Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Doi:10.1016/j.jmb.2006.06.007
J. Mol. Biol. (2006) 361, 140–152
Xenobiotic Reductase A in the Degradation of Quinolineby Pseudomonas putida 86: PhysiologicalFunction, Structure and Mechanism of8-Hydroxycoumarin Reduction
Julia J. Griese1 †, Roman P. Jakob1 †, Stephan Schwarzinger2and Holger Dobbek1⁎
A continuous evolutionary pressure of the biotic and abiotic world has led to
the development of a diversity of microbial pathways to degrade and
Universität Bayreuth,
biomineralize aromatic and heteroaromatic compounds. The heterogeneity
Universitätsstrasse 30,
of compounds metabolized by bacteria like Pseudomonas putida indicates the
95447 Bayreuth, Germany
large variety of enzymes necessary to catalyse the required reactions.
Quinoline, a N-heterocyclic aromatic compound, can be degraded by
Lehrstuhl Biopolymere,
microbes along different pathways. For P. putida 86 quinoline degradation by
Universität Bayreuth, Germany
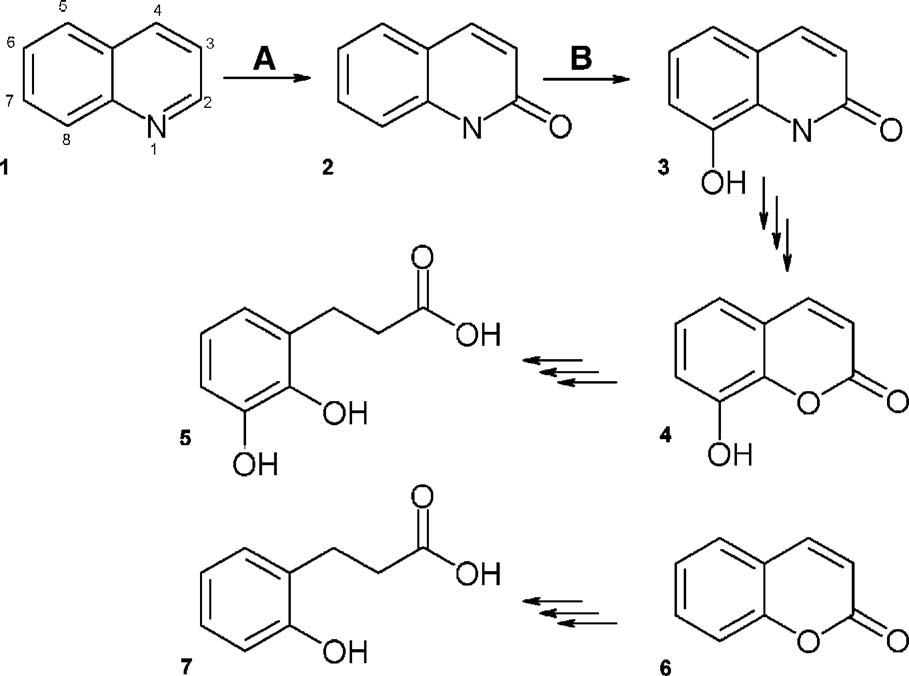
the 8-hydroxycoumarin pathway has been described and several inter-mediates were identified. To select enzymes catalysing the later stages of the8-hydroxycoumarin pathway P. putida 86 was grown with quinoline. TheFMN-containing enzyme xenobiotic reductase A (XenA) was isolated andanalysed for its reactivity with intermediates of the 8-hydroxycoumarinpathway. XenA catalyses the NADPH-dependent reduction of 8-hydro-xycoumarin and coumarin to produce 8-hydroxy-3,4-dihydrocoumarin and3,4-dihydrocoumarin, respectively. Crystallographic analysis of XenA aloneand in complex with the two substrates revealed insights into themechanism. XenA shows a dimeric arrangement of two (β/α)8 barreldomains each binding one FMN cofactor. High resolution crystal structuresof complexes with 8-hydroxycoumarin and coumarin show different modesof binding for these molecules in the active site. While coumarin is ideallypositioned for hydride transfer from N-5 of the isoalloxazine ring to C-4 ofcoumarin, 8-hydroxycoumarin forms a non-productive complex withoxidised XenA. Orientation of 8-hydroxycoumarin in the active site appearsto be dependent on the electronic state of the flavin.
We postulate that XenA catalyses the last step of the 8-hydroxycoumarin
pathway before the heterocyclic ring is hydrolysed to yield 3-(2,3-dihydroxyphenyl)-propionic acid. As XenA is also found in P. putida strainsunable to degrade quinoline, it appears to have more than one physiologicalfunction and is an example of how enzymes with low substrate specificitycan help to explain the variety of degradation pathways possible.
2006 Published by Elsevier Ltd.
Keywords: xenobiotic reductase; flavin; quinoline; Pseudomonas putida 86;
*Corresponding author
Old Yellow Enzyme
† J.J.G. and R.P.J. contributed equally to the work.
Present addresses: J.J. Griese, Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research,
Germany; R.P. Jakob, Lehrstuhl Biochemie, Universität Bayreuth, Germany.
Abbreviations used: XenA, xenobiotic reductase A; OYE, Old Yellow Enzyme; NG, nitroglycerin;
PETN, pentaerythritol tetranitrate.
E-mail address of the corresponding author:
0022-2836/$ - see front matter 2006 Published by Elsevier Ltd.
Structure and Function of XenA
xycoumarin 4 and 3-(2,3-dihydroxyphenyl)-propionic acid 5 (The enzymes producing
Approximately 15 × 106 tons of coal tar are
and converting these two compounds are not
produced worldwide every year and are the source
known. Quinoline degrading P. putida strains do
for condensed aromatics and N-heteroaromatics like
not grow with coumarin as a sole source of carbon
and energy. However, when bacteria that were
have developed strategies to metabolize naturally
grown with quinoline are incubated with coumarin
occurring aromatic compounds and xenobiotics and
6, 3-(2-hydroxyphenyl)-propionic acid 7 accumu-
as they are able to biomineralize potentially toxic
latesCharacteristic reactions of coumarins are
compounds, they can be exploited in the bioreme-
additions to the C-3/C-4 double bond and the
diation of polluted soils and Quinoline is a
nucleophilic opening of the lactone group.Addi-
ubiquitous, soluble, heteroaromatic pollutant with
tion of a hydride and a proton to the C-3/C-4 double
cancerogenic propertiSeveral bacterial species
bond and hydrolysis of the lactone explains the
are capable of degrading quinoline and can grow
observed products 3-(2,3-dihydroxyphenyl)-propio-
with quinoline or its derivatives as sole sources of
nic acid 5 and 3-(2-hydroxyphenyl)-propionic acid 7
carbon, nitrogen and energy. A unique pathway
and defines the activity of the enzymes able to
exists in Pseudomonas putida 86 that was isolated
convert 8-hydroxycoumarin 4 and coumarin 6
from soil near a coal tar factory (Rütgerswerke,
Castrop-Rauxel, Germany) by quinoline enrichment
acid 5 can be further degraded to intermediates of
culture Within the 8-hydroxycoumarin
the tricarboxylic acid 3-(2-hydroxyphenyl)-
pathway of P. putida 86 the N-heterocyclic ring of
propionic acid 7 cannot, explaining why P. putida 86
quinoline 1 is cleaved preferentially to form 8-
does not grow with coumarin as carbon source
hydroxycoumarin 4. Four intermediates (2−5) have
Bacterial enzymes known to catalyse the reduc-
been identified in the 8-hydroxycoumarin path-
tion of the olefinic bond of α,β-unsaturated carbonyl
way,and the enzymes that catalyse the first two
compounds including ketones and esters are a group
steps have been investigated (The
of enzymes called xenobiotic reductaseThese
molybdo-iron-sulfur flavoprotein quinoline oxido-
enzymes constitute a bacterial subgroup of the Old
reductase (QOR) hydroxylates quinoline 1 at C-2 to
Yellow Enzyme (OYE) family and are monomeric,
yield 1H-2-oxoquinoline 2The multicomponent
homodi- or tetrameric, NAD(P)H-dependent, FMN-
enzyme 1H-2-oxoquinoline 8-monooxygenase cata-
containing oxidoreductases with a subunit size of
lyses the NADH-dependent second hydroxylation
∼40 kDa. Like for OYE itself, the physiological
reaction at C-8 to yield 1H-8-hydroxy-2-oxoquino-
oxidant and with it the physiological function is
line 3The next two intermediates are 8-hydro-
unknown for almost all members of the OYE family.
Figure 1. Degradation of quinoline and coumarin by P. putida 86. Intermediates of the 8-hydroxycoumarin pathway are
depicted.The first reaction of the pathway is labelled with A and is catalysed by quinoline 2-oxidoreductase.The secondreaction, labelled with B, is catalysed by 2-oxoquinoline Triple arrows indicate that the reactionsequence between the different intermediates is unclear and multiple steps could be involved. In the following the names ofall substances depicted are given: (1) quinoline, (2) 1H-2-oxoquinoline, (3) 1H-8-hydroxy-2-oxoquinoline, (4) 8-hydroxycoumarin, (5) 3-(2,3-dihydroxyphenyl)-propionic acid, (6) coumarin, and (7) 3-(2-hydroxyphenyl)-propionic acid.
Structure and Function of XenA
Recently, some enzymes of the OYE family were
substrate 2-cyclohexenone was used as positive
implicated in antioxidant defense by detoxification
controQuinoline, 2-quinolinol, 4-quinolinol and
of reactive electrophilic substrates.,Xenobiotic
2,8-quinolinediol did not oxidise reduced XenA
reductases catalyse three types of reactions whereby
(data not shown). Coumarin and 8-hydroxycou-
they accept a broad range of electrophilic, xenobiotic
marin were reduced by XenA, albeit more than
compounds: (i) reduction of the olefinic bond of α,β-
tenfold slower than 2-cyclohexenone. XenA has an
unsaturated carbonyl compounds like 2-cyclohex-
apparent turnover number of 8 s−1 with 2-cyclohex-
enone; (ii) reductive denitration of aliphatic nitro
enone, 0.4 s−1 with coumarin and 0.2 s−1 with 8-
esters like nitroglycerin (NG) or pentaerythritol
hydroxycoumarin (). XenA shows an ∼four-
tetranitrate (PETN); (iii) reduction of nitroaromatic
fold preference for NADPH over NADH. It will also
compounds like 2,4,6-trinitrotoluene.
reduce molecular oxygen with a specific activity of
Xenobiotic reductase A (XenA) was originally
0.2 unit/mg at oxygen saturation of the buffer
identified in a P. putida strain that had been isolated
(263 μM at 25 °C, 1 bar). Therefore, assays were
from NG-contaminated This strain, P. putida II-
conducted under anaerobic conditions. Apparent
B, grows with NG as sole nitrogen source because
values for the Michaelis constant (KM) for all three
XenA denitrates NG. XenA also reduces 2-cyclohex-
substrates are in the same range with 33(±4) μM for 2-
enone to cyclohexanone and the nitro groups of
cyclohexenone, 23(±2) μM for coumarin and 34(±3)
trinitrotoluene to amino groups.XenA is an
μM for 8-hydroxycoumarin. The apparent KM value
NADPH-dependent intracellular enzyme with a
for NADPH, determined with 2-cyclohexenone as
subunit size of 39.8 kDa (for the apoprotein). It
oxidative substrate is 61(±7) μM (
contains one molecule of FMN per subunit. Based on
Real-time NMR spectra were recorded to deter-
gel filtration chromatography it was suggested to be
mine the products of the reactions with 8-hydroxy-
monomeric in solution.
coumarin and coumarin For comparison,
To investigate the late steps of the 8-hydroxycou-
reference spectra of educts (8-hydroxycoumarin,
marin pathway, we were searching for enzymes able
coumarin, and NADPH) and possible products
to convert 8-hydroxycoumarin 4 and coumarin 6,
explaining the reactive principles of the pathway
nic acid and NADP+) were measured. The newly
). We found that XenA is expressed in P.
appearing resonances agreed well with the forma-
putida 86 grown with quinoline as sole carbon,
tion of 8-hydroxy-3,4-dihydrocoumarin and 3,4-
nitrogen and energy source. Ligand titration and
dihydrocoumarin, respectively, and NADP+, while
crystallographic analysis of XenA showed its ability
the resonances disappearing corresponded to 8-
to bind 8-hydroxycoumarin 4 and coumarin 6 in
hydroxycoumarin and coumarin, respectively, and
solution and the crystalline state. Kinetic analysis
NADPH. Approximate rate constants derived for the
proved its ability to reduce the C-3/C-4 double
time-dependent evolution of selected resonances
bond of 8-hydroxycoumarin 4 and coumarin 6.
show similar rates for NADP+ and 8-hydroxy-3,4-dihydrocoumarin production and for NADPH and
Results and Discussion
8-hydroxycoumarin decay (data not shown). Thenearly complete decay of resonances of the eductsshows that the equilibria of both reactions (8-
Expression and purification of XenA
hydroxycoumarin and coumarin) are on the side ofproducts No resonances indicating a
Escherichia coli BL21(DE3) (Stratagene) was used
hydrolysis of the lactone group were detectable
for overexpression of recombinant XenA. Four
during the 5 h of the real time NMR experiment.
column chromatography steps were used to purifymore than 30 mg XenA with a purity exceeding 95%per 10 g of cells (wet weight). The protein solutionwas reconstituted with FMN before the gel filtration
Table 1. Steady-state kinetic properties of xenobiotic
step because E. coli produces XenA to ∼70% in the
apoform, as approximated by the ratio of absorbance
at 280 nm to 464 nm of the protein before and after
reconstitution. The reconstituted recombinant pro-
tein displayed an absorbance ratio A280/A464 of 7.5.
The same ratio was determined for XenA purified
using the same strategy from P. putida 86, indicating
that reconstitution with FMN was complete.
Apparent kinetic constants were derived from steady-state kineticanalyses. The assays were performed at 25 °C in 1 ml of 50 mM
Reactivity of XenA
potassium phosphate buffer (pH 7.0). The compounds 7-hydro-xycoumarin, quinoline, 2-quinolinol, 4-quinolinol, 2,8-quinoline-diol, 2,4-dinitrophenol, picric acid and metronidazole were also
To investigate whether XenA participates in the 8-
tested, but no activity was detected.
hydroxycoumarin pathway, its ability to catalyse the
a Reductive substrate: 150 μM NADPH.
reduction of quinoline, coumarin and several of their
b Oxidative substrate: 300 μM 2-cyclohexenone.
derivatives with NADPH as a reducing substrate
The reaction mixture contained 250 nM XenA.
d The reaction mixture contained 1 μM XenA and 1% DMSO.
was tested. The previously identified oxidative
Structure and Function of XenA
Figure 2. Kinetic analysis of
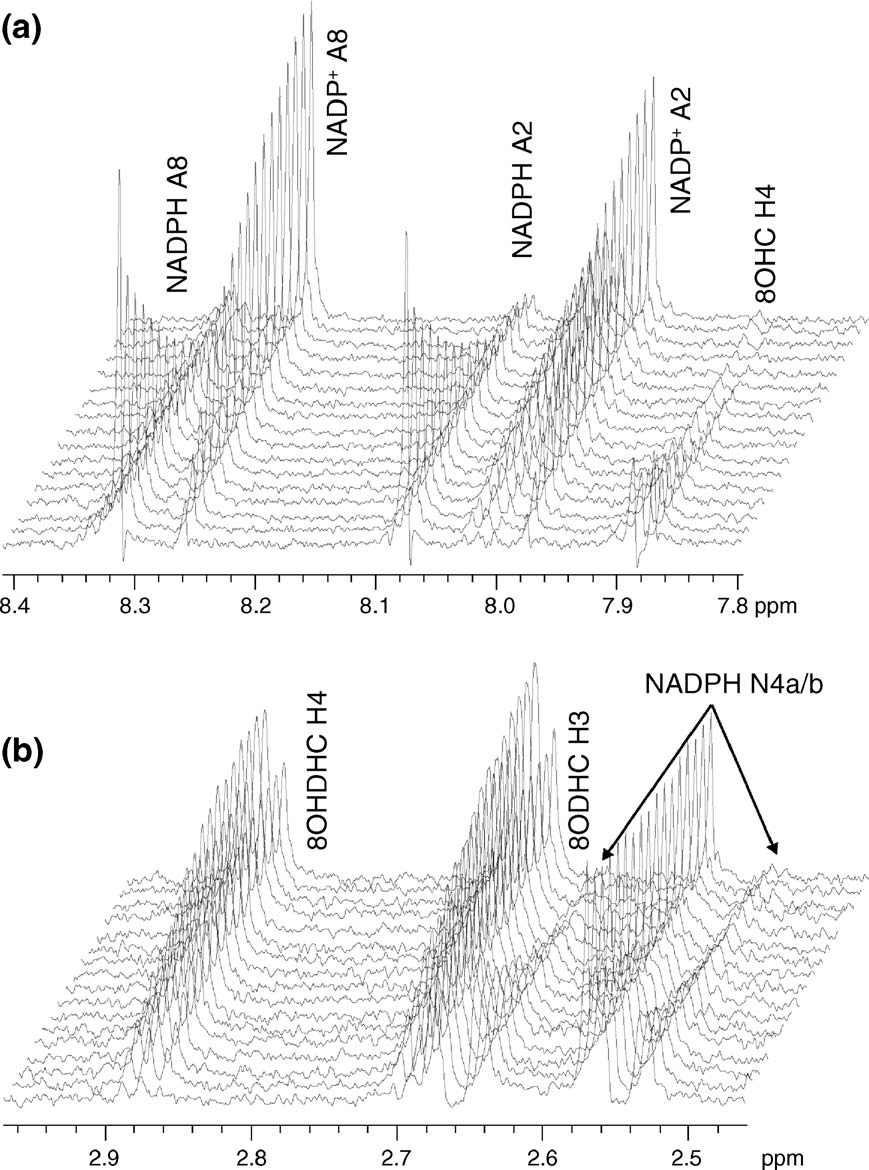
XenA substrate turnover by real-time NMR spectroscopy. Series of1D proton NMR spectra recorded at600 MHz. The time between eachspectrum shown is approximately18 min. The last spectrum shown wasrecorded 288 min after addition ofXenA. (a) A portion of the spectrashowing the decrease and increase ofthe signals corresponding to theadenosine protons A8 and A2 ofNADPH and NADP+, respectively.
A2 of NADP+ overlaps with theincreasing signal of the nicotinamideresonance N5 of NADP+ (labelomitted for clarity). The decayingdoublet at 7.88 ppm corresponds toH4 of the substrate 8-hydroxycou-marin (8OHC). (b) The increasingsignal of protons H3 (2.69 ppm) H4(2.88 ppm) of the enzymatic product,8-hydroxy-3,4-dihydrocoumarin(8OHDHC). H3 partially overlapswith the decaying signal of theprotons at position 4a of the nicotin-amide moiety of NADPH (4a: 2.66and 4b: 2.55 ppm, respectively). Thepositions of H3 and H4 are almostexactly identical to those of 3,4-dihydrocoumarin but different fromthe hydrolysed form, clearly suggest-ing that 8-hydroxy-3,4-dihydrocou-marin is the end product of theenzymatic reaction of XenA. Thereaction with coumarin yields essen-tially identical results, except thatenzymatic turnover is approxi-mately two to threefold higher (datanot shown).
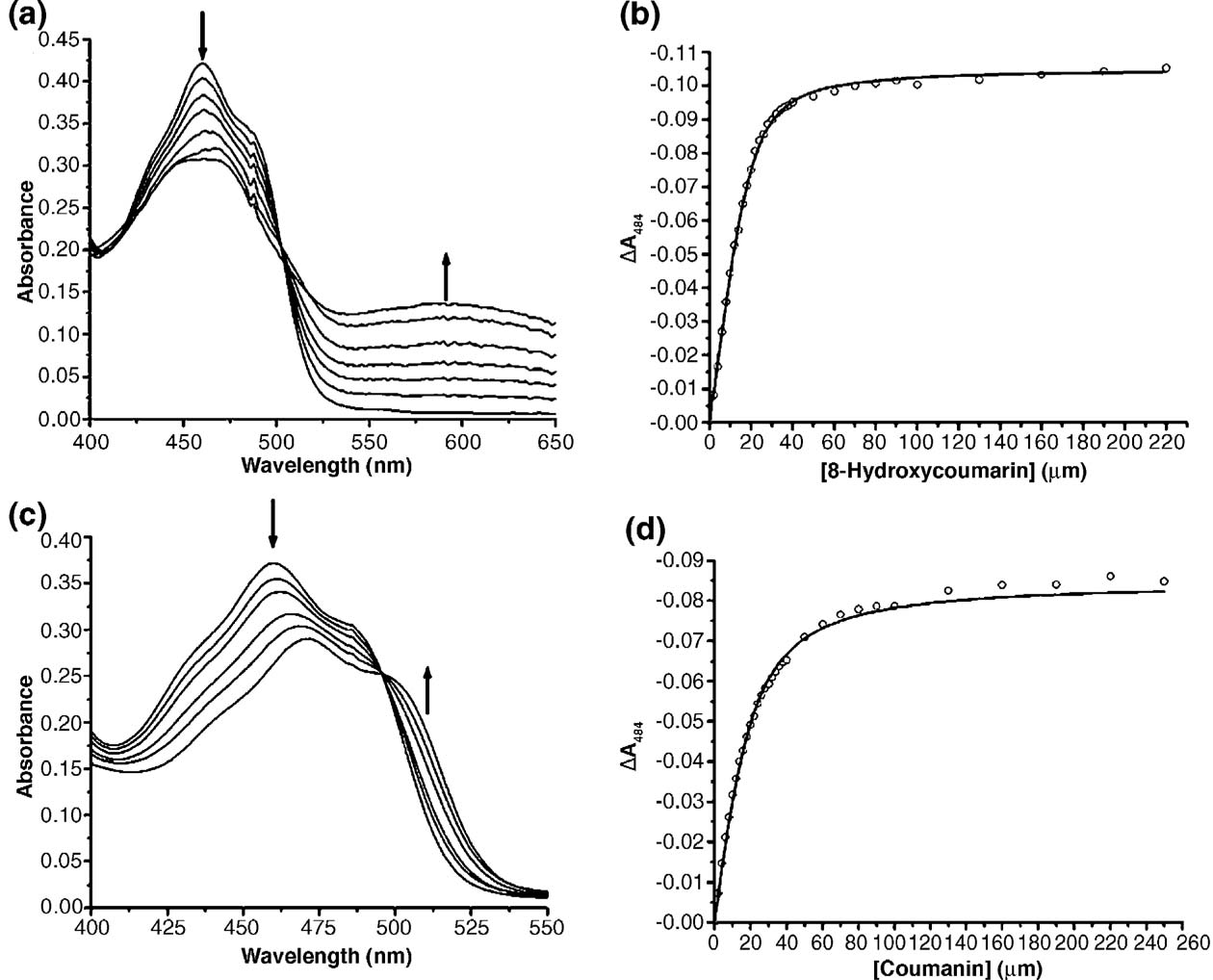
a phenolic group.There is no hyperchromic effectaround 600 nm, and the hypochromic effect at
The titration of oxidised XenA with coumarin and
464 nm is accompanied by a red shift of the entire
8-hydroxycoumarin resulted in perturbations of the
flavin absorbance (c)). Plots of absorption
electronic absorption spectrum of the enzyme-
change at 464 nm versus ligand concentration and
bound FMN and allowed determination of the
fitting to equation (1) gave dissociation constants
dissociation constant of the complexes ).
(Kd) of 2.5(±0.5) μM and 5.0(±1.8) μM for 8-
Optical titration with the two substrates showed
hydroxycoumarin and coumarin, respectively
isosbestic points at 502 nm for 8-hydroxycoumarin
(b) and (d)).
(and 496 nm for coumarin (The absorption changes recorded upon titration of
Overall structure and oligomerisation state
oxidised XenA with 8-hydroxycoumarin are characteristic of charge transfer complexes
The structure of native oxidised XenA from P.
between ligands with a deprotonated phenolic hy-
putida 86 was solved by single isomorphous
droxyl group and the oxidised flavin.The spectra
replacement combined with anomalous dispersion
display a hypochromic effect at 464 nm, corre-
of an iodide derivative ). The resulting
sponding to the flavin absorption maximum, and a
electron density was basically interpretable and
hyperchromic effect at 600 nm probably elicited by
displayed the overall fold of the (β/α)8-barrel. To
the charge transfer interaction. Coumarin causes
optimize automatic model building by using differ-
only a perturbation of the electronic absorption
ent phase sources, a Patterson search was carried out
spectrum of FMN without additional charge
with a search model constructed from the crystal
transfer interactions (c)), as found for
structure of morphinone reductase (PDB-
titrations of OYE with ligands that do not contain
ID:1GWJ)The combined phases from SIRAS
Structure and Function of XenA
Figure 3. Titrations of XenA with 8-hydroxycoumarin and coumarin. Conditions: 30 μM XenA in 50 mM potassium
phosphate buffer (pH 7.0), 20 °C. (a) Spectral changes recorded on titrating XenA with 8-hydroxycoumarin. For clarity,only selected spectra are shown, including the spectra of start and end points of the titration. (b) Plot of absorbance changeat 464 nm versus 8-hydroxycoumarin concentration. The continuous line results from the fit to equation (1). (c) and (d) Asfor (a) and (b), but for titration of XenA with coumarin. Arrows indicate the direction of spectral changes.
phasing and the molecular replacement solution
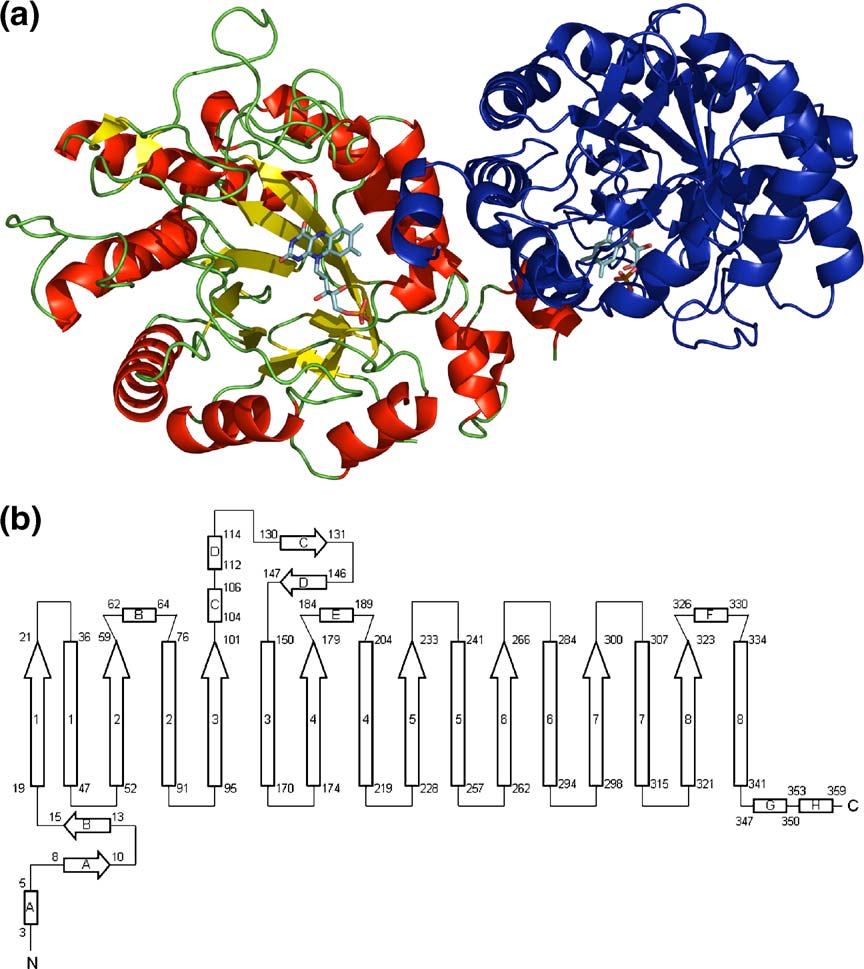
helices surround eight twisted β-strands, forming a
allowed automatic tracing and model building
cylindrical structure (a)). On the N-terminal
including the majority of the water structure. The
side of the β-strands, the barrel is closed by a β-
structure was refined to 1.5 Å resolution with a final
hairpin, whereas on the C-terminal side it is wide
R-factor of 18.2% (). Stereochemical para-
open. The flavin is bound at the C-terminal end.
meters of the model are good except for one residue,
The longer C-terminal loops contain additional
Trp302, which is found in the disallowed region of
secondary structure elements, namely several short
the Ramachandran plot (). The XenA mono-
310-helices, α-helices and two short β-strands
mer adopts a (β/α)8-barrel fold where eight α-
(the C-terminal loops are numbered
Table 2. Phasing statistics
Phasing power.
Friedel mates were treated as independent reflections.
a Heavy atom derivative: 12 iodide sites per a.u.
b Rs=Σh Σi Ii(h)−<I(h)> /Σh Σi Ii(h); where i are the independent observations of reflection h.
c RCullis=Σh ( FPH (h)–FP(h) − FHcalc(h)) /Σh FPH(h)–FP(h) .
Structure and Function of XenA
Table 3. Crystallographic data and refinement
Total/unique refl.
20−1.50 (1.6−1.5)
20−1.42 (1.50−1.42)
20−1.42 (1.50−1.42)
Model R/Rfree-factor (%)b
rms deviation from ideal geometry
Ramachandran statistics (%)
generously allowed/disallowed regions
Friedel mates were merged. XenA: XenA crystal with sulphate ion in the active site. XenA8-OH-Cum: crystal of XenA soaked with 8-hydroxycoumarin. XenACum: crystal of XenA soaked with coumarin. All three crystals belonged to space group I222. The values given inparantheses are for the highest resolution shell. The Ramachandran statistics were calculated with PROCHECK.50
a Rs=Σh Σi Ii(h)−<I(h)> /Σh Σi Ii(h); where i are the independent observations of reflection h.
b The free R-factor was calculated from 5% of the data, which were removed at random before the structure was refined.
according to the preceding β-strands). Loop L3 is the
between helices α1 of the two subunits. Additional
longest loop, consisting of 48 residues, and contains
interactions between the monomers are built up by
most of the barrel's extra secondary structure
the C terminus from helix αF to αH. Trp358 in helix
elements and active site residues.
αH protrudes into the active site of the neighbouring
Like many other OYE family members, XenA
monomer and forms part of its FMN and substrate
forms a homodimer. Monomer A in the asymmetric
binding pocket (see below). The dimer interface
unit is related to monomer B by a crystallographic 2-
shields 10% of the monomer surface (1380 Å2 per
fold axis, resulting in approximately opposite
monomer). It lies on the opposite side of the barrel in
directions for the barrel openings. The crystal-
comparison to the dimer interface of OYE that is
lographic 2-fold axis forming the dimer runs
formed by helices 4, 5, and 6.
Figure 4. Overall structure of
XenA. (a) Ribbon diagram of theXenA dimer. Monomer A is colouredaccording to its secondary structure:red, helices; yellow, β-strands; green,coils. Monomer B is coloured blue.
The FMN cofactors of both mono-mers are shown in stick form withcarbon in cyan, nitrogen in blue,oxygen in red, and phosphorus inorange. (b) Topology of XenA. He-lices are displayed as rectangles,strands as arrows. Helices andstrands of the barrel are numberedaccording to their order in the barrel.
Extra barrel secondary structure ele-ments are designated by letters. Thenumbers at the beginning and end ofeach secondary structure elementaccount for the amino acid number.
Structure and Function of XenA
The structure of XenA is highly similar to that of
occupation of the active site: in the absence of a
other OYE homologues. The closest homologue of
ligand, three different conformations were discern-
XenA is the flavoprotein YqjM from Bacillus
ible in the electron density, whereas in the presence
subtilis,The two proteins share an overall
of a ligand, the γ-sulfhydryl group pointed exclu-
amino acid sequence identity of 40% and similarity
sively to O-4 of the flavin. It was suggested that this
of 54% and are closely related in structure: 292
residue modulates the redox potential of the flavin
residues can be superimposed with a root mean
depending on the presence of substratHow-
square deviation of 1.2 Å.The major structural
ever, in XenA, Cys25 is in hydrogen-bonding
difference in the monomers is in loop L3, which
distance to O-4 of the cofactor in all three structures,
consists of 48 residues in XenA, whereas loop L3 of
indicating a role of Cys25 independent of the
YqjM consists of only 30 residues. YqjM has been
occupation of the active site.
described as a dimer of dimers, while XenA is a
The dimethylbenzene ring of flavin is stabilised
homodimer in the crystal. In place of the active site
by hydrophobic interactions with Met24 on its re
residue Trp358 of XenA, YqjM has an arginine finger
side and Trp358 of the other monomer on the si
protruding into the adjacent active site.Based on
side. Trp358 extends into the active site of the
sequence comparisons of the OYE homologue YqjM
adjacent monomer and closes it on the side of the
from B. subtilis with other OYE family members, a
dimethylbenzene moiety of the cofactor through a
new bacterial subgroup of the OYE family has been
face-on-edge π−π interaction (The term-
suggested.However, several C-terminal residues
inal phosphate group is embedded into an electro-
of YqjM suggested to be invariant in this bacterial
positive groove of the protein formed by loops L7
subgroup are not found in XenA from P. putida 86,
and L8 and the positive end of the macrodipole of
the closest relative of YqjM.Specifically, Arg312,
Gln333, and Arg336 of YqjM align with Ala330,Pro354 and His357 of XenA, respectively. Arg336 is
Ligand complexes in the active site of XenA
the residue that extends into the adjacent active siteof YqjM. In XenA, this residue is functionally
The crystal structure of XenA has been analysed in
replaced by Trp358, resulting in a comparable active
complex with sulphate and the substrates coumarin
site composition from two monomers. However,
other distinctive features of the proposed new
The active site structure of oxidised, uncomplexed
subgroup of the OYE family are found in XenA as
XenA is shown in An anion binds above
well, such as Cys25 and Tyr27, which are conserved
the oxidised flavin as found for other members of
in this subgroup, but not in the OYE family
the OYE family.,A sulphate molecule could bemodelled in the electron density. The sulphate ion is
The FMN binding site
in hydrogen-bonding distance to His178, His181,and Tyr183 ().
A pocket leading to the active site is wide open
The coumarin complex is stabilized by π−π
with an average diameter of around 18 Å measured
stacking interactions between coumarin and the
between the Cβ-atoms of the surrounding amino
isoalloxazine ring (b)). The carbonyl
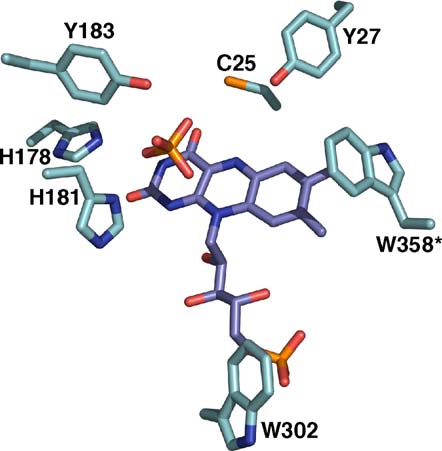
acids. It is formed between the two monomers andhas at its bottom the FMN cofactor in an elongatedconformation (). The FMN cofactor is boundat the C-terminal end of the β-barrel, above β-strands 1 and 8, with its si face exposed to thesolvent. The protein matrix interacts with thepyrimidine ring and the ribityl side-chain of theflavin through hydrogen bonds. Most of the inter-actions between apoprotein and flavin are con-served within the OYE family, for example aglutamine residue (Gln99), a histidine pair (His178and His181), and an arginine residue (Arg231) bindN-3, O-2 and N-1 of the cofactor, respectively. O-4 ofthe FMN is bound by the amide proton of Ala57 andthe γ-sulfhydryl group of Cys25. The amide protonof Cys25 binds N-5. OYE and most of its char-acterised homologues contain a threonine residue ina position equivalent to Cys25 (Thr37 in OYE),reported to increase the redox potential of thecofactor by stabilising the negative charge of the
Figure 5. Active site of oxidised XenA in complex with
reduced flavin through hydrogen bonding with O-
sulphate. All residues are displayed in stick mode with
Based on structural comparisons, the same
carbon atoms of the apoprotein in cyan, carbon atoms of
function can be assigned to Cys25 of XenA. For
the flavin in violet, nitrogen in blue, oxygen in red, and
instance, the corresponding cysteine residue in YqjM
phosphorus and sulphur in orange. The asterisk denotes a
adopts different conformations depending on the
residue from monomer B.
Structure and Function of XenA
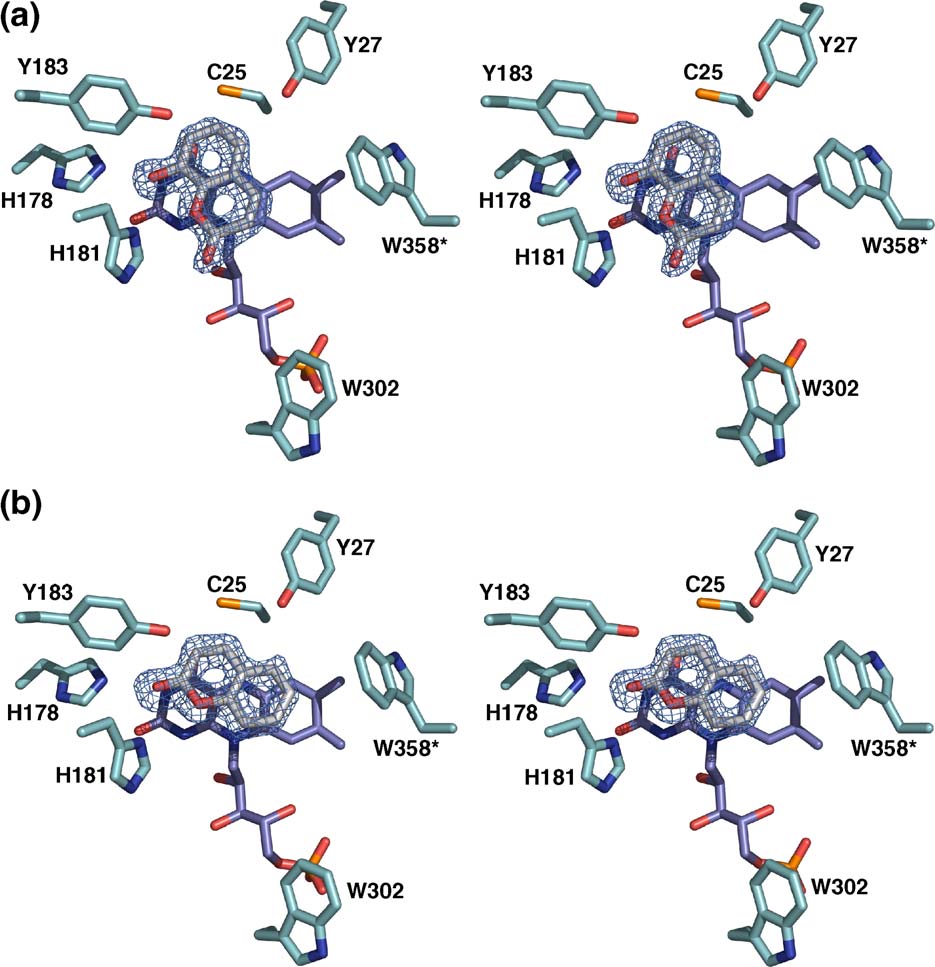
Figure 6. Stereo view of the active site of XenA in complex with (a) 8-hydroxycoumarin, (b) coumarin. All residues are
displayed in stick mode with carbon atoms of the apoprotein in cyan, carbon atoms of the flavin in violet, carbon atoms ofthe ligand in grey, nitrogen in blue, oxygen in red, and phosphorus in orange. Initial Fo−Fc electron density maps for theligands are contoured at 3 σ.
oxygen of coumarin is in hydrogen-bonding dis-
donor. This observation fits the catalytic mechan-
tance to His178 and His181. His181 also binds O-1
ism that has been proposed for OYE with a hydride
of the coumarin. There are no further interactions
transfer from the flavin N-5 to the β-carbon,
with the protein. Notably, Tyr27 does not con-
followed by proton transfer from the active site
tribute to substrate binding. The corresponding
acid Tyr196 to the α-carbon of α,β-unsaturated
residue in YqjM and the C-terminal tyrosine residue
carbonyl substrates,,However, mutational
Tyr375 that occupies the corresponding position in
studies of PETN reductase have shown that the
OYE have been shown to coordinate the carbonyl
corresponding tyrosine residue of this OYE homo-
oxygen atom of the inhibitor p-hydroxybenzalde-
logue is not essential for catalysis,while morphi-
hyde, leading to the assumption that this tyrosine
none reductase contains a cysteine residue in place
residue contributes to substrate specifity. In XenA,
of Tyr196 of OYE, which is not the essential active
the histidine pair His178 and His181 appears to be
site For PETN reductase and morphinone
exclusively responsible for substrate specificity and
reductase, it was suggested that the proton is
orientation by hydrogen-bonding in the active site.
transferred from the solvent to the substrate.,
Through coordination of the carbonyl oxygen of an
The studies on PETN reductase and morphinone
α,β-unsaturated carbonyl substrate like coumarin
reductase show that, despite a conserved active site
by the histidine pair, the reactive olefinic bond is
architecture, the function of individual residues
positioned optimally for proton and hydride
may not be conserved and has to be investigated
transfer and becomes polarised, activating the β-
for each enzyme individually. Furthermore, a
carbon for nucleophilic attack: C-4 (the β-carbon) of
dependence on the type of substrate cannot be
coumarin is in 3.7 Å distance to the hydride donor,
ruled out. Reactivity analysis of XenA mutations
N-5 of the FMN cofactor, whereas C-3 (the α-
will have to reveal if Tyr183 is essential for
carbon) is close to Tyr183, the presumptive proton
protonation of the substrate.
Structure and Function of XenA
In contrast to coumarin, 8-hydroxycoumarin
hydroxycoumarin in reduced XenA, which is in
binds in the active site in a fashion that does not
agreement with the apparent kinetic constants and
allow hydride transfer from flavin N-5 to C-4 of 8-
the product detected by NMR experiments (
hydroxycoumarin, as the two atoms are 4.7 Å
Non-productive binding modes in the oxidised
apart 8-Hydroxycoumarin is flipped
states have also been found for ligand complexes
by 180° around the central C-1a – C-4a axis in
of the related flavoproteins PETN reductasand
comparison to coumarin, so that the phenolic
nitroreductase.It has been suggested that
hydroxyl group at C-8 is hydrogen bonded to
changes in charge distribution upon reduction of
the histidine pair. His181 also coordinates O-1. The
the flavin could help to prevent some molecules
ligand thus does not bind parallel to the long axis
from binding in flavoproteins with broad substrate
of the isoalloxazine ring like coumarin, but is
specificity, like nitroreductases and xenobiotic
rotated by ∼45° relative to this axis. Again, there
are no further interactions with the protein except
Single crystal absorption spectra of uncomplexed
those mediated by the histidine pair and the flavin.
and complexed XenA are comparable to the solution
It has been proposed that phenolic compounds
spectra, and we can conclude that ligand binding in
bind to oxidised OYE in the deprotonated state,
solution and in the crystalline state are equivalent
forming a charge transfer complex with the flavin
(data not shown).
with characteristic These typicalabsorption changes have been seen upon titrationof oxidised XenA with 8-hydroxycoumarin and
Physiological role of XenA
make it likely that it binds in the deprotonatedphenolate state to oxidised XenA. As the phenolate
Evidence has been obtained that XenA can
group is able to form stronger hydrogen bonds
participate in the 8-hydroxycoumarin pathway of
with the histidine pair than the carbonyl oxygen
P. putida 86. XenA is expressed in P. putida 86
the deprotonation would explain why the binding
when grown with quinoline as a sole source of
modes of 8-hydroxycoumarin and coumarin differ
carbon, nitrogen and energy, and it catalyses the
so remarkably. The binding mode of 8-hydroxy-
NADPH-dependent reduction of 8-hydroxycou-
coumarin appears to be non-productive, as the
marin, a known intermediate in the degradation
reactive olefinic bond of 8-hydroxycoumarin does
of quinoline by P. putida Chemical reactivity
not lie above the flavin N-5 or in close proximity
of coumarin and its derivatives, our NMR analysis
to Tyr183. Due to steric clashes, it is unlikely that
of the products formed and the orientation of
re-orientation of the substrate in the active site is
coumarin in the active site complex argue that the
possible. Thus, substrate bound in the unproduc-
C-3/C-4 double bond of 8-hydroxycoumarin is
tive mode would have to be released before
reduced by XenA to yield 8-hydroxy-3,4-dihydro-
binding in the productive mode would be possible.
coumarin. Additionally, the conversion of cou-
The presence of a dominant non-productive binding
marin by P. putida 86 is explained by our kinetic
mode would be expected to result in substantially
analysis of XenA with coumarin as an oxidising
lower apparent KM and kcat values for 8-hydro-
substrate yielding 3,4-dihydrocoumarin, which,
xycoumarin compared to coumarin. However,
when subsequently hydrolysed, produces 2-hydro-
both ligands show comparable apparent KM and
xyphenylpropionic acid, which was found to
kcat values (), arguing for similar binding
accumulate in P. putida 86. NMR clearly shows
geometries in the reduced enzyme. A different
that XenA only catalyses reduction of the C-3/C-4
binding geometry of 8-hydroxycoumarin in the
double bond, but not the hydrolysis of the
reduced enzyme could originate from an influence
of the electron distribution and delocalization of
XenA appears to have several distinct biological
the isoalloxazine ring on the pKa value of the
functions in addition to its participation in the
phenol hydroxyl group of 8-hydroxycoumarin.
degradation of quinoline, as it is also found in the
While oxidised FMN can stabilise the phenolate
genome sequence of P. putida KT2440 that does not
anion by charge transfer interactions, thereby
contain the genes for other enzymes of the 8-
effectively lowering the pKa of the phenol,
hydroxycoumarin pathway. Furthermore, XenA is
FMNH2 will do so to a much smaller extent. It
also highly expressed in P. putida II-B grown in the
appears likely that in oxidised XenA 8-hydroxy-
absence of coumarins, constituting ∼14% of the total
coumarin binds in the phenolate form while in the
soluble protein.The broad substrate specificity of
reduced state the phenol would be more stable. As
XenA makes it an ideal candidate for participation
the orientation of the ligands in the active site is
in the degradation of several heteroaromatic and
dominated by the strength of the hydrogen
potentially toxic compounds. Enzymes with such a
bonding interaction with the donating histidine
broad substrate specificity can to some extent
pair, the deprotonated form would bind preferen-
explain the wide variety of aromatic, and often
tially with the phenolate oxygen while the proto-
xenobiotic, substances that are degraded by bacteria
nated 8-hydroxycoumarin would bind with the
like P. putida 86. Remarkably, XenA is to our
carbonyl oxygen of the lactone group to the
knowledge the first OYE homologue implicated in
histidine pair. The consequence would be the
the in vivo degradation of heteroaromatic com-
prevalence of a productive binding mode of 8-
Structure and Function of XenA
Materials and Methods
FMN at its absorption maximum (464 nm) was calculatedfrom the known extinction coefficient of 12.2 mM–1cm–1 at450 nm for free FMN.
Substrate specificity
All chemicals used were of analytical grade. Coumarin
and 2-cyclohexenone were obtained from Fluka. 8-Hydroxycoumarin was prepared essentially as described
Apparent steady-state kinetic constants were
by Cerqueira et All chromatography materials were
recorded by following the oxidation of NADPH at
from Amersham Biosciences.
340 nm in a Specord 30 spectrophotometer (AnalytikJena) while systematically varying the substrate con-centration. Reactions were initiated by addition of en-
Growth of P. putida 86
zyme and monitored for 2 min. All experiments wereconducted three times and results were averaged. One
P. putida 86 was cultured aerobically in 35 l fermentors at
unit of enzyme activity was defined as the oxidation
30 °C in a quinoline minimal Whenever quinoline
of 1 μmol NADPH per min at 25 °C in the assay
and 2-oxoquinoline were undetectable in the fermentation
broth, further portions of quinoline (0.5 ml/l) were added. At
The reaction mixture was made anaerobic by flush-
an optical density (600 nm) of approximately 3.5, cells were
ing with nitrogen. Enzyme assays were measured at
harvested by centrifugation, frozen in liquid nitrogen, and
25 °C in 1 ml of 50 mM potassium phosphate buffer
stored at −30 °C until further use.
(pH 7.0), containing 150 μM NADPH, and 250 nMXenA, where 2-cyclohexenone was used as oxidativesubstrate, or 1 μM XenA, where coumarin or 8-
PCR, cloning, sequencing and expression
hydroxycoumarin were used as oxidative substrate.
Apparent steady-state kinetic constants for NADPH
The nucleotide sequence of xenA from P. putida KT2440
were determined with 300 μM 2-cyclohexenone as
was obtained from GenBank (accession no. AF154061).
oxidative substrate. 8-Hydroxycoumarin and coumarin
The open reading frame of P. putida 86 xenA was amplified
were dissolved at 100-fold concentration in dimethyl
by polymerase chain reaction, using a P. putida 86 colony
sulphoxide (DMSO), keeping the DMSO concentration
as template, and cloned into pET-11a (Novagen). The
in the reaction mixture constant at 1%. The software
construct was verified by sequence analysis and was
GraFit version 5.0.10 (Erithacus Software Limited) was
transformed into E. coli BL21(DE3) (Stratagene) for
used for evaluation of the data.
expression. E. coli strain BL21(DE3) harbouring plasmidpET-11a with the insertion of xenA was grown aerobically
at 25 °C in 4 l of LB medium containing 100 μg/mlampicillin after 1% inoculation. Expression was inducedwith 0.1 mM IPTG at an optical density (600 nm) of ∼0.7.
1D-proton NMR spectra of the individual substances
Cells were harvested by centrifugation 18 h after induc-
tion, frozen in liquid nitrogen, and stored at −30 °C until
3-(2-hydroxyphenyl)-propionic acid, NADPH, and
further use.
NADP+) in an aqueous buffer (pH 7.0), containing50 mM potassium phosphate and 5% 2H2O weremeasured on a Bruker Avance 400 spectrometer
Purification of XenA
equipped with a HCN-triple-resonance probe headwith z-axis gradients at 293 K. The residual water
The enzyme heterologously expressed in E. coli BL21
signal was suppressed using double-pulsed field
(DE3) was purified by four column chromatography steps:
gradient echo The concentration of each
anion exchange chromatography, hydrophobic interaction
substance was 100 μM. 256 transients with 16.384
chromatography, gel filtration and a second anion
complex data points were recorded and zero-filled to
exchange chromatography. All chromatography steps
64,000 data points prior to Fourier transformation.
were carried out at 16 °C; in between the protein solution
Signal assignments for NADPH, NADP+, and coumarin
was kept on ice. After the hydrophobic interaction
were taken from the literature.Assignments for
chromatography step XenA-containing fractions were
the other compounds were derived from
incubated with 5 mM FMN on ice over night.
spectra and interpretation of 1D-spectra. 2D-NOESY
From P. putida 86, XenA was purified in essentially the
experiments with a mixing time of 800 ms and
same way, except that the protein was not reconstituted
with double-pulsed field gradient echos for solvent
suppressionwere recorded at 600 MHz with4000 complex data points in the direct dimensionand 256 data points in the indirect dimension. The
Determination of protein concentration
assignments were used to identify compounds in thereaction mixture during kinetic experiments (data not
The concentration of XenA holoprotein was determined
using a millimolar extinction coefficient of 12.2 mM−1cm−1
Kinetic NMR experiments of the turnover of cou-
at 464 nm. The extinction coefficient of XenA-bound FMN
marin and 8-hydroxycoumarin by XenA were recorded
was evaluated as follows: a spectrum of the native protein
on a Bruker DRX 600 spectrometer equipped with a
was recorded. The protein was then denatured by the
HCN-triple resonance probe head with x,y,z-axis
addition of 0.1 vol. 50% trichloroacetic acid, and the
gradients at 288 K. The concentration of the substrate
precipitated protein removed by centrifugation. The
was 100 μM and 150 μM the cofactor NADPH in each
supernatant was neutralised with solid sodium bicarbo-
case. After the first data point enzyme was directly
nate and the spectrum recorded against a reagent blank
added into the NMR-sample tube to a final concen-
without XenA. The extinction coefficient of XenA-bound
tration of 0.8 μM. The dead time for mixing and
Structure and Function of XenA
equilibration was approximately 5 min. A series of 50
approximately 30 s in 400 mM potassium iodide dissolved
experiments with 128 transients was recorded, each
in the harvesting buffer. The crystals were immediately
experiment requiring approximately 6 min. Spectra
frozen after soaking with iodide. Iodide sites were localized
were evaluated with MESTREC The time-
using SHELXInitial phases were calculated using
dependent intensities of selected signals were fit using
SHAR(), and modified with SOLOMONTo
Microcal™ Origin® version 6.0 (Microcal Software, Inc.)
allow for automatic model building the modified experi-
assuming exponential decay/build up to extract
mental phases were combined with phases calculated from
approximate rates. All chemical shifts were referenced
a positioned model of morphinone reductase (PDB-ID.:
relative to internal TSP.
1GWJ)using AMoRfor the Patterson search. Thecombined phases were further modified using the auto-matic building routine of wARP.The model was built
Ligand binding titrations
using MAIN 2000 , and atomic positions and B-factorswere refined with C).
30 μM oxidised XenA in 50 mM potassium phosphate
buffer (pH 7.0), at 20 °C, was titrated by systematicallyvarying the ligand concentration. Spectra were recorded
Protein Data Bank accession codes
between 240 and 650 nm with a 8452 A diode arrayspectrophotometer (Hewlett Packard). Spectral changes
The coordinates and structure factor amplitudes are
evoked by the addition of ligand to XenA agree with a
deposited in the RCSB Protein Data Bank with ID codes:
1:1 binding stoichiometry, and the isosbestic points
2H8X, 2H8Z, and 2H90.
during the titration are attestive of a single step process.
Absorption changes at two selected wavelengths withthe largest spectral perturbations were plotted againstligand concentration. The data were fitted with thesoftware Microcal™ Origin® version 6.0 (Microcal
Software, Inc.) to the quadratic function (equation (1))to calculate the dissociation constant (Kd) for the
The authors thank Susanne Fetzner for donation
enzyme−ligand complex:
of the P. putida 86 strain and helpful discussions,
Juan Manuel Urbina-González and Ellen Wiede-
mann for assistance in the preparation of 8-
hydroxycoumarin, Ilme Schlichting for the mea-
surement of single crystal absorption spectra and
T þ ET þ KdÞ� ðLT þ ET þ KdÞ2�ð4LT ET Þ
Paul Roesch for access to the NMR facilities. R.P.J. is
supported by a PhD fellowship of the Fonds derChemischen Industrie. H.D. acknowledges the
where ΔAmax is the maximum absorption change at the
Deutsche Forschungsgemeinschaft (DO-785/2) for
selected wavelength, LT is the total ligand concentration,and E
financial support.
T is the total enzyme concentration.
Protein crystallisation and structure determination
XenA was crystallised by vapour diffusion using the
1. Fetzner, S. (1998). Bacterial degradation of pyridine,
hanging-drop setup at 16 °C. The reservoir solution (500 μl)
indole, quinoline, and their derivatives under different
contained 1.4 – 1.7 M ammonium sulphate in 0.1 M Hepes
redox conditions. Appl. Microbiol. Biotecholn. 49,
buffer (pH 7.5). Two drops were set up per reservoir by
mixing 2 μl of protein solution (10 and 15 mg/ml in 10 mM
2. Fetzner, S., Tshisuaka, B., Lingens, F., Kappl, R. &
Tris-HCl (pH 8.0), respectively) with 2 μl of reservoir
Huttermann, J. (1998). Bacterial degradation of quino-
line and derivatives - Pathways and their biocatalysts.
For determination of the uncomplexed structure, single
Ang. Chem.-Int. Ed. 37, 577–597.
crystals were transferred to a harvesting buffer containing
3. Schwarz, G., Senghas, E., Erben, A., Schafer, B.,
ammonium sulphate at 1.5-fold the concentration of the
Lingens, F. & Hoke, H. (1988). Microbial-metabolism
reservoir in 0.1 M Hepes buffer (pH 7.5). To soak XenA
of quinoline and related-compounds .1. Isolation and
crystals with coumarin, 8-hydroxycoumarin and 7-hydro-
characterization of quinoline-degrading bacteria. Syst.
xycoumarin, crystals were cross-linked with 0.025% glu-
Appl. Microbiol. 10, 185–190.
taraldehyde in harvesting buffer for 1 h, and then
4. Shukla, O. P. (1986). Microbial transformation of
transferred to harvesting buffer containing 5 mM of the
quinoline by a Pseudomonas sp. Appl. Environ. Micro-
respective ligand and 5% DMSO for at least 30 min. The
biol. 51, 1332–1342.
crystals belong to space group I222 with cell dimensions
5. Blase, M., Bruntner, C., Tshisuaka, B., Fetzner, S. &
a = 57.9 Å, b = 83.4 Å, c = 156.8 Å, α = β = γ= 90° and contain
Lingens, F. (1996). Cloning, expression, and sequence
one molecule per asymmetric unit. Visible absorption
analysis of the three genes encoding quinoline 2-
spectra of single XenA crystals were recorded using a
oxidoreductase, a molybdenum-containing hydroxy-
micro spectrophotometer (4DXray Systems AB). The XenA
lase from Pseudomonas putida 86. J. Biol. Chem. 271,
crystals were mounted in a loop and kept at 100 K during
6. Kappl, R., Huttermann, J. & Fetzner, S. (2002). The
The structure of XenA was determined by SIRAS phasing
molybdenum-containing hydroxylases of quinoline,
with a potassium iodide derivative, which is routinely being
isoquinoline, and quinaldine. Met. Ions Biol. Syst. 39,
carried out with protein crystals of unknown structure. The
derivative has been prepared by incubating the crystals for
7. Bonin, I., Martins, B. M., Purvanov, V., Fetzner, S.,
Structure and Function of XenA
Huber, R. & Dobbek, H. (2004). Active site geometry
Bourenkov, G. P., Macheroux, P. & Clausen, T. (2005).
and substrate recognition of the molybdenum hydro-
The 1.3 A crystal structure of the flavoprotein YqjM
xylase quinoline 2-oxidoreductase. Structure, 12,
reveals a novel class of Old Yellow Enzymes. J. Biol.
Chem. 280, 27904–27913.
8. Rosche, B., Tshisuaka, B., Fetzner, S. & Lingens, F.
25. Holm, L. & Sander, C. (1993). Protein structure
(1995). 2-Oxo-1,2-dihydroquinoline 8-monooxygen-
comparison by alignment of distance matrices.
ase, a two-component enzyme system from Pseudo-
J. Mol. Biol. 233, 123–138.
monas putida 86. J. Biol. Chem. 270, 17836–17842.
26. Xu, D., Kohli, R. M. & Massey, V. (1999). The role of
9. Martins, B. M., Svetlitchnaia, T. & Dobbek, H.
threonine 37 in flavin reactivity of the old yellow
(2005). 2-Oxoquinoline 8-monooxygenase oxygenase
enzyme. Proc. Natl Acad. Sci. USA, 96, 3556–3561.
component: active site modulation by Rieske-[2Fe-
27. Barna, T. M., Khan, H., Bruce, N. C., Barsukov, I.,
2S] center oxidation/reduction. Structure, 13,
Scrutton, N. S. & Moody, P. C. (2001). Crystal structure
of pentaerythritol tetranitrate reductase: "flipped"
10. Eicher, T. & Hauptmann, S. (2005). The Chemistry of
binding geometries for steroid substrates in different
Heterocycles. Wiley-VCH GmbH, Weinheim.
redox states of the enzyme. J. Mol. Biol. 310, 433–447.
11. Fetzner, S. (2000). Enzymes involved in the aerobic
28. Vaz, A. D., Chakraborty, S. & Massey, V. (1995). Old
bacterial degradation of N-heteroaromatic com-
Yellow enzyme: aromatization of cyclic enones and the
pounds: molybdenum hydroxylases and ring-opening
mechanism of a novel dismutation reaction. Biochem-
2,4-dioxygenases. Naturwissenschaften, 87, 59–69.
istry, 34, 4246–4256.
12. Binks, P. R., French, C. E., Nicklin, S. & Bruce, N. C.
29. Brown, B. J., Deng, Z., Karplus, P. A. & Massey, V.
(1996). Degradation of pentaerythritol tetranitrate by
(1998). On the active site of Old Yellow Enzyme. Role
Enterobacter cloacae PB2. Appl. Environ. Microbiol. 62,
of histidine 191 and asparagine 194. J. Biol. Chem. 273,
13. Fitzpatrick, T. B., Amrhein, N. & Macheroux, P. (2003).
30. Khan, H., Barna, T., Bruce, N. C., Munro, A. W., Leys,
Characterization of YqjM, an Old Yellow Enzyme
D. & Scrutton, N. S. (2005). Proton transfer in the
homolog from Bacillus subtilis involved in the oxida-
oxidative half-reaction of pentaerythritol tetranitrate
tive stress response. J. Biol. Chem. 278, 19891–19897.
reductase. Structure of the reduced enzyme-proges-
14. Blehert, D. S., Knoke, K. L., Fox, B. G. & Chambliss,
terone complex and the roles of residues Tyr186,
G. H. (1997). Regioselectivity of nitroglycerin denitra-
His181, His184. FEBS J. 272, 4660–4671.
tion by flavoprotein nitroester reductases purified from
31. Messiha, H. L., Bruce, N. C., Sattelle, B. M., Sutcliffe,
two Pseudomonas species. J. Bacteriol. 179, 6912–6920.
M. J., Munro, A. W. & Scrutton, N. S. (2005). Role of
15. Snape, J. R., Walkley, N. A., Morby, A. P., Nicklin, S. &
active site residues and solvent in proton transfer and
White, G. F. (1997). Purification, properties, and
the modulation of flavin reduction potential in bacterial
sequence of glycerol trinitrate reductase from Agro-
morphinone reductase. J. Biol. Chem. 280, 27103–27110.
bacterium radiobacter. J. Bacteriol. 179, 7796–7802.
32. Race, P. R., Lovering, A. L., Green, R. M., Ossor, A.,
16. Miura, K., Tomioka, Y., Suzuki, H., Yonezawa, M.,
White, S. A., Searle, P. F. et al. (2005). Structural and
Hishinuma, T. & Mizugaki, M. (1997). Molecular
mechanistic studies of Escherichia coli nitroreductase
cloning of the nemA gene encoding N-ethylmaleimide
with the antibiotic nitrofurazone. Reversed binding
reductase from Escherichia coli. Biol. Pharm. Bull. 20,
orientations in different redox states of the enzyme. J.
Biol. Chem. 280, 13256–13264.
17. French, C. E. & Bruce, N. C. (1994). Purification and
33. Cerqueira, N. M. F. S. A., Oliveira-Campos, A. M. F.,
characterization of morphinone reductase from Pseu-
Coelho, P. J., de Carvalho, L. H. M., Samat, A. &
domonas putida M10. Biochem. J. 301, 97–103.
Guglielmetti, R. (2002). Synthesis of photochromic
18. Rohde, B. H., Schmid, R. & Ullrich, M. S. (1999).
dyes based on annulated coumarin systems. Helv.
Thermoregulated expression and characterization of
Chim. Acta, 85, 442–450.
an NAD(P)H-dependent 2-cyclohexen-1-one reduc-
34. Tshisuaka, B., Kappl, R., Huttermann, J. & Lingens, F.
tase in the plant pathogenic bacterium Pseudomonas
(1993). Quinoline oxidoreductase from Pseudomonas
syringae pv. glycinea. J. Bacteriol. 181, 814–822.
putida 86: an improved purification procedure and
19. Kohli, R. M. & Massey, V. (1998). The oxidative half-
electron paramagnetic resonance spectroscopy. Bio-
reaction of Old Yellow Enzyme. The role of tyrosine
chemistry, 32, 12928–12934.
196. J. Biol. Chem. 273, 32763–32770.
35. Whitby, L. G. (1953). A new method for preparing
20. Blehert, D. S., Fox, B. G. & Chambliss, G. H. (1999).
flavin-adenine dinucleotide. Biochem. J. 54, 437–442.
Cloning and sequence analysis of two Pseudomonas
36. Hwang, T. L. & Shaka, A. J. (1995). Water suppression
flavoprotein xenobiotic reductases. J. Bacteriol. 181,
that works - excitation sculpting using arbitrary wave-
forms and pulsed-field gradients. J. Magn. Reson. Ser.
21. Abramovitz, A. S. & Massey, V. (1976). Interaction of
A, 112, 275–279.
phenols with old yellow enzyme. Physical evidence
37. Auchere, F., Bertho, G., Artaud, I., Girault, J. P. &
for charge-transfer complexes. J. Biol. Chem. 251,
Capeillere-Blandin, C. (2001). Purification and struc-
ture of the major product obtained by reaction of
22. Barna, T., Messiha, H. L., Petosa, C., Bruce, N. C.,
NADPH and NMNH with the myeloperoxidase/
Scrutton, N. S. & Moody, P. C. (2002). Crystal structure
hydrogen peroxide/chloride system. Eur. J. Biochem.
of bacterial morphinone reductase and properties of
268, 2889–2895.
the C191A mutant enzyme. J. Biol. Chem. 277,
38. Miura, S. & Ichikawa, Y. (1994). Interaction of
NADPH-adrenoferredoxin reductase with NADP+
23. Fox, K. M. & Karplus, P. A. (1994). Old yellow enzyme
and adrenoferredoxin. Equilibrium and dynamic
at 2 A resolution: overall structure, ligand binding, and
properties investigated by proton nuclear magnetic
comparison with related flavoproteins. Structure, 2,
resonance. J. Biol. Chem. 269, 8001–8006.
39. Martinez-Martinez, F. J., Padilla-Martinez, I. I. &
24. Kitzing, K., Fitzpatrick, T. B., Wilken, C., Sawa, J.,
Trujillo-Ferrara, J. (2001). H-1 and C-13 NMR
Structure and Function of XenA
assignments of 2-oxo-2H-1-benzopyran-3-acyl and-3-
The CCP4 Suite: programs for protein crystallogra-
amide derivatives. Magn. Reson. Chem. 39, 765–767.
phy. Acta Crystallog. sect. D, 50, 760–763.
40. Jeener, J., Meier, B. H., Bachmann, P. & Ernst, R. R.
46. Navaza, J. (1994). AMoRe: an automated package for
(1979). Investigation of exchange processes by 2-di-
molecular replacement. Acta Crystallog. sect. A, 50,
mensional NMR-spectroscopy. J. Chem. Phys. 71,
47. Perrakis, A., Sixma, T. K., Wilson, K. S. & Lamzin, V. S.
41. Cobas, J. C. & Sardina, F. J. (2003). Nuclear magnetic
(1997). wARP: Improvement and extension of crystal-
resonance data processing. MestRe-C: a software
lographic phases by weighted averaging of multiple-
package for desktop computers. Concepts Magnetic
refined dummy atomic models. Acta Crystallog. sect.
Reson. 19A, 80–96.
D, 53, 448–455.
42. Schneider, T. R. & Sheldrick, G. M. (2002). Substruc-
48. Turk, D. (1992). Weiterentwicklung eines Programms
ture solution with SHELXD. Acta Crystallog. sect. D,
für Molekülgraphik und Elektronendichte-Manipula-
58, 1772–1779.
tion und seine Anwendung auf verschiedene Protein-
43. Uson, I., Schmidt, B., von Bulow, R., Grimme, S., von
Strukturaufklärungen, TU München.
Figura, K., Dauter, M. et al. (2003). Locating the
49. Brünger, A. T., Adams, P. D., Clore, G. M., Delano,
anomalous scatterer substructures in halide and sulfur
W. L., Gros, P., Grosse Kunstleve, R. W. et al. (1998).
phasing. Acta Crystallog. sect. D, 59, 57–66.
Crystallography and NMR system: a new software
44. La Fortelle, E. D., Irwin, J. J. & Bricogne, G.
suite for macromolecular structure determination.
(1997). SHARP: A maximum-likelihood heavy-
Acta Crystallog. sect. D, 54, 905–921.
atom parameter refinement and phasing program
50. Laskowski, R. A., MacArthur, M. W., Moss, D. S. &
for the MIR and MAD methods. Crystallog.
Thornton, J. M. (1993). PROCHECK: a program to
Comput. 7, 1–9.
check the stereochemical quality of protein structures.
45. Collaborative Computational Project No. 4. (1994).
J. Appl. Crystallog. 26, 283–291.
Edited by R. Huber
(Received 4 April 2006; received in revised form 1 June 2006; accepted 7 June 2006)
Available online 21 June 2006
Source: http://btcpx9.biomac.uni-bayreuth.de/Publications/Papers/2006/Jakob_Griese_Schwarzinger_Dobbek_JMB2006.pdf
Université de Poitiers Faculté de Médecine et Pharmacie ANNEE 2013 Thèse n° POUR LE DIPLOME D'ETAT DE DOCTEUR EN MEDECINE (décret du 16 janvier 2004) présentée et soutenue publiquement le 2 juillet 2013 à Poitiers par Monsieur Paul LOUMAIGNE
Keeping the Auckland Airport community informed Issue 73 April 2008 ISSN1176-9432 for airport emergency teamInside this issue: • Golf day benefits charity • Airport wins bronze award • Greening the airport • Auckland Cup race day • Plus much more… Cover: Brian Chase (left) and Tony Beattie (right) of the Airport









