Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Dev.chestpubs.org
Original Research
Population Pharmacodynamic Model
of Bronchodilator Response to Inhaled
Albuterol in Children and Adults With
Asthma*
Kathryn Blake, PharmD; Rajanikanth Madabushi, PhD;Hartmut Derendorf, PhD; and John Lima, PharmD
Background: Because interpatient variability in bronchodilation from inhaled albuterol is large
and clinically important, we characterized the albuterol dose/response relationship by pharma-
codynamic modeling and quantified variability.
Methods: Eighty-one patients with asthma (24% African American [AA]; 8 to 65 years old; baseline
FEV1, 40 to 80% of predicted) received 180
g of albuterol from a metered-dose inhaler (MDI),
and then 90
g every 15 min until maximum improvement or 540
g was administered; all then
received 2.5 mg of nebulized albuterol. FEV1 was measured 15 min after each dose. The
population cumulative dose/response data were fitted with a sigmoid maximum effect of albuterol
(Emax) [maximum percentage of predicted FEV1 effect] model by nonlinear mixed-effects
modeling. The influence of covariates on maximum percentage of predicted FEV1 reached after
albuterol administration (Rmax) and cumulative dose of albuterol required to bring about 50% of
maximum effect of albuterol (ED50) and differences between AA and white patients were explored.
Results: ED50 was 141
g, and Emax was 24.0%. Coefficients of variation for ED50 and Emax were
40% and 56%, respectively. Ethnicity was a statistically significant covariate (p < 0.05). AA and white
patients reached 82.4% and 91.9% of predicted FEV1, respectively (p ⴝ
0.0004); and absolute
improvement in percentage of predicted FEV1 was 16.6% in AA patients vs 26.7% in white patients
(p < 0.0003). There were no baseline characteristic differences between AA and white patients.
Nebulized albuterol increased FEV >
200 mL in 21% of participants. Heart rate and BP were
unchanged from baseline after maximal albuterol doses.
Conclusions: Our model predicts that 180
g of albuterol by MDI produces a 14.4% increase in
percentage of predicted FEV1 over baseline (11.7% in AA patients, and 17.5% in white patients).
Emax varies widely between asthmatic patients. AA patients are less responsive to maximal doses of
inhaled albuterol than white patients.
(CHEST 2008; 134:981–989)
Key words: African American; albuterol; asthma; bronchodilator; cumulative dose of albuterol required to bring about 50%
of maximum effect of albuterol; ethnicity; maximum effect of albuterol; metered-dose inhaler; nebulizer; pharmacodynamic
model; white race
Abbreviations: ED ⫽
cumulative dose of albuterol required to bring about 50% of maximum effect of albuterol;
Emax ⫽ maximum effect of albuterol; ␥ ⫽ Hill coefficient that describes the steepness of the dose/response relation;
ICS ⫽ inhaled corticosteroids; MDI ⫽ metered-dose inhaler; PPK/PD ⫽ population pharmacokinetic/pharmacodynamic;
baseline percentage of predicted FEV1; Rmax ⫽ maximum percentage of predicted FEV1 reached after albuterol
administration; SABA ⫽ short-acting 2-agonist
Inhaled short-acting 2-agonists (SABAs) are the been associated with significant interpatient variabil-
most potent bronchodilators used today to treat
ity in response.3–9 Many studies3–12 have character-
acute symptoms of asthma1; and albuterol, a partial
ized the SABA dose to bronchodilator response
2-agonist, is the most frequently prescribed asthma
relationship under controlled conditions. However,
medication in the United States.2 Although univer-
few studies have explored the magnitude and sources
sally used for acute asthma symptoms, SABAs have
of bronchodilator response variability, and no studies
CHEST / 134 / 5 / NOVEMBER, 2008
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
Cumulative
Dose by MDI
Cumulative Dose by
MDI + Nebulizer
Figure 1. Number of participants who received each cumulative dose of albuterol. After baselinespirometry, participants received 180 g of albuterol and then 90 g every 15 min until maximum
improvement or 540 g was administered; all then received 2.5 mg of nebulized albuterol.
have characterized the dose to bronchodilator re-
Materials and Methods
sponse relationship using population pharmacoki-netic/pharmacodynamic (PPK/PD) modeling.
In a patient-care setting, the purpose of PPK/PD
modeling is to gain a better understanding of the
Participants of any ethnicity 8 to 65 years old with a well-
defined history of physician-diagnosed asthma; a baseline pre-
quantitative guidelines for dosage individualization
bronchodilator FEV1 of 40% to 80% predicted for age, height,
and optimization. Additionally, PPK/PD modeling
and gender15,16; who denied oral corticosteroid use, emergency
allows one to identify and quantify fixed and random
department visits, or hospitalizations within the previous 3
sources of variability that characterize the dose (or
months; who were nonsmokers or had ⬍ 5–pack-year history with
concentration) vs response relationship in the target
no smoking in the previous year; and who had a normal physicalexamination and no confounding diseases were selected. Partic-
population to be treated with the drug.13
ipants had to withhold inhaled SABAs or inhaled anticholinergic
American Thoracic Society guidelines state that an
drugs for 8 h, oral antihistamines for 5 days, theophylline for 24 h,
and cromolyn, nedocromil, and inhaled corticosteroids (ICS) for
1 of 12 to 15% above baseline
measured 15 min following inhalation of 100 to 400
2 h prior to the study. Inhaled salmeterol and formoterol and
g of a SABA, such as albuterol, by metered-dose
leukotriene modifiers were not available in the United Stateswhen this study was conducted. Participants were recruited from
inhaler (MDI) "suggest a significant bronchodilata-
our asthma research clinic database or newspaper advertise-
tion."14 Additionally, this measurement is a criterion
ments. The study was approved by our local Institutional Review
used to diagnose asthma.1 However, it is not clear if
Board, and written informed consent was obtained.
these dosing recommendations will achieve maximalbronchodilator response in patients.
Study Design and Drug Administration
In the present study, we characterized the albu-
terol dose to bronchodilator response relationship in
This was an open-label study conducted over 1 to 2 h for each
participant on a single day. After we obtained baseline spirome-
81 children and adults with moderate-to-severe per-
try, heart rate, and BP measurements, participants received two
sistent asthma using a population pharmacodynamic
inhalations of albuterol (90 g per inhalation) from an MDI
approach. The purpose was to obtain estimates of the
attached to a holding chamber (InspirEase; Schering-Plough
pharmacodynamic parameters that characterize the
Corporation; Kenilworth, NJ). Additional inhalations of 90 g
albuterol dose/bronchodilator response curve, quan-
through the holding chamber were administered every 15 min,with spirometry, heart rate, and BP measured immediately prior
tify and identify sources of interpatient pharmacody-
to each dose. When there was no further improvement in FEV1
namic variability, and determine the additional bron-
(⬍ 100 mL change from the highest FEV1 obtained after the
chodilator effect of a single dose of nebulized
previous dose), each participant received a single 2.5-mg dose of
albuterol after maximal dosing from an MDI.
nebulized albuterol. Final spirometry, heart rate, and BP mea-surements were obtained 15 min after nebulized albuterol. Thecumulative doses of albuterol administered from the MDI were
*From the Center for Clinical Pediatric Pharmacology Research
180 g, 270 g, 360 g, 450 g, 540 g; and the cumulative
(Drs. Blake and Lima), Nemours Children's Clinic, Jacksonville;
doses from the MDI plus nebulizer were 270 g MDI plus 2,500
and Department of Pharmaceutics (Drs. Madabushi and Deren-
g nebulized (2,770 g); 360 g MDI plus 2,500 g nebulized
dorf), College of Pharmacy, University of Florida, Gainesville, FL.
All work was performed in the Center for Clinical Pediatric
g); 450 g MDI plus 2,500 g nebulized (2,950 g); and
Pharmacology Research, Nemours Children's Clinic, Jackson-
540 g MDI plus 2,500 g nebulized (3,040 g). The number of
participants receiving each dose and the cumulative administered
The authors have no conflicts of interest to report.
doses (MDI and MDI plus nebulized) are shown in Figure 1.
Manuscript received December 13, 2007; revision accepted May
These doses represent the amount of drug administered to the
patient from each device.
Reproduction of this article is prohibited without written permission
Prior to the first dose of study drug, two actuations from the
from the American College of Chest Physicians (www.chestjournal.
albuterol MDI were discharged into the holding chamber to
prime the MDI and to neutralize the electrostatic charge present
Correspondence to: Kathryn Blake, PharmD, Center for Clinical
in the plastic holding chamber.17,18 The holding chamber was
Pediatric Pharmacology Research, Nemours Children's Clinic, 807Childrens Way, Jacksonville, FL 32207; e-mail: [email protected]
collapsed and expanded several times in a location away from the
participant to remove any aerosolized albuterol from the interior
Original Research
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
chamber of the InspirEase. For each single inhalation of albu-
dicted FEV1 between whites and African Americans, and dura-
terol from the MDI, including the initial two inhalations, partic-
tion of asthma between whites and African Americans. The
ipants were instructed to actuate the inhaler to release one dose
proportion of whites vs African Americans who used ICS, the
(90 g) of albuterol into the holding chamber, breathe in slowly
proportion of whites vs African Americans who used regularly
through the mouthpiece without sounding the reed (inhalation
scheduled inhaled albuterol for daily asthma management, and
rate of 0.3 L/s is not exceeded), and breath hold for 5 s.
the proportion of ICS users vs nonusers who reported regularly
Participants were instructed to exhale into the chamber and
scheduled inhaled albuterol therapy for daily asthma manage-
inhale a second time followed by a 5-s breath hold.19
ment were compared by 2 analysis. Paired
t tests (two-tailed)
Albuterol for nebulization (inhalation solution 0.5%) was
were used to test for differences in BP and heart rate after
diluted with saline solution to a final volume of 3.5 mL. The
maximal albuterol doses compared with baseline. A p value
dose was administered by tidal breathing to nebulizer sputter-
⬍ 0.05 was considered significant.
ing after repeated tamping of the nebulizer bowl using an opensystem consisting of a nebulizer (Puritan-Bennett; OverlandPark, KS) connected to a mouthpiece by a T joint and driven
by an air compressor (Medi-mist; Mountain Medical Equip-ment; Littleton, CO).
Spirometry (MultiSpiro; Irvine, CA) was performed with the
participant standing and wearing a nose clip, and up to eight
Of 107 asthmatics screened, 81 patients met the
efforts were recorded after each dose to obtain the two highest
inclusion criteria. Participant characteristics are pre-
FEV1 measurements within 100 mL of each other; the highest
sented in Table 1. Race and ethnicity were deter-
FEV1 was recorded. Polgar reference equations were used fordetermining predicted FEV
mined by self-report from the adults and by parental
1 values in girls and boys ⬍ 19 years
of age; Knudson reference equations were used for men and
report for children ⬍ 18 years old. Thirty-seven
women ⱖ 19 years of age.15,16 BP and heart rate were measured
percent of participants were ⬍ 20 years old, 40%
with the participant seated (Dinamap BP monitor; Critikon, GE
were 20 to 45 years old, and 23% were 46 to 65 years
Healthcare; Bucks, UK).
old. Baseline FEV1 was from 60 to 80% of predictedin 68% of participants, classifying them as having
Population Pharmacodynamic Analysis
Population analysis was performed on the cumulative albuterol
dose/response data.13 A sigmoid maximum effect of albuterol
(Emax) model was used to describe the relationship betweenalbuterol dose and bronchodilator response as determined by the
change in percentage of predicted FEV1 from baseline (R0).20
(Rmax ⫺ R0) ⫻ D␥/(ED50
17 (21.0)/12 (14.8)
27 (33.3)/5 (6.2)
where D is cumulative albuterol dose, Rmax is the maximum
13 (16.0)/3 (3.7)
percentage of predicted FEV1 reached after albuterol adminis-
tration, ␥ is the Hill coefficient that describes the steepness of the
Duration of asthma, % of patient's life
dose/response relation, and ED50 is the cumulative dose of
albuterol required to bring about 50% of Emax.
Emax ⫽ Rmax ⫺ R
Baseline FVC, % of predicted
Mean and between subject coefficient of variation values for
Baseline FEV1, % of predicted
population parameters were obtained by nonlinear mixed-effects
Baseline FEF25–75, % of predicted
modeling (NONMEM Project Group; San Francisco, CA). The
between-participant variability was assumed to follow a log-
ICS current users‡
normal distribution, while an additive error model was used to
ICS not used currently or never used
explain the random residual error. The method of estimation was
Oral corticosteroid: current users (20 mg qod)
first-order conditional estimation, which uses conditional esti-
Dose of ICS (n ⫽ 45)§
mates of the random interindividual variability while estimating
the population parameters.
21,22 The first-order conditional esti-
mation method is applicable for nonlinear data and as the amount
of data per individual increases.
Duration of ICS therapy (n ⫽ 45), yr
Gender, ethnicity, age, years diagnosed with asthma, and
current ICS use (yes/no) were incorporated into the model as
covariates. A power function was used to test the influence of
continuous covariates normalized to their median values for
numerical stability on the model parameters. Categorical covari-
*Data are presented as mean ⫾ SD or No. (%) unless otherwise
ates that take numerical values were tested using a linear model.
forced expiratory flow, midexpiratory phase.
Two-sample
t tests (two-tailed, unequal variance) were used to
†There was one Hispanic patient in each age group and one other
test for significant differences in baseline pulmonary function
ethnicity in the 20-to 45-year age group.
(R0), Rmax, baseline percentage of predicted FEV1 between
‡ICS included beclomethasone, flunisolide, and triamcinolone.
users and nonusers of ICS, duration of asthma between users and
§Low-, medium-, and high-dose levels as specified in National
nonusers of ICS, absolute improvement in percentage of pre-
Asthma Education and Prevention Program.1
CHEST / 134 / 5 / NOVEMBER, 2008
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
moderate persistent asthma.1 By patient report onthe day of the study visit, 26 patients (8 AfricanAmericans) stated that they used two inhalations ofalbuterol on a regular schedule either once daily(n ⫽ 5), twice daily (n ⫽ 5), three times daily(n ⫽ 8), or four times daily (n ⫽ 8) [African Ameri-cans vs whites, p ⫽ 0.40]. All other patients reportedusing albuterol at two inhalations on an as-neededbasis. Quantification of SABA use during the weekimmediately prior to the study day was not obtained.
Forty percent of participants were using low-doseICS, 14% were using medium-dose ICS, and 2%were using high-dose ICS; one pediatric patient wastreated with prednisone every other day.1 All partic-ipants had been using ICS for at least 3 months, butthe majority had used ICS for ⱕ 2 years. Clinicalcharacteristics were similar between users and non-users of ICS (baseline FEV
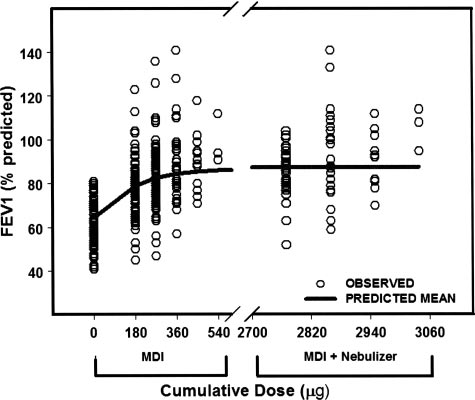
Figure 2. Fitted dose/response after cumulative doses of albu-
1 percentage of predicted
[p ⫽ 0.89]; duration of asthma [p ⫽ 0.89]; and fre-
terol (solid line). Open circles represent individual patient re-sponses. Response is percentage of predicted FEV
quency of SABA use [p ⫽ 0.48]). Thirty-two patients
lative doses of albuterol administered from the MDI were 180
were using a theophylline product, and 1 patient was
g, 270 g, 360 g, 450 g, and 540 g; and the cumulative
using cromolyn sodium. Of these 32 patients, 21
doses from the MDI plus nebulizer were 2,770 g (270 g MDI
plus 2,500 g nebulized); 2,860 g (360 g MDI plus 2,500 g
patients were also using an ICS. All participants had
nebulized); 2,950 g (450 g MDI plus 2,500 g nebulized); and
long-standing asthma with an asthma diagnosis for
3,040 g (540 g MDI plus 2,500 g nebulized).
64%, 63%, and 56% of their lives in the age groups
⬍ 20 years, 20 to 45 years, and 46 to 65 years,
whites and African Americans demonstrated no dif-
ference in ED50, but Rmax was 91.9% of predictedFEV1 in whites and 82.4% of predicted in African
Modeled Data
Americans (p ⫽ 0.0004) [Fig 3]. Consistent with ourmodeled data of difference in Rmax between ethnic
Population modeling of the albuterol dose/re-
groups, whites had an absolute improvement in
sponse relationship is shown in Figure 2. A Hill
percentage of predicted FEV
coefficient (␥) of 2 was found to best fit the data and
1 of 26.7% from base-
line after receiving maximum albuterol doses
had lowest objective function value. In preliminary
compared with 16.6% in African Americans
studies, several pharmacodynamic models were fit-
(p ⬍ 0.0003). Using the modeled parameters, two
ted to the albuterol dose vs FEV1 relationship. The
inhalations of albuterol (180 g) would increase
sigmoid Emax model with ␥ ⫽ 2 provided the best fit
percentage of predicted FEV
to the data. Subsequent analyses setting ␥ as a
1 by 11.7% in African
Americans vs 17.5% in whites. Baseline percentage
parameter resulted in unreasonable pharmacody-
namic and variability estimates suggesting that fitting
was not different between
whites and African Americans (65.2 ⫾ 11.4% vs
the sigmoid Emax model with four parameters re-
65.8 ⫾ 8.3%, respectively; p ⫽ 0.81), nor was du-
sulted in overparameterization. Therefore, we set
ration of asthma (p ⫽ 0.31) nor the proportion
2 and fit the model with thee parameters. The
using ICS (p ⫽ 0.61).
modeled parameters, ED50 of 141 g and Emax of24.0%, predict that the standard of administeringtwo inhalations of albuterol (180 g) is sufficient to
Table 2—Population Modeling of Albuterol
produce a 14.4% increase in percentage of predicted
FEV1 from baseline and to reach 60.0% of Emax
(Table 2). There were 40% and 56% variabilities
associated with ED50 and Emax, respectively.
Incorporating gender, age, years diagnosed with
asthma, and current ICS use (yes/no) as covariates
64.3% of predicted FEV1
87.6% of predicted FEV1
for R0, Rmax, and ED50 did not account for signifi-
24.0% of predicted FEV1
cant between-participant variability. Including eth-
nicity as a covariate on Emax decreased the interpa-
tient variability by 1% (p ⬍ 0.05). Modeled data for
Residual error (SD)
3.2% of predicted FEV1
Original Research
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
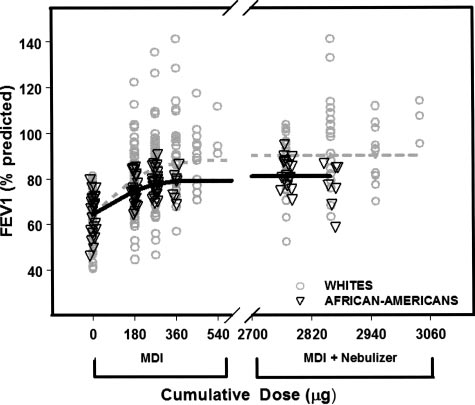
Table 3—BP and Heart Rate at Baseline and After
Maximal Albuterol Doses*
After Maximal Albuterol
Systolic BP, mm Hg†
Diastolic BP, mm Hg‡
Heart rate, beats/min§
*Data are presented as mean ⫾ SD.
†p ⫽ 0.53.
‡p ⫽ 0.06.
§p ⫽ 0.13.
response to SABAs has been observed to be highlyvariable between patients.3–9 The results of thepresent study support these findings and extendthem to include quantification of the variability in
Figure 3. Fitted dose/response after cumulative doses of albu-
bronchodilator response using a pharmacodynamic
terol in African Americans (solid line) and whites (dotted line).
population model. This is the first study to our knowl-
Open triangles are individual patient responses for African
edge to use population pharmacodynamic modeling to
Americans. Open circles are individual patient responses forwhites. Response is percentage of predicted FEV
characterize the dose/response relationship and vari-
tive doses of albuterol administered from the MDI were 180 g,
ability associated with bronchodilator response after
270 g, 360 g, 450 g, and 540 g; and the cumulative doses
inhaled albuterol in physician-diagnosed asthmatic pa-
from the MDI plus nebulizer were 2,770 g (270 g MDI plus
2,500 g nebulized); 2,860 g (360 g MDI plus 2,500 g
tients with moderate-to-severe persistent disease. With
nebulized); 2,950 g (450 g MDI plus 2,500 g nebulized); and
an Emax of 24% of predicted FEV1 (absolute change
3,040 g (540 g MDI plus 2,500 g nebulized).
in percentage of predicted FEV1 over baseline) andan ED50 of 141 g (dose of albuterol that producedhalf-maximal bronchodilation, ie, 12% [0.5 ⫻ 24%]),
Effect of Nebulized Albuterol After Maximum
these modeled data predict that two inhalations of
Doses From the MDI
albuterol (180 g) would increase the percentage ofpredicted FEV
All participants reached maximum improvement
1 by 14.4%. The results of this study
cannot be applied to the management of acute exacerba-
in FEV1 (⬍ 100 mL change from highest FEV1
tions of asthma, which may have a predominantly
obtained after previous dose) after six inhalations or
inflammatory component. Inflammation is known to
less of albuterol from the MDI (Fig 2). The population-
impair the response to inhaled 
modeled data in Figure 2 demonstrate that addition
2-agonists, which
would result in different 
of nebulized albuterol to maximal doses from an
MDI (from 2,770 to 3,040 g) does not further
Patients were considered stable and enrolled in the
increase bronchodilator response. However, nebu-
study if they had not had an asthma exacerbation
lized albuterol provided additional bronchodilation
requiring treatment with oral steroids, emergency de-
(ⱖ 200 mL)14 in 11 participants (21%) [mean ⫾ SD
partment care, or hospitalization in the past 3 months
improvement, 366.5 ⫾ 126.1 mL; range, 200 to 610
and were not having an acute worsening of their
mL]. After receiving nebulized albuterol, six par-
symptoms (albuterol use for acute symptom control
ticipants had a fall in FEV ⬎
100 mL (range, 3.3
was unchanged over previous days). It is likely that
some patients would be considered as having poorlycontrolled but stable asthma based on their baseline
Adverse Effects
pulmonary function, lack of antiinflammatory therapy,
There was no change from baseline in BP or heart
and regularly scheduled use of inhaled albuterol. Ques-
rate after maximal albuterol doses (Table 3). There
tionnaires currently used to assess asthma stability such
were no complaints of tremor.
as the Asthma Control Test, the Childhood AsthmaControl Test, the Asthma Control Questionnaire, andthe Asthma Therapy Assessment Questionnaire control
index1 were not available at the time this study wasconducted.
Inhaled albuterol is the most extensively used
Bronchodilator response in the present study was
medication for patients with asthma. Bronchodilator
extremely variable (Fig 2). Nevertheless, the results
CHEST / 134 / 5 / NOVEMBER, 2008
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
of our study clearly show the typical pharmacologic
study, the covariates of gender, ethnicity, age, years
pattern for the bronchodilator response to albuterol
diagnosed with asthma, and current ICS use (yes/no)
(steep increase in response at low doses followed by
did not further explain the variability observed. We
flattening of the response curve at high doses). The
were surprised that ICS use did not influence our
between-subject coefficient of variation for R0 and
findings because corticosteroids increase -adrenergic
Rmax was approximately 16%, and for Emax was
expression and could affect ED50 and Emax.36 How-
56.2% (Table 2). The similar coefficient of variation
ever, only a little over half of patients were using
for R0 and Rmax compared with the large variability
ICS, and 73% were using a low dose. There were no
in Emax suggests that variability associated with
significant differences between ICS users (including
Emax is due to R0, ED50, and Rmax, and that the
the pediatric patient on prednisone) and nonusers
change from baseline (Emax) between individuals is
for baseline FEV1 percentage of predicted nor du-
highly variable. Thus, the Emax 56.2% coefficient of
ration of asthma. In addition, a similar proportion of
variation predicts that certain asthmatics may be
ICS users and nonusers reported albuterol use on a
particularly responsive to the bronchodilator effects
regular schedule (two inhalations once to four times
of albuterol and may experience relief of symptoms
daily). Thus, there were not any obvious clinical
with relatively low doses of inhaled albuterol. This
differences between ICS users and nonusers that
individual variation in bronchodilator responsiveness
would be expected to influence the ED50 or Emax
is clearly evident in Figure 2. However, the variabil-
findings. It is possible that inclusion of participants
ity in the ED50, although high (41%), is not clear
with differing genotypes or haplotypes for genes in
from the graph and indicates that the relationship
the 2-receptor pathway could have contributed to
between the dose of inhaled albuterol to achieve a
variability.37–43 However, a limitation of our study is
targeted reversibility is highly variable.
that we did not collect DNA.
The actual doses of albuterol delivered to the
Additionally, we do not know the extent of prior
lungs of the study participants would be lower
inhaled SABA usage in the week immediately prior
than the doses we used in the pharmacodynamic
to the study day. Twenty-six patients reported regu-
model for the MDI (90 g) and nebulizer (2,500
lar use of albuterol one to four times daily as part of
g). Of the 100 g present in the valve of an
their asthma self-management. Frequent use of in-
albuterol MDI (90 g delivered from the mouth-
haled SABAs and long-acting 2-agonists can induce
piece of the actuator), only 25 to 40 g is delivered
2-receptor desensitization, causing patients to be-
to the patient in an in vitro model when the MDI
come less bronchodilator responsive to additional
is attached to a holding chamber.25–27 Likewise,
SABA doses.44–46 In addition, frequent use is con-
only 400 to 500 g of a 2,500-g dose of albuterol
sidered a marker of airways inflammation and in-
inhalation solution placed in the nebulizer used in
flammatory mediators impair 2-receptor activation
this study is available for inhalation.28 As we did
by 2-agonists.24,31 Thus, the variability in broncho-
not measure the amount of drug available to the
dilator response observed in this study could reflect
patient from the devices, we chose to use the MDI
dysfunction of the 2-receptor induced by overuse of
and nebulizer doses most commonly recognized by
albuterol and the presence of underlying airway
inflammation in some patients.
Patients in this study had a wide range of baseline
While patients included in our study had been
FEV1 values (40 to 80% of predicted FEV1). Base-
nonsmokers for at least the previous year, patients
line FEV1 influences the bronchodilator response to
with ⬍ 5–pack-year history were allowed to partici-
inhaled 2-agonists such that larger doses are re-
pate. Smoking is known to increase responsiveness to
quired in patients with lower baseline values to reach
albuterol and methacholine, and even former smok-
maximum bronchodilation compared with patients
ing increases the risk of having poorly controlled
who have higher baseline values.29 Reasons for this
asthma.47–49 It is possible that the inclusion of a mix
phenomenon could include the presence of endoge-
of former and never-smokers in our study could have
nous functional antagonists acting at the 2-receptor, or
contributed to the variability in bronchodilator
desensitization of the 2-receptor due to either the
action of inflammatory mediators at the receptor
Our data demonstrate an ethnic difference in
or from excessive exposure (due to overuse) to
bronchodilator reversibility in white vs African-
2-agonist drugs.24,30,31 Baseline FEV1 was not in-
American patients with moderate-to-severe, stable
cluded as a covariate in our analysis because it is
asthma. Ethnicity as a covariate accounted for only
included as a parameter in the pharmacodynamic
1% of the variability in Emax (p ⬍ 0.05), but when
bronchodilator response was stratified by ethnicity,
Other studies5,7,8,35 have also noted significant
differences became clinically important. White pa-
interpatient variability in response. In the present
tients achieved a 9.5% higher maximum percentage
Original Research
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
of predicted FEV1 than African-American patients
consistent with a study in hospitalized asthmatics in
(91.9% vs 82.4%, p ⫽ 0.0004), and the pharmacody-
which approximately seven inhalations of albuterol
namic model predicts that African-American pa-
produced maximal bronchodilation and the addition
tients would increase percentage of predicted FEV1
of a single 5.0-mg dose of nebulized albuterol in-
by 11.7%, compared with 17.5% for white patients
creased FEV1 by only 44 ⫾ 8.7 mL.55 Examination
after a standard dose of albuterol (two inhalations of
of individual responses indicates that there are asth-
90 g from an MDI). There was no difference in
matics who benefit with treatment by nebulizer
baseline FEV1 nor duration of asthma to explain this
following maximal MDI dosing (Fig 2). Further
finding, and 50% of the African Americans were re-
research is needed to identify which patients would
ceiving ICS therapy. It is possible that there were
require additional dosing by nebulization.
genetic differences between white and Africans-
Both the asthma and COPD diagnosis and man-
American patients in our study population that influ-
agement guidelines recommend up to a 400-g dose
enced response.41 Our data could have important
of a short-acting inhaled bronchodilator to assess the
clinical implications for 2-agonist treatment in African
degree of bronchodilator response as an indicator of
Americans, in which higher doses of albuterol oradditional or alternative drugs such as anticholinergics
disease severity.1,56 Our findings do not alter this
may be required for sufficient bronchodilation.
recommendation for asthmatics because we found
Three previous studies34,50,51 compared broncho-
that two to four inhalations of albuterol (as predicted
dilator response to albuterol between white and
by the ED50 of 141 g) could increase FEV1
African-American asthmatics. Our data are consis-
percentage of predicted by 12% in patients with
tent with Hardie et al,50 who found that maximum
moderate-to-severe persistent disease. However, af-
bronchodilation in mild asthmatics was lower in
ter maximal bronchodilation was achieved by dosing
African-American compared to white patients after
with the MDI, 21% of our study patients had further
multiple doses of albuterol by MDI following
clinically relevant increases in FEV1 with a 2.5-mg
methacholine-induced bronchoconstriction. Of the
nebulized dose of albuterol. Therefore, our results
other two studies,34,51 one was underpowered to
suggest that if adequate bronchodilation is not
detect a difference between ethnic groups, and the
achieved with the MDI, administration of a nebu-
other was conducted in emergency department
lized dose may be warranted. Nebulized albuterol is
patients (whose clinical features differ from stable
known to cause palpitations, sinus tachycardia, anx-
asthmatics), and patients were not dosed with
iety, tremor, and increased BP.
albuterol to maximal bronchodilation. Differences
A small number of participants had bronchocon-
in response between other ethnic groups have
striction after nebulized albuterol. The formulation
been observed.52,53
of albuterol solution used at the time this study was
The finding from our modeled data that 180 g of
conducted contained 50 g of benzalkonium chlo-
albuterol is sufficient to increase percentage of pre-
ride per 2.5-mg dose. Inhaled benzalkonium chlo-
dicted FEV1 14.4% from baseline and to reach
ride administered in the absence of an SABA is
60.0% of Emax is supported by results from other
known to induce bronchospasm in asthmatics.57,58
studies5,8,11 in which four inhalations (90 g per
However, aside from case reports, there are no
inhalation) or less achieve near-maximal bronchodi-
controlled studies to indicate that the dose of ben-
lation. Studies that found that larger doses were
zalkonium chloride used in albuterol solutions for
required to reach a maximum bronchodilator re-
nebulization approved by the Food and Drug Ad-
sponse included patients with severe bronchocon-
ministration cause clinically relevant bronchocon-
striction, which is known to shift the albuterol dose/
response curve to the right54 (larger doses needed to
Our pharmacodynamic population model of albu-
achieve the same effect), or demonstrated relatively
terol bronchodilator response data is important to
small incremental increases (⬍ 100 mL) in FEV1 at
the practicing clinician because we found that the
higher doses.7,9,11
majority of stable asthmatics with moderate-to-severe
Nebulizer treatment is often used in patients with
asthma having symptoms will achieve a 12% increase
insufficient response to multiple inhalations of albu-
in percentage of predicted FEV1 with two to four
terol from an MDI. Our modeled population data
inhalations of albuterol from their MDI. However,
predict that nebulized albuterol does not add to the
the maximum percentage of predicted FEV1 will
bronchodilator effects from maximal albuterol dos-
vary widely between patients with additional doses.
ing from an MDI as shown by the flat dose/response
Importantly, the reduced bronchodilator response to
curve for all cumulative doses, which include the
albuterol in African-American asthmatics compared
2.5-mg nebulized albuterol dose (2,770 g, 2,860 g,
to whites suggests that African Americans may require
2,950 g, 3,040 g) [Fig 1, 2]. These data are
more aggressive treatment than whites to ensure the
CHEST / 134 / 5 / NOVEMBER, 2008
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
bronchodilator and dose used will be effective in
20 Gabrielsson J, Weiner D. Pharmacodynamic concepts: phar-
macokinetic and pharmacodynamic data analysis; conceptsand applications. Stockholm, Sweden: Swedish Pharmaceuti-cal Press, 1997; 172–250
21 Jonsson EN, Wade JR, Karlsson MO. Nonlinearity detection:
advantages of nonlinear mixed-effects modeling. AAPS
1 National Asthma Education and Prevention Program. Expert
PharmSci 2000; 2:E32
panel report 3: guidelines for the diagnosis and management
22 Beal SL, Scheiner LB. NONMEM users guide, NONMEM
of asthma. Bethesda, MD: U.S. Department of Health and
Project Group. San Francisco, CA: University of California at
Human Services, Public Health Service, National Institutes of
San Francisco, 1989
Health, National Heart, Lung, and Blood Institute, 2007;
23 Beal SL, Scheiner LB. NONMEM users guide. Part VII:
publication 08 – 4051
Conditional estimation methods. San Francisco, CA: Univer-
2 Top 200 generic drugs by units 2005. Drug Topics Maga-
sity of California at San Francisco, 1992
24 Shore SA, Moore PE. Regulation of -adrenergic responses
3 Lehmann S, Bakke PS, Eide GE, et al. Bronchodilator
in airway smooth muscle. Respir Physiol Neurobiol 2003;
reversibility testing in an adult general population; the im-
portance of smoking and anthropometrical variables on the
25 Barry PW, O'Callaghan C. Inhalational drug delivery from
response to a 2-agonist. Pulm Pharmacol Ther 2006; 19:
seven different spacer devices. Thorax 1996; 51:835– 840
26 Wilkes W, Fink J, Dhand R. Selecting an accessory device
4 Dales RE, Spitzer WO, Tousignant P, et al. Clinical interpre-
with a metered-dose inhaler: variable influence of accessory
tation of airway response to a bronchodilator: epidemiologic
devices on fine particle dose, throat deposition, and drug
considerations. Am Rev Respir Dis 1988; 138:317–320
delivery with asynchronous actuation from a metered-dose
5 Kradjan WA, Driesner NK, Abuan TH, et al. Effect of age on
inhaler. J Aerosol Med 2001; 14:351–360
bronchodilator response. Chest 1992; 101:1545–1551
27 Proventil prescribing information [package insert]. Kenilworth,
6 Turner DJ, Landau LI, LeSouef PN. The effect of age on
NJ: Schering-Plough Corporation, 2007
bronchodilator responsiveness. Pediatr Pulmonol 1993; 15:
28 Hess D, Fisher D, Williams P, et al. Medication nebulizer
performance: effects of diluent volume, nebulizer flow, and
7 Chaieb J, Belcher N, Rees PJ. Maximum achievable bron-
nebulizer brand. Chest 1996; 110:498 –505
chodilatation in asthma. Respir Med 1989; 83:497–502
29 Barnes PJ, Pride NB. Dose-response curves to inhaled
8 Hendeles L, Beaty R, Ahrens R, et al. Response to inhaled
-adrenoceptor agonists in normal and asthmatic subjects.
albuterol during nocturnal asthma. J Allergy Clin Immunol
Br J Clin Pharmacol 1983; 15:677– 682
2004; 113:1058 –1062
30 Lemoine H, Overlack C, Kohl A, et al. Formoterol, fenoterol,
9 Lipworth BJ, Clark RA, Dhillon DP, et al. -Adrenoceptor
and salbutamol as partial agonists for relaxation of maximally
responses to high doses of inhaled salbutamol in patients with
contracted guinea pig tracheae: comparison of relaxation with
bronchial asthma. Br J Clin Pharmacol 1988; 26:527–533
receptor binding. Lung 1992; 170:163–180
10 Fishwick D, Bradshaw L, Macdonald C, et al. Cumulative and
31 Nijkamp FP, Engels F, Henricks PA, et al. Mechanisms of
single-dose design to assess the bronchodilator effects of
-adrenergic receptor regulation in lungs and its implications
2-agonists in individuals with asthma. Am J Respir Crit Care
for physiological responses. Physiol Rev 1992; 72:323–367
Med 2001; 163:474 – 477
32 Lima JJ, Krukemyer JJ, Boudoulas H. Drug- or hormone-
11 Lotvall J, Palmqvist M, Arvidsson P, et al. The therapeutic
induced adaptation: model of adrenergic hypersensitivity.
ratio of R-albuterol is comparable with that of RS-albuterol
J Pharmacokinet Biopharm 1989; 17:347–364
in asthmatic patients. J Allergy Clin Immunol 2001; 108:
33 Lalonde RL, Straka RJ, Pieper JA, et al. Propranolol phar-
macodynamic modeling using unbound and total concentra-
12 Blake KV, Hoppe M, Harman E, et al. Relative amount of
tions in healthy volunteers. J Pharmacokinet Biopharm 1987;
albuterol delivered to lung receptors from a metered-dose
inhaler and nebulizer solution: bioassay by histamine bron-
34 Lima JJ, Mohamed MH, Self TH, et al. Importance of
choprovocation. Chest 1992; 101:309 –315
(2)adrenergic receptor genotype, gender and race on
13 Ette EI, Williams PJ. Population pharmacokinetics: I. Back-
albuterol-evoked bronchodilation in asthmatics. Pulm Phar-
ground, concepts, and models. Ann Pharmacother 2004;
macol Ther 2000; 13:127–134
35 Tinkelman DG, Avner SE, Cooper DM. Assessing broncho-
14 Pellegrino R, Viegi G, Brusasco V, et al. Interpretative
dilator responsiveness. J Allergy Clin Immunol 1977; 59:109–114
strategies for lung function tests. Eur Respir J 2005;
36 Barnes PJ. -Adrenergic receptors and their regulation. Am J
Respir Crit Care Med 1995; 152:838 – 860
15 Polgar G, Promadhat V. Standard values: pulmonary function
37 Martinez FD, Graves PE, Baldini M, et al. Association
testing in children; techniques and standards. Philadelphia,
between genetic polymorphisms of the 2-adrenoceptor and
PA: WB Saunders, 1971; 87–212
response to albuterol in children with and without a history of
16 Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the
wheezing. J Clin Invest 1997; 100:3184 –3188
normal maximal expiratory flow-volume curve with growth
38 Lima JJ, Thomason DB, Mohamed MH, et al. Impact of
and aging. Am Rev Respir Dis 1983; 127:725–734
genetic polymorphisms of the 2-adrenergic receptor on
17 Blake KV, Harman E, Hendeles L. Evaluation of a generic
albuterol bronchodilator pharmacodynamics. Clin Pharmacol
albuterol metered-dose inhaler: importance of priming the
Ther 1999; 65:519 –525
MDI. Ann Allergy 1992; 68:169 –174
39 Small KM, Brown KM, Theiss CT, et al. An Ile to Met
18 Kenyon CJ, Thorsson L, Borgstrom L, et al. The effects of
polymorphism in the catalytic domain of adenylyl cyclase type
static charge in spacer devices on glucocorticosteroid aerosol
9 confers reduced 2-adrenergic receptor stimulation. Phar-
deposition in asthmatic patients. Eur Respir J 1998; 11:606–610
macogenetics 2003; 13:535–541
19 InspirEase prescribing information [package insert]. Ken-
40 Tantisira KG, Small KM, Litonjua AA, et al. Molecular
ilworth, NJ: Schering-Plough Corporation, 2007
properties and pharmacogenetics of a polymorphism of ad-
Original Research
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
enylyl cyclase type 9 in asthma: interaction between beta-
ness: FEV(1) and symptom differences in whites and African
agonist and corticosteroid pathways. Hum Mol Genet 2005;
Americans with mild asthma. J Asthma 2007; 44:621– 628
51 El Ekiaby A, Brianas L, Skowronski ME, et al. Impact of race
41 Hawkins GA, Tantisira K, Meyers DA, et al. Sequence,
on the severity of acute episodes of asthma and adrenergic
haplotype, and association analysis of ADR2 in a multiethnic
responsiveness. Am J Respir Crit Care Med 2006; 174:508–513
asthma case-control study. Am J Respir Crit Care Med 2006;
52 Choudhry S, Ung N, Avila PC, et al. Pharmacogenetic
differences in response to albuterol between Puerto Ricans
42 Drysdale CM, McGraw DW, Stack CB, et al. Complex
and Mexicans with asthma. Am J Respir Crit Care Med 2005;
promoter and coding region 2-adrenergic receptor haplo-
types alter receptor expression and predict in vivo respon-
53 Naqvi M, Thyne S, Choudhry S, et al. Ethnic-specific differ-
siveness. Proc Natl Acad Sci U S A 2000; 97:10483–10488
ences in bronchodilator responsiveness among African Amer-
43 Silverman EK, Kwiatkowski DJ, Sylvia JS, et al. Family-based
icans, Puerto Ricans, and Mexicans with asthma. J Asthma
association analysis of 2-adrenergic receptor polymorphisms
2007; 44:639 – 648
in the Childhood Asthma Management Program. J Allergy
54 Kelly HW, Murphy S. -Adrenergic agonists for acute, severe
Clin Immunol 2003; 112:870 – 876
asthma. Ann Pharmacother 1992; 26:81–91
44 Hancox RJ, Aldridge RE, Cowan JO, et al. Tolerance to
55 Tarala RA, Madsen BW, Paterson JW. Comparative efficacy
-agonists during acute bronchoconstriction. Eur Respir J
of salbutamol by pressurized aerosol and wet nebulizer in
1999; 14:283–287
acute asthma. Br J Clin Pharmacol 1980; 10:393–397
45 Haney S, Hancox RJ. Tolerance to bronchodilation during
56 Global Initiative for Chronic Obstructive Lung Disease.
treatment with long-acting -agonists: a randomised con-
Global Strategy for the Diagnosis, Management, and Preven-
trolled trial. Respir Res 2005; 6:107
tion of Chronic Obstructive Pulmonary Disease. Bethesda,
46 van der Woude HJ, Winter TH, Aalbers R. Decreased
MD: National Institutes of Health, National Heart, Lung, and
bronchodilating effect of salbutamol in relieving methacho-
Blood Institute, and the World Heath Organization, 2007
line induced moderate to severe bronchoconstriction during
57 Asmus MJ, Sherman J, Hendeles L. Bronchoconstrictor
high dose treatment with long acting 2 agonists. Thorax
additives in bronchodilator solutions. J Allergy Clin Immunol
2001; 56:529 –535
1999; 104:S53–S60
47 Boskabady MH, Farhadi H. Relation of airway responsiveness
58 Beasley R, Fishwick D, Miles JF, et al. Preservatives in
to salbutamol and to methacholine in smokers. Med Sci
nebulizer solutions: risks without benefit. Pharmacotherapy
Monit 2005; 11:CR344 –CR350
1998; 18:130 –139
48 Pedersen SE, Bateman ED, Bousquet J, et al. Determinants
59 Ponder RD, Wray BB. A case report: sensitivity to benzalko-
of response to fluticasone propionate and salmeterol/flutica-
nium chloride. J Asthma 1993; 30:229 –231
sone propionate combination in the Gaining Optimal Asthma
60 Spooner LM, Olin JL. Paradoxical bronchoconstriction with
Control study. J Allergy Clin Immunol 2007; 120:1036 –1042
albuterol administered by metered-dose inhaler and nebu-
49 Boulet LP, Lemiere C, Archambault F, et al. Smoking and
lizer solution. Ann Pharmacother 2005; 39:1924 –1927
asthma: clinical and radiologic features, lung function, and
61 Boucher M, Roy MT, Henderson J. Possible association of
airway inflammation. Chest 2006; 129:661– 668
benzalkonium chloride in nebulizer solutions with respiratory
50 Hardie GE, Brown JK, Gold WM. Adrenergic responsive-
arrest. Ann Pharmacother 1992; 26:772–774
CHEST / 134 / 5 / NOVEMBER, 2008
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
Source: http://dev.chestpubs.org/data/Journals/CHEST/22078/zcb01108000981.pdf
Case Report of Eosinophilic Gastroenteropathy and a Sandra Roberto A,1, Rómulo Bonilla G, MD,2 Gabriel Pérez G, MD.3 1 Fourth year medical student at the Hospital Universitario de Santander of the Universidad Introduction: Eosinophilic gastroenteropathy is a rare disease characterized by infiltration of eosinophils into one Industrial de Santander in Bucaramanga, Colombia
Malawi HIV and AIDS Monitoring and Evaluation Report 2005-2006 Estimated Population based HIV prevalence per Region Acknowledgements The National AIDS Commission (NAC) is grateful to the following organizations for providing data used to compile this report; all CBOs that reported to district assemblies in the 2005-6 Financial Year; NGOs, FBOs, public sector and private sector institutions that submitted reports to NAC, and District Assemblies during the 2005-2006 Financial Year; District AIDS Coordinators and numerous individuals that assisted in data abstraction from data source institutions, namely: Mr. J. Ghobede (Population Services International) Mrs. Veronica Chirwa, Mr James Gondwe and Mr. J. Zingeni (Ministry of Health, John Snow Incorporated Project); Mr. Chris Moyo and Mr. Naphini (Ministry of Health, HMIS); Dr Felix Salaniponi (Ministry of Health, TB Control Programme); Mr George Bello (Ministry of Health-CHSU); Dr. Eric Schouten (Ministry of Health, HIV Co-ordinator); Professor Tony Harries (Ministry of Health, HIV and AIDS Unit), Tupochele Mtila and Felistas Sibweza (Banja la Mtsogolo), Mrs Kanyuka and Mr. Derick Zanera (National Statistics Office); Mr. Wellington Limbe and Mr Tapson Ndundu (Malawi AIDS Counselling and Rehabilitation Organisation); Mr. D. Runganaikaloo (National AIDS Commission- Financial Management Agency); HIV Sentinel Surveillance Technical Working Group; Dr Kalanda (MASAF); National Health Accounts and HIV Resource Tracking Technical Working Group; and, the Malawi Demographic and Health Survey Steering Committee,





