ar-turmerone at 50 mg/kg. ‘Control A' column corresponds to
after treatment with turmeric oil or vehicle only. Data are expressed as the mean±SD
vehicle-treated controls for α,β-turmerone; ‘Control B' column corresponds to
(n=5). Graphical depiction of tabulated results from (A) turmeric oil at 50 mg/kg and
vehicle-treated controls for ar-turmerone. Data are expressed as the mean ± SD
(B) at 100 mg/kg. Results are expressed as relative values compared to control (set as
(n = 5). For sake of clarity, SDs are not depicted in the graphs but are indicated in
100%). Statistically significant differences between sample (dark gray) and control group
the tables. Results are expressed as relative values compared to control (set as 100%).
(light gray) are labeled as * for pb 0.05 and ** for pb 0.01 (unpaired Student's t-test). For
Statistically significant differences between sample (dark gray) and control group
sake of clarity, SDs are not depicted in the graphs but are indicated in the tables. However,
(light gray) are labeled as * for p b 0.05 and ** for p b 0.01 (unpaired Student's t-test).
the coefficient of variation never exceeded 28% (unpaired Student's t-test).
For the sake of clarity, SDs are not depicted. However, the coefficient of variationnever exceeded 28% and 37% in the case of ar-turmerone and α,β-turmerone,respectively.
is known that some of their main constituents are monoterpenes, phe-nylpropenes and sesquiterpenes. These compounds present in essentialoils, including the bisabolene sesquiterpenoids from turmeric, show a
The identification of the pharmacophore/s (functional chemical
wide variety of functional groups and differences on their basic carbon
group/s) responsible for the anticonvulsant activity of ar-turmerone
skeletons. Since the identified chemical structures of any of the bisabo-
and α,β-turmerone will further lead to their target and the probable
lene sesquiterpenoids are not similar to the structure of the available
mechanisms involved in exerting their activity. It would be of partic-
AEDs, this chemical variability may perhaps increase the chances of
ular interest if these pharmacophores display their action through a
identifying compounds acting through different mechanisms of action.
novel mechanism since this is the major aim of AED discovery. The
With regard to the mechanism of action of the bisabolene sesqui-
main limitation regarding the discovery of novel AEDs is that most
terpenoids, previous studies on the neuroprotective activity of tur-
of them have been identified using the same models, especially MES
meric oil have shown that these compounds can suppress oxidative
This is probably the main cause for a misguided orientation
DNA damage and lipid peroxidation in rodent models . Penty-
that has led to the discovery of drugs that act against new-onset epi-
lenetetrazole has been shown to increase glutamatergic transmission
lepsy but not against the refractory types. Therefore, it is currently in
that, in turn, leads to an increase in intracellular calcium and cell
our interest to additionally assess the activity of the bisabolene ses-
death . Even though the mechanisms of neuroprotection of
quiterpenoids in other model/s of epilepsy such as the 6-Hz seizure
AEDs have not yet been fully elucidated, several studies have revealed
test in mice, the hippocampal kindled rat model of temporal lobe ep-
the potential of lamotrigine, levetiracetam, topiramate and zonisa-
ilepsy (TLE), etc.
mide to limit DNA damage and restrict the extent of neuronal loss
With regard to its toxicity profile, turmeric has been widely used
It is, therefore, tempting to speculate that the antioxidant prop-
as a food condiment predominantly in India for centuries and is con-
erties of the bisabolene sesquiterpenoids are in some way related to
sidered safe for human consumption . Furthermore, toxicity stud-
their anticonvulsant activities. Clearly, further experiments are war-
ies performed in healthy human patients and in silico analysis
ranted in order to support this hypothesis.
have predicted ar-turmerone as a safe potential candidate for
A.M. Orellana-Paucar et al. / Epilepsy & Behavior 24 (2012) 14–22
further drug development. The absence of toxic effects was also
a novel bioenhanced preparation of curcumin. Indian J Pharm Sci 2008;70(4):445–9.
confirmed in our experiments. Larval zebrafish exposed (for up to
[17] Sayyah M, Valizadeh J, Kamalinejad M. Anticonvulsant activity of the leaf essential
24 h) to either turmeric oil or any one of the bisabolene sesquiterpe-
oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-
noids displayed normal heart rate and showed no signs of motor
induced seizures. Phytomedicine 2002;9(3):212–6.
[18] Quintans-Júnior LJ, Souza TT, Leite BS, et al. Phytochemical screening and anticon-
impairment as evidenced by a normal touch/escape response
vulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in
upon application of a tactile stimulus, no balance defects, and no
rodents. Phytomedicine 2008;15(8):619–24.
signs of hypoactivity.
[19] Dohare P, Varma S, Ray M. Curcuma oil modulates the nitric oxide system re-
In conclusion, in this study, we demonstrate the usefulness of a
sponse to cerebral ischemia/reperfusion injury. Nitric Oxide 2008;19(1):1–11.
[20] Dohare P, Garg P, Sharma U, Jagannathan NR, Ray M. Neuroprotective efficacy and
zebrafish seizure model for rapid bioactivity-guided fractionation of
therapeutic window of curcuma oil: in rat embolic stroke model. BMC Comple-
natural products and their purified compounds for the identification
ment Altern Med 2008;8:55.
of novel small molecules with anticonvulsant activity in vivo. Our
[21] Rathore P, Dohare P, Varma S, Ray A, Sharma U, Jagannathan NR. Curcuma oil: re-
duces early accumulation of oxidative product and is anti-apoptogenic in tran-
findings suggest that the main bisabolene sesquiterpenoids of tur-
sient focal ischemia in rat brain. Neurochem Res 2008;33(9):1672–82.
meric could be considered as interesting novel AED candidates that
[22] Crawford AD, Esguerra CV, de Witte PA. Fishing for drugs from nature: zebrafish
deserve further exploration. Current studies are now underway to
as a technology platform for natural product discovery. Planta Med 2008;74(6):624–32.
test these compounds in additional seizure models and to identify
[23] Tan JL, Zon Ll. Chemical screening in zebrafish for novel biological and therapeutic
the pharmacophore/s responsible for their anticonvulsant activity
discovery. Methods Cell Biol 2011;105:493–516.
through structure–activity relationship (SAR) analysis.
[24] Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebra-
fish: a genetic system for large-scale screening. Pharmacol Biochem Behav
Supplementary materials related to this article can be found on-
[25] Zon L, Peterson R. In vivo drug discovery in the zebrafish. Nat Rev 2005;4(1):35–44.
[26] Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes
in zebrafish behavior, neural activity and c-fos expression. Neuroscience
[27] Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a pri-
The authors wish to thank R. Ansaloni for her assistance regarding
mary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res2007;75(1):18–28.
the microscopic characterization of turmeric used in the present
[28] Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin J-P, Hutchinson TH. Vali-
study and N. Cuzco for her work in preparation of turmeric volatile
dation of a larval zebrafish locomotor assay for assessing the seizure liability of
oil. We are also grateful to I. Smolders and R. Clinckers for sharing
early-stage development drugs. J Pharmacol Toxicol Methods 2008;57(3):176–87.
their knowledge and experience with regard to performing the
[29] Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: a
mouse PTZ assay and P. Verstreken and R. Habets for providing advice
comparative analysis with s.c. PTZ and MES models of anticonvulsant screening
and access to their electrophysiology equipment. AMOP was funded
in mice. Seizure 2007;16(7):636–44.
[30] Sills GJ, Butler E, Thompson GG, Brodie MJ. Pharmacodynamic interaction studies
through a fellowship from the Vlaamse Interuniversitaire Raad
with topiramate in the pentylenetetrazol and maximal electroshock seizure
(VLIR)—"Pharmacological Characterization of Medicinal Plants from
models. Seizure 2004;13(5):287–95.
the South of Ecuador" Project.
[31] Jackson B, Snowdon D. Atlas of microscopy of medicinal plants, culinary herbs and
spices. London, U.K.: Belhaven Press; 1990. p. 257 p.
[32] Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish
(Danio rerio). 4th ed. Eugene, Oregon: University of Oregon; 2000.
[33] He X-G, Lin L-Z, Lian L-Z, Lindenmaier M. Liquid chromatography–electrospray
mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric
[1] World Health Organization. Atlas: epilepsy care in the world. Geneva, Switzerland:
(Curcuma longa). J Chromatogr A 1998;818(1):127–32.
WHO Press; 2005. 90 p.
[34] Giardina WJ, Gasior M. Acute seizure tests in epilepsy research: electroshock- and
[2] Schmidt D. Drug treatment of epilepsy: options and limitations. Epilepsy Behav
chemical-induced convulsions in the mouse. In: Enna SJ, Williams M, Barret JF,
Ferkany JW, Kenakin T, Porsolt RD, editors. Current protocols in pharmacology.
[3] Arroyo S, de la Morena A. Life-threatening adverse events of antiepileptic drugs.
Hoboken, NJ, USA: Wiley & Sons; 2009. p. 27–30.
Epilepsy Res 2001;47(1–2):155–74.
[35] Scallier A, Massie A, Loyens E, et al. vGLUT2 heterozygous mice show more susceptibil-
[4] Carpay JA, Aldenkamp AP, van Donselaar CA. Complaints associated with the use
ity to clonic seizures induced by pentylenetetrazol. Neurochem Int 2009;55(1–3):
of antiepileptic drugs: results from a community-based study. Seizure
[36] Kamal A, Shaheer Malik M, Azeeza S, Bajee S, Shaik AA. Total synthesis of (R)- and
[5] Arif H, Buchsbaum R, Weintraub D, Pierro J, Jr. SRR, Hirsch LJ. Patient-reported
(S)-turmerone and (7S,9R)-bisacumol by an efficient chemoenzymatic approach.
cognitive side effects of antiepileptic drugs: predictors and comparison of all
Tetrahedron Asymmetr 2009;20(11):1267–71.
commonly used antiepileptic drugs. Epilepsy Behav 2009;14(1):202–9.
[37] Uehara S-I, Yasuda I, Takeya K, Itokawa H. New bisabolane sesquiterpenoids from
[6] Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy
the rhizomes of Curcuma xanthorrhiza (Zingiberaceae). Chem Pharm Bull
[7] Schachter SC. Botanicals and herbs: a traditional approach to treating epilepsy.
[38] Friesen RW, Blouin M. Preparation of γ,δ‐unsaturated β‐ketophosphonates from
tertiary α‐allenic alcohols. The synthesis of (+/−)‐(E)‐α‐atlantone. J Org Chem
[8] Nsour W, Lau CB-S, Wong IC. Review on phytotherapy in epilepsy. Seizure
[39] Bharal N, Sahaya K, Jain S, Mediratta PK, Sharma KK. Curcumin has anticonvulsant
[9] Malawska B. New anticonvulsant agents. Curr Top Med Chem 2005;5(1):69–85.
activity on increasing current electroshock seizures in mice. Phytother Res
[10] Willmore LJ, Losigamone, Dr Willmar Schwabe. Curr Opin Investig Drugs
[40] Ribeiro MCP, de Ãvila DS, Schneider CYM, et al. alpha-Tocopherol protects against
[11] World Health Organization. WHO monographs on selected medicinal plants.
pentylenetetrazol- and methylmalonate-induced convulsions. Epilepsy Res
Geneva, Switzerland: WHO library cataloguing in publication data; 1999. 289 p.
[12] Jyoti A, Sethi P, Sharma D. Curcumin protects against electrobehavioral progres-
[41] Lores Arnaiz S, Travacio M, Llesuy S, Rodríguez de Lores Arnaiz G. Regional vulner-
sion of seizures in the iron-induced experimental model of epileptogenesis.
ability to oxidative stress in a model of experimental epilepsy. Neurochem Res
Epilepsy Behav 2009;14(2):300–8.
[13] Jithendra C, Murthy TE, Upadyay L. Protective role of curcumin in maximal elec-
[42] Willmore LJ. Antiepileptic drugs and neuroprotection: current status and future
troshock induced seizures, memory impairment and neurotransmitters in rat
roles. Epilepsy Behav 2005;7(Suppl. 3):S25–8.
brain. JPCCR 2008;2(1):35–9.
[43] Löscher W, Schmidt D. Modern antiepileptic drug development has failed to de-
[14] Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Watanabe H, Matsumoto K.
liver: ways out of the current dilemma. Epilepsia 2011;52(4):657–78.
Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell
[44] Villegas I, Sánchez-Fidalgo S, Alarcón de la Lastra C. New mechanisms and thera-
damage in the rat hippocampus by manganese complexes of curcumin and diace-
peutic potential of curcumin for colorectal cancer. Mol Nutr Food Res 2008;52:
tylcurcumin. Life Sci 2006;78(16):1884–91.
[15] Mehla J, Reeta KH, Gupta P, Gupta YK. Protective effect of curcumin against sei-
[45] Joshi J, Ghaisas S, Vaidya A, et al. Early human safety study of turmeric oil
zures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat
(Curcuma longa oil) administered orally in healthy volunteers. J Assoc Physicians
model. Life Sci 2010;87(19–22):596–603.
[16] Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over
[46] Balaji S, Chempakam B. Toxicity prediction of compounds from turmeric
study to evaluate human oral bioavailability of BCM-95®CG (Biocurcumax™),
(Curcuma longa L). Food Chem Toxicol 2010;48(10):2951–9.
Source: https://lifekey.com.au/wp-content/uploads/2015/09/Anticonvulsant-activity-of-bisabolene-sesquiterpenoids-of-Curcuma-longa-in-zebrafish.pdf
Reisen und Gesundheit Prophylaxe Vorbeugung ist die wichtigste Schutzmaßnahme vor Infektionskrankheiten. Die wichtigsten vorbeugenden Maßnahmen aus Sicht der Tropenmedizin finden Sie hier zusammengestellt. Wozu Prophylaxe? Vorbeugen ist besser als Heilen. Das gilt auch bei Reisen in ferne Länder. Die Vorbeugung richtet sich dabeim nach Reiseziel und Aufenthaltsdauer, nach Reisestil, Jahreszeit und individuellen Bedürfnissen.
Phillip A. Glogoza, Extension EntomologistDean K. McBride, Professor EmeritusAlbin W. Anderson, Professor Emeritus North Dakota State UniversityFargo, North Dakota 58105 At least 43 species of mosquitoes are known tooccur in North Dakota. Fortunately, only a few species cause annoyance. Nevertheless, their presence affects people engaged in outdoor activities during the warm months of the year.







