Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma


Contents lists available at
The Veterinary Journal
Biology, diagnosis and treatment of canine appendicular osteosarcoma:Similarities and differences with human osteosarcoma
Emanuela Morello , Marina Martano, Paolo Buracco
School of Veterinary Medicine, Department of Animal Pathology, via Leonardo da Vinci 44, 10095 Grugliasco, Italy
Osteosarcoma (OSA) is the most common primary bone tumour in dogs. The appendicular locations are
Accepted 28 August 2010
most frequently involved and large to giant breed dogs are commonly affected, with a median age of
Available online xxxx
7–8 years. OSA is a locally invasive neoplasm with a high rate of metastasis, mostly to the lungs. Dueto similarities in biology and treatment of OSA in dogs and humans, canine OSA represents a valid and
important tumour model. Differences between canine and human OSAs include the age of occurrence
(OSA is most commonly an adolescent disease in humans), localisation (the stifle is the most common
site of localisation in humans) and limited use of neoadjuvant chemotherapy in canine OSA.
Ó 2010 Elsevier Ltd. All rights reserved.
extends into surrounding soft tissues and has a high rate of metas-tasis. Metastasis occurs mainly to the lungs via the haematogenous
Spontaneous tumours in dogs may serve as models for human
route, as well as to other bones, visceral organs, the brain, subcuta-
cancer biology and translational cancer therapeutics, having great-
neous tissue and skin ().
er similarity than many current experimental tumour models. Ca-
Lymph nodes are involved less commonly, with reported frequen-
nine osteosarcoma (OSA) is a suitable model for OSA in humans
cies of 4.4–9.0%
due to the relatively high incidence of the tumour in dogs, similar-
The use of chemotherapy as part of standard
ities in biological behaviour, common molecular features, large
curative-intent treatment is associated with an increase in the rate
body size of breeds more frequently affected and sharing of the
of bone and soft tissue metastases
same environment. The lack of specific chemotherapeutic drugs
In dogs, appendicular OSAs most often affect the metaphyses of
in veterinary medicine and the sometimes prohibitive costs of
long bones. The fore limbs are affected twice as often as the hind
treatment for the management of cancer in dogs allow early access
limbs, with the distal radius and proximal humerus being the most
to novel therapeutics. Dogs have a naturally shorter life span, with
frequent sites, followed by the distal femur and proximal and distal
more rapid progression and early metastatic failure of cancer,
tibia (). Large and giant
permitting more rapid completion of clinical trials in this species
breeds are more commonly affected; only 5% of OSAs occur in dogs
compared to human patients. This paper gives an overview of the
<15 kg ). Greyhounds, Rottweilers, Great Danes,
biology and treatment options of OSA in dogs and humans.
Saint Bernards, Doberman Pinschers, Irish Setters, Golden Retriev-ers and German Shepherds have an increased risk of developingOSA, even though the predisposition seems to be related to size
Clinical presentation of appendicular osteosarcoma
rather than breed (Afamilial pattern of occurrence has been observed in the Saint Ber-
Canine OSA accounts for 85–98% of all canine bone tumours
nard, Rottweiler and Scottish Deerhound (
). Appendicular locations
are more common (75%), but OSA can also affect the axial skeleton
Males are more often affected than females (ratio 1.5:1), but
(24%) and, occasionally, soft tissues (1%). Appendicular OSA is a lo-
this finding is not consistent among publications
cally aggressive malignant neoplasm which destroys bone locally,
Affected females mainly belong
to the Great Dane, Saint Bernard and Rottweiler breeds. The age at
Corresponding author. Tel.: +39 011 6709062; fax: +39 011 6709165.
E-mail address: (E. Morello).
presentation has a bimodal distribution; a first peak is reported at
1090-0233/$ - see front matter Ó 2010 Elsevier Ltd. All rights reserved.
doi:
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010),
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
18–24 months, but most dogs are 7–9 years old
plates) after orthopaedic procedures have been associated with
). Neutered dogs have twice the risk of developing OSA com-
the development of OSA
pared with sexually intact dogs A study on 683
Rottweilers undergoing gonadectomy before 1 year of age found
Hypotheses to explain this phenomenon include a direct effect of
a strong inverse association between lifetime exposure to gonadal
metal implants, infection, instability of the implant and corrosion
hormones and incidence of OSA, suggesting that sex hormones
). However, given the large number of orthopae-
may play a role in tumour development
dic surgical implants routinely applied and the fact that no conclu-
Human OSA is the most common primary solid bone tumour in
sions have been drawn on the role of metallic implants in sarcoma
childhood and adolescence (
development, the occurrence of malignant lesions at the same site
). The incidence is higher in the second decade of life, during
may be no more than a coincidence (). Under-
periods of rapid bone turnover, with a median age of 16 years
lying diseases, such as spontaneous or post-orthopaedic surgery
). After 10 years of age, males are more frequently af-
bone infarcts and osteochondritis dissecans, have also been re-
fected than females. A second peak in incidence occurs in older pa-
ported as possible causative factors in dogs
tients, usually associated with underlying bone pathology, such as
Paget's disease, medullary infarcts or prior irradiation (
). A study by on two groups of dogs
). The metaphysis of long bones is the primary site in more
of different sizes (<15 kg; >25 kg) failed to demonstrate increased
than 80% of cases. OSAs develop most commonly at sites of rapid
microdamage in the distal metaphyseal radius in the large size
bone turnover, such as the distal femur, proximal tibia and proxi-
group, suggesting that microdamage is unlikely to be an important
risk factor for OSA.
Metastases are clinically detectable in approximately 20% of hu-
man patients on initial presentation and
Genetic alterations
metastatic spread is usually by the haematogenous route. The
In one study, 27 p53 tumour suppressor gene mutations (20
lungs are the most common metastatic site (80–85%), followed
point mutations and 7 deletions) were observed in 24/59 (40.7%)
by bone (10%), which is usually involved only after pulmonary
canine OSAs Cases of OSA with mutated
metastasis ). Less frequent sites of metasta-
p53 had a decreased survival time compared to dogs without p53
ses include lymph nodes (<10%), liver, adrenal glands, central ner-
alterations (In two other studies, p53 was
vous system, muscle and skin ). Patients without
over-expressed in the majority of canine OSAs and alterations in its
clinically detectable metastases are presumed to have micrometa-
expression correlated with highly aggressive tumour behaviour
and higher tumour grade Mutations in p53 have also been observed in other studies
Aetiology and risk factors for osteosarcoma
of canine OSA by and .
The aetiology of most OSAs remains unknown both in humans
Over-expression of erb-B2, which encodes human epidermal
and dogs. Some factors have been identified as possibly being in-
growth factor receptor 2 (HER-2), was observed in 86% and 40%
volved in development of canine OSA.
of canine OSA cell lines and tissue samples, respectively ). Deletions, mutations and down-regulation of the
PTEN tumour suppressor gene have also been detected in OSA celllines and tumour samples Hepatocyte growth
Ionising radiation
factor (HGF) and its receptor c-Met were expressed in most OSA
In both therapeutic and experimental settings, exposure to ion-
ising radiation can induce OSA. Beagles administered aerosols con-
A role for insulin-like growth factor-1 (IGF-1) and its recep-
taining plutonium dioxide developed OSAs in the lungs, skeleton
tor (IGF-1R) in cell growth and invasion in OSA canine cell lines has
and liver, beginning about 3 years after exposure (
been demonstrated (Matrix metalloprotein-
). Skeletal malignancies, most of which were OSAs,
ases 2 and 9, which may contribute to local disease progression
were documented among 234 young adult beagles given single
and metastatic spread, are expressed in OSA cell lines and tissues
intravenous injections of monomeric 239Plutonium citrate (
). Similarly, ezrin, a
). In another experimental study, 36/117 young adult
membrane cytoskeleton linker also potentially involved in metas-
beagles injected with 241Americium developed OSA (
tasis, was detected in 83% of primary canine OSAs and its presence
was associated with a shorter median disease-free interval com-
Several reports of OSA as a late complication of radiation ther-
pared to OSAs with low ezrin expression (). Con-
apy in dogs have been described. A vertebral OSA occurred in a
dog 5 years after 60Cobalt teletherapy for a spinal cord tumour
transcription 3 (STAT3) was present in a subset of canine OSA tu-
(Secondary OSAs developed within the field
mours and cell lines, but not in normal canine osteoblasts (
of megavoltage irradiation 1.7–5 years after treatment in 3/87
(3.4%) of spontaneous tumour-bearing dogs irradiated for soft tis-sue sarcomas (). OSA has also been reported
after orthovoltage irradiation of oral acanthomatous epulis (). In an experimental study, 21% of dogs
Factors associated with the development of OSA in humans in-
undergoing intra-operative radiation therapy (>25 Gy) to the ver-
clude the faster growth rate of bone at puberty, exposure to
tebral column, followed in some cases by external beam radiation,
developed OSA 4–5 years post-treatment
hereditary retinoblastoma (mutations in the RB gene), Li-Fraumenisyndrome (mutations in the p53 gene), Bloom syndrome,
Minor chronic trauma
Rothmund–Thomson syndrome and Werner's syndrome
Long-standing metallic implants (e.g. Jonas intramedullary
Radiation-induced OSAs are rare and
splints and older generation tibial plateau levelling osteotomy
typically occur in adults because of the long interval (5–20 years)
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010), doi:
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
between radiation exposure and neoplastic transformation
scintigraphy overestimated the local extent of OSA more than radi-
ography, providing a larger margin of safety when determining the
In human OSAs, tumorigenesis has been associated with altera-
site of the proximal osteotomy, but also decreasing candidates for
tions in tumour suppressor proteins (p53, Rb, PTEN), alterations in
limb-sparing surgery
oncogene expression (erbB-2, MET) and dysregulated cell signal-
Thoracic radiographs are performed to evaluate metastatic
ling and kinase pathways, such as vascular endothelial growth fac-
spread to the lungs. Only a few patients (<5–10%) are positive for
tor (VEGF), platelet-derived growth factor (PDGF), mTOR, c-Kit,
radiographic lung metastasis at presentation, but OSA is consid-
metalloproteinases and ezrin
ered to be a tumour with a high metastatic potential, since approx-
imately 90% of dogs with OSA treated by amputation only die ofmetastatic disease, usually to the lung, within 1 year of diagnosis(). CT of the thorax is superior to radiography
History and physical examination
in detecting smaller lung lesions (). Using nu-clear scintigraphy, the incidence of occult bone metastasis at the
time of diagnosis of the primary tumour is 7.9%; lesions appearas non-specific areas of increased uptake of radiopharmaceutical
Dogs with appendicular OSA are referred for the onset of pro-
that should be verified by radiographs or biopsy
gressive lameness and leg swelling Lameness
is usually intermittent and initially mild, but progresses to becomepersistent and severe. The mass at the primary site is usually firmand often painful on palpation. Acute non-weight bearing lame-
ness is typically associated with a pathological fracture.
In humans, at least two orthogonal radiographic views are re-
quired when a bone lesion is suspected. The classical radiographic
appearance is of ill-defined borders, an osteoblastic and/or osteo-lytic lesion and an associated soft tissue mass. MRI represents
Human patients with OSA present with pain of several months'
the primary mode of evaluation of OSA in humans and can clearly
duration (2–4 months before diagnosis), usually related to strenu-
demonstrate the extent of tumour invasion of the surrounding soft
ous exercise or trauma, and the pain interferes with sleep. On clin-
tissue, neurovascular involvement, extent of bone marrow replace-
ical examination, a visible swelling with a hard painful mass,
ment and presence of discontinuous metastases (
decreased joint mobility or localised warmth or erythema may
MRI is also useful to assess the possibility of limb salvage.
be present. Approximately 5–10% of patients with OSA present
CT-guided core biopsy is frequently used for tissue biopsy for his-
with a pathological fracture
topathological diagnosis ). A CT scan of the
chest and a nuclear scintigraphy bone scan are recommended torule out metastasis to the lungs and bone. Interest in the use of
Diagnosis and staging
positron-emission tomography (PET) for staging OSAs and moni-toring treatment is increasing ().
A diagnosis of primary malignant bone tumour is often sug-
gested by clinical presentation and radiographic findings. Initial
diagnosis can be attempted by fine needle aspiration and cytology
Dogs with appendicular OSA can be managed with either palli-
Alkaline phosphatase (ALP) staining of cytology samples is useful
ative- or curative-intent therapy. Curative-intent treatment is
for differentiating between OSA and other primary bone tumours
aimed at local tumour control and prevention or delay of meta-
Bone biopsy can be performed via closed
static disease.
(Jamshidi needle or Michelle's trephine) or open techniquesThe diagnostic quality of cytological or histo-logical bone samples can be improved by image-guided techniques
Limb amputation remains the current standard of care for local
Diagnostic imaging plays an important role in diagnosis and
management (). It
staging of dogs with OSA. Cranio-caudal and latero-medial radio-
avoids the risk of pathological fracture, eliminates pain and is a
graphic views of the primary lesion, including the joint above
well tolerated procedure, with minimal complications; even large
and below the affected bone, are required. The radiographic
breed dogs show good functional results and the majority of own-
appearance of OSA in long bones includes cortical bone lysis and/
ers are satisfied with the pet's quality of life (
or a proliferative sunburst pattern, periosteal proliferation, sub-
). Contraindications to amputation
periosteal new bone formation and soft tissue swelling, with calci-
may be severe obesity or concurrent debilitating orthopaedic or
fication extending into surrounding soft tissue.
neurological diseases; however, each case needs to be evaluated
Several studies have been performed to evaluate the accuracy of
on an individual basis.
radiography, bone scintigraphy, computed tomography (CT) andmagnetic resonance imaging (MRI) in assessing the local extent
Limb-sparing surgery
of appendicular OSA (
Limb-sparing in dogs can be achieved with surgical and/or radi-
). In one study, MRI was recognised as the best modality
ation techniques. Good results have been reported with recon-
for preoperative assessment of intramedullary extent of appendic-
structive limb-sparing procedures for OSA of the distal radius,
ular OSA when limb-sparing was an option (
whereas limb-sparing surgery at other sites is associated with a
Another study comparing radiographs, MRI and CT in 10 post-
higher complication rate and poor limb function (
amputation OSA cases failed to identify a superior modality in pre-
). Conversely, a lower complication rate
dicting extent of tumour infiltration ). Nuclear
and good restoration of limb function have been reported after
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010),
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
ablative limb-sparing techniques for OSA in the ulna, scapula, met-acarpus, metatarsus and ischium.
Several surgical techniques have been used to preserve the limb
and they represent a valid alternative to amputation. After surgicalresection of the OSA, the defect may be filled with a frozen corticalallograft (an endoprosthesis ) or with theresected
(), autoclaved(or irradiated().
Bone or metallic implants are fixed in position with a bone plateand screws, and arthrodesis of the adjacent joint is performed. Acombination of allograft and prosthesis has been used to preservethe limb for OSA of the proximal femur (Themore common complications associated with cortical allograft,pasteurised autograft and endoprosthesis include local tumourrecurrence (15–28%), infection (31–60%) and implant failure orloosening (11–40%) ().
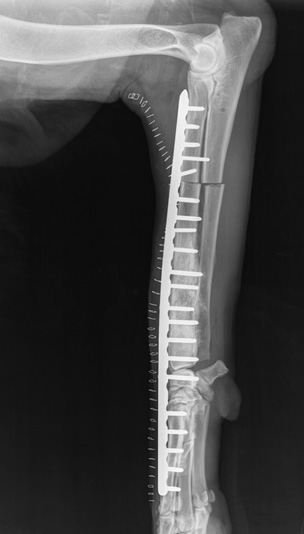
Fig. 2. Post-operative lateral radiograph of a dog with osteosarcoma treated by
After excision of OSAs from the distal radius or tibia, large sur-
limb-sparing surgery after pasteurised autograft replacement and plate fixation.
gical defects have been replaced by vascularised, viable, regener-ated bone by single (or double transport osteogenesis(
Transverse ulnar bone transport osteogenesis has also beeninvestigated experimentally (These procedurescan achieve good limb function and absence of infection, but prob-lems include local tumour recurrence, owner compliance in dis-tracting the apparatus several times per day and apparatusfailure. Limb-sparing surgery has also been performed by rollingthe distal ulna into the distal radial defect after tumour excision,thus using the ulna as a vascularised transposition autograft). However, the procedure is more likely to havebiomechanical complications compared to the standard corticalallograft technique (
Surgery alone is considered to be palliative. The reported mean
survival time after surgery alone is 103–175 days ). The 1- and 2-year survival is 11–20% and 2–4%, respectivelyThere are no statistical differences in sur-vival between amputation and limb-sparing surgery if adequatesystemic chemotherapy is given ). Animprovement in survival with limb-sparing is only evident if thesurgical field becomes infected; the median survival time for dogswith infected surgical sites after limb-sparing is 685 days com-pared to 289 days in the absence of infection (). Similar findings have also been reportedin humans with limb-sparing surgery
Adjuvant chemotherapy can improve survival of dogs with OSA
when associated with surgery and/or radiotherapy. Chemotherapyprotocols include doxorubicin, cisplatin, carboplatin and lobaplatinused alone or in combination ). A local cisplatin deliverysystem has been described (). Administration of chemotherapy in addi-tion to surgery and/or radiotherapy increases the median survivaltime from 103–175 days to 262–450 days. The 1- and 2-year sur-vival rates with chemotherapy range from 31–48% to 10–26%,respectively. Survival times for dogs treated with single agentplatinum compounds are similar to those reported with combined
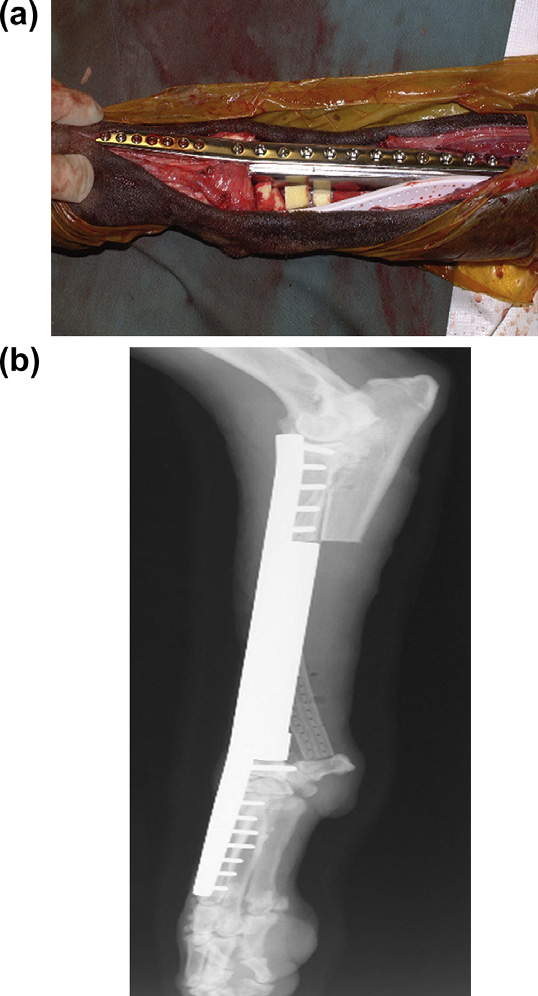
Fig. 1. Distal aspect of the radius of a dog with osteosarcoma treated by limb-
protocols ().
sparing surgery with an endoprosthesis. (a) Intra-operative view showing the
The most efficacious chemotherapeutic agent and the ideal tim-
biodegradable cisplatin-impregnated open-cell polylactic acid implant and closed
ing to start adjuvant chemotherapy have not been identified. How-
continuous suction drain. (b) Post-operative lateral radiograph. Images courtesy ofDr. Julius M. Liptak.
ever, there is no substantial advantage in early post-operative
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010), doi:
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
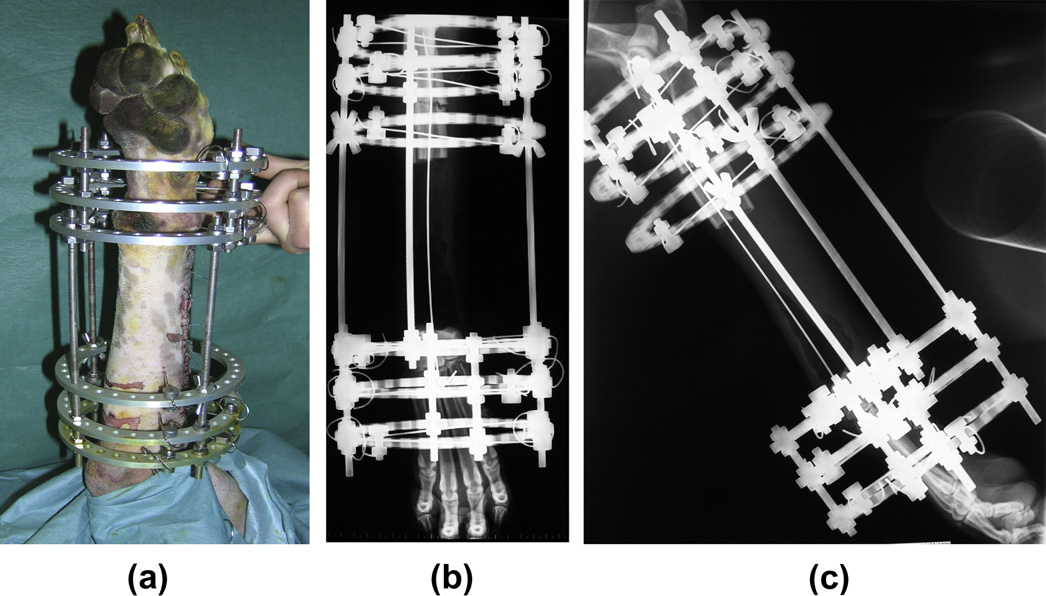
Fig. 3. Single bone transport osteogenesis limb-sparing surgery in the distal radius of a dog with osteosarcoma. Post-operative image of the circular fixator (a). Post-operativelateral (b) and cranio-caudal (c) radiographs of distal radius.
metastatectomy resulted in significantly prolonged survival in se-
Table 1Chemotherapeutic agents used for dogs with appendicular osteosarcoma.
lected patients (
Immunotherapy has been combined with chemotherapy to
Anti-tumour activity was observed when the immunomodulatory
agent L-muramyl tripeptide-phosphatidylethanolamine (L-MTP-
PE) was administered to dogs with OSA after limb amputation or
limb-sparing surgery and completion of cisplatin treatment(L-MTP-PE adminis-
Cisplatin + doxorubicin
tered with doxorubicin enhanced canine monocyte activation in-
duced by doxorubicin or L-MTP-PE alone () and
induced cytotoxic activity of pulmonary alveolar macrophages
against OSA cells when compared to dogs treated with doxorubicin
or L-MTP-PE alone (Kurzman et al., 1999).
Radiation therapy has a role in curative-intent local treatment
of canine appendicular OSA. proposed a full-
Carboplatin + doxorubicin
course fractionated external beam protocol in conjunction with
cisplatin, but there was no substantial improvement over a pallia-
tive protocol. A single fraction of 70 Gy given intra-operatively
Carboplatin + doxorubicin + piroxicam
after exteriorisation of the tumoral bone segment has been used
in combination with chemotherapy (
Post-operative complications may be high (69%) and
include deep infection, fracture of irradiated bone, implant failure
and local recurrence. This procedure should only be performed in
a NR, not reported.
dogs bearing appendicular OSAs at sites in which limb-sparingtechniques are not an option.
A stereotactic radiosurgery protocol has also been used, in
which dogs are irradiated with a single large targeted dose (30–
chemotherapy so it is better
50 Gy), with or without chemotherapy (This al-
to wait an adequate time to allow the patient to recover from sur-
lows normal tissue to be spared and avoids the need for surgery in
gery and early healing of the surgical wound
some cases, although pathological fractures may occur.
Chemotherapy is usually less effective in the presence of macro-scopic metastatic disease (The efficacy of aer-
osol-delivered gemcitabine has been investigated by
The aim of palliative-intent treatment is to alleviate pain. Radi-
in dogs with pulmonary metastatic OSA. Pulmonary
ation therapy is a valid method of palliation for appendicular OSA,
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010),
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
inducing relief of pain, reduced lameness and improving quality of
In humans, 5-year survival of 10–20% has been reported after
life, whereas radiation-induced acute side effects are rare. Radia-
amputation without chemotherapy, whereas a 5-year survival
tion therapy protocols include a two-fraction protocol
rate of 60–78% has been reported for non-metastatic patients
three-fraction protocols
when surgery is combined with systemic multi-agent chemo-
), four-fraction protocol
therapy (). Despite multimodality treatment,
() and expedited protocol (
30–40% of OSA patients still experience relapses within 3 years
). The effectiveness and duration of analgesia among these
of treatment.
protocols range from 50–93% and 53–180 days, respectively. Most
An increase in 5-year survival has been obtained by combining
dogs died or were euthanased because of local disease progression,
L-MTP-PE immunotherapy with chemotherapy in non-metastatic
metastatic disease or pathological fractures.
patients. Radiation therapy is mainly used as palliation for unre-
It is not clear if dogs with OSA benefit from more durable pain
sectable tumours, as well as for incompletely resected tumour
relief when chemotherapy is combined with radiotherapy
excision margins Pain palliation has also
been achieved by use of radiopharmaceuticals such as 153Samar-
ported that longer survival (median survival time 130 days) can be
ium (Extracorporeal irradiation, includ-
achieved in dogs with metastatic (stage III) appendicular OSA using
ing stereotactic radiosurgery or surgically exposed, irradiated and
palliative radiation compared with surgery alone. Palliative-intent
reimplanted bone, are among the more innovative and promising
treatment for canine OSA has also been attempted by intravenous
curative-intent uses of radiation therapy
administration of 153Samarium
Bisphosphonates have been proposed for palliative-intent treat-
ment in dogs with OSA. Clinical applications include therapy for tu-
Prognostic factors
mour-related hypercalcaemia, inhibition of bone metastasis andpain relief. Zoledronic acid pamidronate
() and alendronate () pro-vided pain palliation in some treated dogs. However, when com-
Negative prognostic indicators associated with a shorter sur-
bined with chemotherapy, pamidronate did not improve pain
vival time in dogs with appendicular OSA include young age
alleviation (). Some success in providing pain palli-
ation in dogs with metastatic appendicular OSA has been achieved
pre-treatment total and bone-specific serum ALP activities
using metronomic chemotherapy with doxycycline, piroxicam and
cyclophosphamide (
), metastatic spread to regional lymph nodes high histological grade (grade III) (
stage III OSA (distantmetastases to bone and/or other sites)
In humans, multimodality treatment is recommended for OSA.
proximal humeral, rib or scapular involvement
For high grade OSAs, preoperative (neoadjuvant) chemotherapy,
), higher body weight
wide surgical resection and post-operative chemotherapy are used.
incompleteness of excision () and tumour vol-
The most effective chemotherapeutic agents are doxorubicin, cis-
ume ). Percent tumour necrosis induced
platin, methotrexate and ifosfamide. Combined treatment serves
by chemotherapy or radiation therapy is predictive of local tumour
to avoid chemoresistance and to increase the degree of tumour
necrosis. There is usually a delay of 3–4 weeks after the last admin-istration of neoadjuvant chemotherapy before the tumour is excised.
Adjuvant chemotherapy is usually started 2 weeks after surgery.
Tumour necrosis after administration of preoperative chemo-
In humans, negative prognostic factors include metastases at
therapy is an important factor in determining the post-operative
presentation, metastatic spread to lymph nodes, poor response to
chemotherapy regimen Patients with
preoperative chemotherapy, large tumour volume, increased activ-
P90% tumour necrosis at the time of surgery (good responders)
ities of ALP and lactate dehydrogenase (LDH) in serum, primary
will usually receive the same treatment regimen post-operatively
localisation in the axial skeleton and inadequate surgical margins
as pre-operatively. In patients with <90% tumour necrosis (poor
responders), post-surgery treatment usually includes a salvage
). In one study, time to relapse was longer in
regimen, either an increased dose or duration of the same chemo-
patients treated by neoadjuvant chemotherapy than in those given
therapeutic agents or a different protocol, but neither have been
adjuvant chemotherapy (
shown to improve survival ().
Amputation and limb-sparing procedures are the two principal
surgical options in humans. No significant differences in survival
rates and local recurrence (2.8–6%) are reported when amputationor limb-sparing surgery are used. Limb salvage is possible in more
Dogs with OSA represent a unique model for OSA in humans
than 85% of human appendicular OSAs (
due to their similar clinical presentation and molecular features,
but is contraindicated when resection
along with the relatively high number of dogs diagnosed with
with wide surgical margins is not feasible, in cases of neurovascu-
OSA each year. Differences and similarities between human and ca-
lar involvement or with pathological fractures. The options
nine OSA are summarised in . Improvements in diagnostic
available for limb salvage include tumour removal and endopros-
and imaging techniques, chemotherapy and surgical procedures
thetic replacement, rotationplasty, allografts and autografts.
have improved outcomes in both human and canine patients.
Limb-sparing complications include infection (11%) and implant
However, there is still a need for effective treatment of OSA, mainly
failure. Tumour excision usually includes resection of both primary
to control metastatic disease. Important comparative advances
and metastatic sites; excision of all clinically detectable tumours is
have been made in the study of tumour biology and progression,
associated with a 5-fold increase in survival compared with
risk factors and the evaluation of novel cancer strategies. There is
excision of the primary tumour alone.
likely to be increasing interest from the human cancer drug
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010), doi:
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
Table 2Similarities and differences between human and canine appendicular osteosarcomas.
>8000 cases/year
Middle-aged to older dogs
Adolescent disease
Peak of incidence 7–9 years
Peak of incidence at 10–20 years
Second small peak at 18–24 months
Median peak age at 16 years
Median peak age at 7 years
Large/giant breeds
Familiar pattern in Saint Bernard, Rottweiler and Scottish Deerhound
Males slightly more than females: Ratio 1.1–1:5:1
Males more than females: Ratio 1.6:1
75% appendicular skeleton, metaphysis of long bones, mainly distal radius, proximal
Metaphysis or diaphysis of long bones (80–90%)
humerus, distal femur and proximal and distal tibia
Bones of the knee joint (50%)Proximal humerus (25%)
Not completely known
Not completely known
IGF-1/IGF-1R: Over-expressed; Poor clinical outcome
IGF-1/IGF-1R: Over-expressed; Poor clinical outcome
HGF/c-Met: Over-expressed; Contributes to malignant phenotype
HGF/C-Met: Over-expressed; Contributes to malignantphenotype
ErbB-2/HER-2: Over-expressed; Poor clinical outcome
ErbB-2/HER-2: Over-expressed; Poor clinical outcome
PTEN: Mutated or down-regulated
PTEN: Mutated or down-regulated
Ezrin: Detected; Contributes to malignant phenotype
Ezrin: Detected; Contributes to malignant phenotype
Matrix metalloproteinases: Expressed
Matrix metalloproteinases: Expressed
PDGF-b: Expressed
PDGF-b: Expressed
Hard painful mass
Hard painful mass
Uncommon pathological fracture (3%)
Uncommon pathological fracture
10% of cases with metastasis at diagnosis: Lung, bone (7.4%)
20% of cases with metastasis at diagnosis: Lung, bone
Regional lymph node metastasis (4.4–9.0%)
Regional lymph node metastasis < 10%
Limb-sparing techniques (90% cases)
Limb-sparing techniques
Amputation (rare)
Adjuvant chemotherapy: Doxorubicin, platinum
Neoadjuvant chemotherapy: Doxorubicin,methotrexate, isofosfamide, platinum and adjuvantpost-surgery
60% survival at 1 year with chemotherapy
70% survival at 5 years with chemotherapy
Negative prognostic
Metastasis at diagnosis: Lungs, bones, lymph nodes
Metastasis at diagnosis: Lungs, bones, lymph nodes
High serum ALP, LDH activities
High serum ALP, LDH activities
Age: Youngest affectedPoor response to neoadjuvant chemotherapy: % tumournecrosis
Positive prognostic
Post-operative limb-sparing infection
Post-operative limb-sparing infection
High percentage of tumor necrosis induced by chemotherapy or radiotherapy
High percentage of tumor necrosis induced bychemotherapy or radiotherapy
IGF-1, insulin-like growth factor-1; IGF-1R, IGF-1 receptor; HGF, hepatocyte growth factor, HER-2, human epidermal growth factor receptor 2; PTEN, phosphatase and tensinhomolog, PDGF-b, platelet-derived growth factor-b; VEGF, vascular endothelial growth factor; P-gp, P-glycoprotein; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
industry in conducting clinical trials in dogs with OSA before or
Bacon, N.J., Ehrhart, N.P., Dernell, W.S., Lafferty, M., Withrow, S.J., 2008. Use of
concomitantly with trials in human patients.
alternating administration of carboplatin and doxorubicin in dogs withmicroscopic metastases after amputation for appendicular osteosarcoma: 50cases (1999–2006). Journal of the American Veterinary Medical Association232, 1504–1510.
Conflict of interest statement
Bailey, D., Erb, H., Williams, L., Ruslander, D., Hauck, M., 2003. Carboplatin and
doxorubicin combination chemotherapy for the treatment of appendicularosteosarcoma in the dog. Journal of Veterinary Internal Medicine 17, 199–205.
None of the authors of this paper has a financial or personal
Barnard, S.M., Zuber, R.M., Moore, A.S., 2007. Samarium Sm 153 lexidronam for the
relationship with other people or organisations that could inappro-
palliative treatment of dogs with primary bone tumors: 35 cases (1999–2005).
priately influence or bias the content of the paper.
Journal of the American Veterinary Medical Association 230, 1877–1881.
Bateman, K.E., Catton, P.A., Pennock, P.W., Kruth, S.A., 1994. 0–7–21 radiation
therapy for the palliation of advanced cancer in dogs. Journal of VeterinaryInternal Medicine 8, 394–399.
Berg, J., Lamb, C.R., O'Callaghan, W., 1990. Bone scintigraphy in the initial evaluation
of dogs with primary bone lesions. Journal of the American Veterinary Medical
Bacci, G., Longhi, A., Versari, M., Mercuri, M., Briccoli, A., Picci, P., 2006. Prognostic
Association 196, 917–920.
factors for osteosarcoma of the extremity treated with neoadjuvant
Berg, J., Gebhardt, M.C., Rand, W.M., 1997. Effect of timing of postoperative
chemotherapy. Cancer 106, 1154–1161.
chemotherapy on survival of dogs with osteosarcoma. Cancer 79, 1343–1350.
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010),
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
Berg, J., Weinstein, M.J., Shelling, S.H., Rand, W.M., 1992. Treatment of dogs with
Ferracini, R., Angelini, P., Cagliero, E., Linari, A., Martano, M., Wunder, J., Buracco, P.,
osteosarcoma by administration of cisplatin after amputation and limb sparing:
2000. MET oncogene aberrant expression in canine osteosarcoma. Journal of
22 cases (1987–1990). Journal of the American Veterinary Medical Association
Orthopedic Research 18, 253–256.
200, 2005–2008.
Fieten, H., Spee, B., Ijzer, J., Kik, M.J., Penning, L.C., Kirpensteijn, J., 2009. Expression
Berg, J., Weinstein, M.J., Springfield, D.S., Rand, W.M., 1995. Results of surgery and
of hepatocyte growth factor and the proto-oncogenic receptor c-Met in canine
doxorubicin chemotherapy in dogs with osteosarcoma. Journal of the American
osteosarcoma. Veterinary Pathology 46, 869–877.
Veterinary Medical Association 206, 1555–1560.
Flint, A.F., U'Ren, L., Legare, M.E., Withrow, S.J., Dernell, W.S., Hanneman, W.H.,
Bergman, P.J., MacEwen, E.G., Kurzman, I.D., Henry, C.J., Hammer, A.S., Knapp, D.W.,
2004. Overexpression of the erB-2 proto-oncogene in canine osteosarcoma cell
Hale, A., Kruth, S.A., Klein, M.K., Klausner, J., Norris, A.M., McCaw, D., Straw, R.C.,
lines and tumors. Veterinary Pathology 41, 291–296.
Withrow, S.J., 1996. Amputation and carboplatin for treatment of dogs with
Fossey, S.L., Liao, A.T., McCleese, J.K., Bear, M.D., Lin, J., Li, P.K., Kisseberth, W.C.,
osteosarcoma: 48 cases (1991–1993). Journal of Veterinary Internal Medicine
Cheryl, A., London, C.A., 2009. Characterization of STAT3 activation and
10, 76–81.
expression in canine and human osteosarcoma. BMC Cancer 9, 1–15.
Boston, S.E., Ehrhart, N.P., Dernell, W.S., Lafferty, M., Withrow, S.J., 2006. Evaluation
Garzotto, C.K., Berg, J., Hoffmann, W.E., Rand, W.M., 2000. Prognostic significance of
of survival time in dogs with stage III osteosarcoma that undergo treatment: 90
serum alkaline phosphatase activity in canine appendicular osteosarcoma.
cases (1985–2004). Journal of the American Veterinary Medical Association
Journal of Veterinary Internal Medicine 14, 587–592.
228, 1905–1908.
Gellasch, K.L., Kalscheur, V.L., Clayton, M.K., Muir, P., 2002. Fatigue microdamage in
Boston, S.E., Duerr, F., Bacon, N., LaRue, S., Ehrhart, E.J., Withrow, S., 2007.
the radial predilection site for osteosarcoma in dogs. American Journal of
Intraoperative radiation for limb sparing of the distal aspect of the radius
Veterinary Research 63, 896–899.
without transcarpal plating in five dogs. Veterinary Surgery 36, 314–323.
Gillette, S.M., Gillette, E.L., Powers, B.E., Withrow, S.J., 1990. Radiation-induced
Boudrieau, R.J., McCarthy, R.J., Sisson, R.D., 2005. Sarcoma of the proximal portion of
osteosarcoma in dogs after external beam or intraoperative radiation therapy.
the tibia in a dog 5.5 years after tibial plateau leveling osteotomy. Journal of the
Cancer Research 50, 54–57.
American Veterinary Medical Association 227, 1613–1617.
Gorman, E., Barger, A.M., Wypij, J.M., Pinkerton, M.E., 2006. Cutaneous metastasis of
Britt, T., Clifford, C., Barger, A., Moroff, S., Drobatz, K., Thacher, C., Davis, G., 2007.
primary appendicular osteosarcoma in a dog. Veterinary Clinical Pathology 35,
Diagnosing appendicular osteosarcoma with ultrasound-guided fine-needle
aspiration: 36 cases. Journal of Small Animal Practice 48, 145–150.
Green, E.M., Adams, W.M., Forrest, L.J., 2002. Four fraction palliative radiotherapy
Brodey, R.S., Abt, D.A., 1976. Results of surgical treatment in 65 dogs with
for osteosarcoma in 24 dogs. Journal of the American Animal Hospital
osteosarcoma. Journal American Veterinary Medical Association 168, 1032–
Association 38, 445–451.
Hahn, K.A., Hurd, C., Cantwell, H.D., 1990. Single-phase methylene diphosphate
Buracco, P., Martano, M., Morello, E., Vasconi, M.E., 2002. Pasteurized tumoral
bone scintigraphy in the diagnostic evaluation of dogs with osteosarcoma.
autograft as a novel procedure of limb sparing in dogs: a clinical report in a
Journal of the American Veterinary Medical Association 196, 1483–1486.
canine distal radial osteosarcoma. Veterinary Surgery 31, 525–532.
Hammer, A.S., Weeren, F.R., Weisbrode, S.E., Padgett, S.L., 1995. Prognostic factors in
Carberry, C.A., Harvey, H.J., 1986. Owner satisfaction with limb amputation in dogs
dogs with osteosarcomas of the flat or irregular bones. Journal of the American
and cats. Journal of the American Animal Hospital Association 23, 227–232.
Animal Hospital Association 31, 321–326.
Carrle, D., Bielack, S.S., 2006. Current strategies of chemotherapy in osteosarcoma.
Harasen, G.L.G., Simko, E., 2008. Histiocytic sarcoma of the stifle in a dog with
International Orthopaedics 30, 445–451.
cranial cruciate ligament failure and TPLO treatment. Veterinary and
Chun, R., Garret, L.D., Henry, C., Wall, M., Smith, A., 2005. Toxicity and efficacy of
Comparative Orthopaedics and Traumatology 21, 375–377.
cisplatin and doxorubicin combination chemotherapy for the treatment of
Hillers, K.R., Dernell, W.S., Lafferty, M., Withrow, S.J., Lana, S.E., 2005. Incidence and
canine osteosarcoma. Journal of the American Animal Hospital Association 41,
prognostic importance of lymph node metastases in dogs with appendicular
osteosarcoma: 228 cases (1986–2003). Journal of the American Veterinary
Cooley, D.M., Beranek, B.C., Schlittler, D.L., Glickman, N.W., Glickman, L.T., Waters,
Medical Association 226, 1364–1367.
D.J., 2002. Endogenous gonadal hormone exposure and bone sarcoma risk.
Holmberg, B.J., Farese, J.P., Taylor, D., Uhl, E.W., 2004. Osteosarcoma of the humeral
Cancer Epidemiology Biomarkers and Prevention 1, 1434–1440.
head associated with osteochondritis dissecans in a dog. Journal of the
Davis, G.J., Kapatkin, A.S., Craig, L.E., Heins, G.S., Wortman, J.A., 2002. Comparison of
American Animal Hospital Association 40, 246–249.
radiography, computed tomography, and magnetic resonance imaging for
Jankowski, M.K., Steyn, P.F., Lana, S.E., Dernell, W.S., Blom, C.M., Uhrig, J.L., Lafferty,
evaluation of appendicular osteosarcoma in dogs. Journal of the American
M., Withrow, S.J., 2003. Nuclear scanning with 99mTc-HDP for the initial
Veterinary Medical Association 220, 1171–1176.
evaluation of osseous metastasis in canine osteosarcoma. Veterinary and
De Maria, R., Miretti, S., Iussich, S., Olivero, M., Morello, E., Bertotti, A., Christensen,
Comparative Oncology 1, 152–158.
J.G., Biolatti, B., Levine, R.A., Buracco, P., Di Renzo, M.F., 2009. Met oncogene
Jehn, C.T., Lewis, D.D., Farese, J.P., Ferrell, E.A., Conley, W.G., Ehrhart, N., 2007.
activation qualifies spontaneous canine osteosarcoma as a suitable pre-clinical
Transverse ulnar bone transport osteogenesis: a new technique for limb salvage
model of human osteosarcoma. Journal of Pathology 218, 399–408.
for the treatment of distal radial osteosarcoma in dogs. Veterinary Surgery 36,
Dernell, W.S., Ehrhart, N.P., Straw, R.C., Vail, D.M., 2007. Tumors of the skeletal
system. In: Withrow, S.J., Vail, D.M. (Eds.), Withrow and MacEwen's Small
Jeys, L.M., Grimer, R.J., Carter, S.R., Tillman, R.M., Abudu, A., 2007. Post operative
Animal Clinical Oncology. Saunders, Elsevier, St. Louis, MI, USA, pp. 540–582.
infection and increased survival in osteosarcoma patients: are they associated?
Dickinson, P.J., McEntee, M.C., Lipsitz, D., Keel, K., LeCouteur, R.A., 2001. Radiation
Annals of Surgical Oncology 14, 2887–2895.
induced vertebral osteosarcoma following treatment of an intradural
Kent, M.S., Strom, A., London, C.A., Seguin, B., 2004. Alternating carboplatin and
extramedullary spinal cord tumor in a dog. Veterinary Radiology and
doxorubicin as adjunctive chemotherapy to amputation or limb-sparing
Ultrasound 42, 463–470.
surgery in the treatment of appendicular osteosarcoma in dogs. Journal of
Dubielzig, R.R., Biery, D.N., Brodey, R.S., 1981. Bone sarcomas associated with
Veterinary Internal Medicine 18, 540–544.
multifocal medullary bone infarction in dogs. Journal of the American
Khanna, C., Wan, X., Bos, S., Cassaday, R., Olomu, O., Mendoza, A., Yeung, C., Gorlick,
Veterinary Medical Association 79, 64–68.
R., Hewitt, S.M., Helman, L.J., 2004. The membrane cytoskeleton linker ezrin is
Ehrhart, N., Dernell, W.S., Hoffmann, W.E., Weigel, R.M., Powers, B.E., Withrow, S.J.,
necessary for osteosarcoma metastasis. Nature Medicine 10, 182–186.
1998. Prognostic importance of alkaline phosphatase activity in serum from
Kirpensteijn, J., van den Bos, R., Endenburg, N., 1999. Adaptation of dogs to the
dogs with appendicular osteosarcoma: 75 cases (1990–1996). Journal of the
amputation of a limb and their owners' satisfaction with the procedure.
American Veterinary Medical Association 213, 1002–1006.
Veterinary Record 144, 115–118.
Ehrhart, N., 2005. Longitudinal bone transport for treatment of primary bone
Kirpensteijn, J., Kik, M., Rutteman, G.R., Teske, F., 2002a. Prognostic significance of a
tumors in dogs: technique description and outcome in 9 dogs. Veterinary
new histologic grading system for canine osteosarcoma. Veterinary Pathology
Surgery 34, 24–34.
39, 240–246.
Fan, T.M., de Lorimer, L.P., Charney, S.C., Hintermesister, J.G., 2005. Evaluation of
Kirpensteijn, J., Teske, E., Kik, M., Klenner, T., Rutteman, G.R., 2002b. Lobaplatin as an
intravenous pamidronate administration in 33 cancer-bearing dogs with
adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a
primary or secondary bone involvement. Journal of Veterinary Internal
phase II evaluation. Anticancer Research 22, 2765–2770.
Medicine 19, 74–80.
Kirpensteijn, J., Kik, M., Teske, E., Rutteman, G.R., 2008. TP53 gene mutations in
Fan, T.M., de Lorimier, L.P., O'Dell-Anderson, K., Lacoste, H.I., Charney, S.C., 2007.
canine osteosarcoma. Veterinary Surgery 37, 454–460.
Single-agent pamidronate for palliative therapy of canine appendicular
Knapp-Hoch, H.M., Fidel, J.L., Sellon, R.K., Gavin, P.R., 2009. An expedited palliative
osteosarcoma bone pain. Journal of Veterinary Internal Medicine 21, 431–439.
radiation protocol for lytic or proliferative lesions of appendicular bone in dogs.
Fan, T.M., Charney, S.C., de Lorimier, L.P., Garrett, L.D., Griffon, D.J., Gordon-Evans,
Journal of the American Animal Hospital Association 45, 24–32.
W.J., Wypij, J.M., 2009. Double-blind placebo-controlled trial of adjuvant
Kraegel, S.A., Madewell, B.R., Simonson, E., Gregory, C.L., 1991. Osteogenic sarcoma
pamidronate with palliative radiotherapy and intravenous doxorubicin for
and cisplatin chemotherapy in dogs: 16 cases (1986–1989). Journal of the
canine appendicular osteosarcoma bone pain. Journal of Veterinary Internal
American Veterinary Medical Association 199, 1057–1059.
Medicine 23, 152–160.
Kuntz, C.A., Asselin, T.L., Dernell, W.S., Powers, B.E., Straw, R.C., Withrow, S.J., 1998.
Farese, J.P., Milner, R., Thompson, M.S., Lester, N., Cooke, K., Fox, L., Hester, J., Bova,
Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17
F.J., 2004. Stereotactic radiosurgery for treatment of osteosarcomas involving
dogs. Veterinary Surgery 27, 417–422.
the distal portions of the limbs in dogs. Journal of the American Veterinary
Kurzman, I.D., MacEwen, E.G., Rosenthal, R.C., Fox, L.E., Keller, E.T., Helfand, S.C.,
Medical Association 225, 1567–1572.
Vail, D.M., Dubielzig, R.R., Madewell, B.R., Rodriguez, C.O., Obradovich, J., Fidel,
Federman, N., Bernthal, N., Eilber, F.C., Tap, W.D., 2009. The multidisciplinary
J., Rosemberg, M., 1995. Adjuvant therapy for osteosarcoma in dogs: results of
management of osteosarcoma. Current Treatment Options in Oncology 10, 82–
randomized clinical trials using combined liposome-encapsulated muramyl
tripeptide and cisplatin. Clinical Cancer Research 1, 1595–1601.
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010), doi:
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
Kurzman, I.D., Shi, F., Vail, D.M., MacEwen, E.G., 1999. In vitro and in vivo
Messerschmitt, P.J., Garcia, R.M., Abul-Karim, F.W., Greenfield, E.M., Getty, P.J., 2009.
enhancement of canine pulmonary alveolar macrophage cytotoxic activity
Osteosarcoma. Journal of the American Academy of Orthopaedic surgeons 17,
against canine osteosarcoma cells. Cancer Biotherapy and Radiopharma-
ceuticals 14, 121–128.
Misdorp, W., 1980. Skeletal osteosarcoma. Animal model: Canine osteosarcoma.
Lana, S.E., Ogilvie, G.K., Hansen, R.A., Powers, B.E., Dernell, W.S., Withrow, S.J., 2000.
American Journal of Pathology 98, 285–288.
Identification of matrix metalloproteinases in canine neoplastic tissue.
Misdorp, W., Hart, A.A., 1979. Some prognostic and epidemiologic factors in canine
American Journal Veterinary Research 61, 111–114.
osteosarcoma. Journal of the National Cancer Institute 62, 537–545.
Langova, V., Straw, R., Mutsaers, A.J., Thamm, D., 2004. Treatment of eight dogs with
Moore, A.S., Dernell, W.S., Ogilvie, G.K., Kristal, O., Elmslie, R., Kitchell, B., Susaneck,
nasal tumours with alternating doses of doxorubicin and carboplatin in
S., Rosenthal, R., Klein, M.K., Obradovich, J., Legendre, A., Haddad, T., Hahn, K.,
conjunction with oral piroxicam. Australian Veterinary Journal 82, 676–680.
Powers, B.E., Warren, D., 2007. Doxorubicin and BAY 12-9566 for the treatment
LaRue, S.M., Withrow, S.J., Wrigley, R.H., 1986. Radiographic bone surveys in the
of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study.
evaluation of primary bone tumors in dogs. Journal of the American Veterinary
Journal of Veterinary Internal Medicine 21, 783–790.
Medical Association 188, 514–516.
Morello, E., Buracco, P., Martano, M., Peirone, B., Capurro, C., Valazza, A., Cotto, D.,
LaRue, S.M., Withrow, S.J., Powers, B.E., Wrigley, R.H., Gillette, E.L., Schwarz, P.D.,
Ferracini, R., Sora, M., 2001. Bone allografts and adjuvant cisplatin as treatment
Straw, R.C., Ritcher, S.L., 1989. Limb-sparing treatment for osteosarcoma in
of canine appendicular osteosarcoma: 18 dogs (1991–1996). Journal of Small
dogs. Journal of the American Veterinary Medical Association 195, 1734–1744.
Animal Practice 42, 61–66.
Lascelles, B.D., Dernell, W.S., Correa, M.T., Lafferty, M., Devitt, C.M., Kunz, C.A., Straw,
Morello, E., Vasconi, E., Martano, M., Peirone, B., Buracco, P., 2003. Pasteurized
R.C., Withrow, S.J., 2005. Improved survival associated with postoperative
tumoral autograft and adjuvant chemotherapy for the treatment of canine
wound infection in dogs treated with limb-salvage surgery for osteosarcoma.
distal radial osteosarcoma: 13 cases. Veterinary Surgery 32, 539–544.
Annals of Surgical Oncology 12, 1073–1083.
Mueller, F., Poirier, V., Melzer, K., Nitzl, D., Roos, M., Kaser-Hotz, B., 2005. Palliative
Leibman, N.F., Kunz, C.A., Steyn, P.F., Fettman, M.J., Powers, B.E., Withrow, S.J.,
radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. In Vivo
Dernell, W.S., 2001. Accuracy of radiography, nuclear scintigraphy, and
19, 713–716.
histopathology for determining the proximal extent of distal radius
Mueller, F., Fuchs, B., Kaser-Hotz, B., 2007. Comparative biology of human and
osteosarcoma in dogs. Veterinary Surgery 30, 240–245.
canine osteosarcoma. Anticancer Research 27, 155–164.
Levine, R.A., Forest, T., Smith, C., 2002. Tumor suppressor PTEN is mutated in canine
Muggenburg, B.A., Guilmette, R.A., Mewhinney, J.A., Gillett, N.A., Mauderly, J.L.,
osteosarcoma cell lines and tumors. Veterinary Pathology 39, 372–378.
Griffith, W.C., Diel, J.H., Scott, B.R., Hahn, F.F., Boecker, B.B., 1996. Toxicity of
Liptak, J.M., Dernell, W.S., Straw, R.C., Jameson, V.J., Lafferty, M.H., Rizzo, S.A.,
inhaled plutonium dioxide in beagle dogs. Radiation Research 145, 361–381.
Withrow, S.J., 2004a. Intercalary bone grafts for joint and limb preservation in
Murphy, S.T., Parker, R.B., Woodard, J.C., 1997. Osteosarcoma following total hip
17 dogs with high-grade malignant tumors of the diaphysis. Veterinary Surgery
arthroplasty in a dog. Journal of Small Animal Practice 38, 263–267.
33, 457–467.
Nemanic, S., London, C.A., Wisner, E.R., 2006. Comparison of thoracic radiographs
Liptak, J.M., Dernell, W.S., Ehrhart, N.P., Withrow, S.J., 2004b. Canine appendicular
and single breath-hold helical CT for detection of pulmonary nodules in dogs
osteosarcoma: diagnosis and palliative treatment. Compendium of Continuing
Education for the Practising Veterinarian 26, 172–182.
Liptak, J.M., Dernell, W.S., Lascelles, B.D., LaRue, S.M., Jameson, V.J., Powers, B.E.,
O'Brien, M.G., Straw, R.C., Withrow, S.J., Powers, B.E., Jameson, V.J., Lafferty, M.,
Huber, D.J., Withrow, S.J., 2004c. Intraoperative extracorporeal irradiation for
Ogilvie, G.K., LaRue, S.M., 1993. Resection of pulmonary metastases in canine
limb sparing in 13 dogs. Veterinary Surgery 33, 446–456.
osteosarcoma: 36 cases (1983–1992). Veterinary Surgery 22, 105–109.
Liptak, J.M., Pluhar, G.E., Dernell, W.S., Withrow, S.J., 2005. Limb-sparing surgery in a
O'Day, K., Gorlick, R., 2009. Novel therapeutic agents for osteosarcoma. Expert
dog with osteosarcoma of the proximal femur. Veterinary Surgery 34, 71–77.
Reviews in Anticancer Therapy 9, 511–523.
Liptak, J.M., Dernell, W.S., Ehrhart, N., Lafferty, M.H., Monteith, G.J., Withrow, S.J.,
Ogilvie, G.K., Straw, R.C., Jameson, V.J., Walters, L.M., Lafferty, M., Powers, B.E.,
2006. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs
Withrow, S.J., 1993. Evaluation of single agent chemotherapy for treatment of
with distal radial osteosarcoma: a prospective clinical comparison of two
clinically evident osteosarcoma metastases in dogs: 45 cases (1987–1991).
different limb-sparing techniques. Veterinary Surgery 35, 518–533.
Journal of the American Veterinary Medical Association 202, 304–306.
Lloyd, R.D., Taylor, G.N., Angus, W., Bruenger, F.W., Miller, S.C., 1993. Bone cancer
Phillips, J.C., Stephenson, B., Hauck, M., Dillberger, J., 2007. Heritability and
occurrence among beagles given 239Pu as young adults. Health Physics 64, 45–
segregation analysis of osteosarcoma in the Scottish deerhound. Genomics 90,
Lloyd, R.D., Taylor, G.N., Angus, W., Miller, S.C., Boecker, B.B., 1994. Skeletal
Phillips, B., Powers, B.E., Dernell, W.S., Straw, R.C., Khanna, C., Hogge, G.S., Vail, D.M.,
malignancies among beagles injected with 241Am. Health Physics 66, 172–177.
2009. Use of single agent carboplatin as adjuvant or neoadjuvant therapy in
Loukopoulos, P., Robinson, W.F., 2007. Clinicopathological relevance of tumour
conjunction with amputation for appendicular osteosarcoma in dogs. Journal of
grading in canine osteosarcoma. Journal of Comparative Pathology 136, 65–73.
the American Animal Hospital Association 45, 33–38.
Loukopoulos, P., Thornton, J.R., Robinson, W.F., 2003. Clinical and pathologic
Pooya, H.A., Séguin, B., Mason, D.R., Walsh, P.J., Taylor, K.T., Kass, P.H., Stover, S.M.,
relevance of p53 index in canine osseous tumors. Veterinary Pathology 40, 237–
2004. Biomechanical comparison of cortical radial graft versus ulnar
transposition graft limb-sparing techniques for the distal radial site in dogs.
Loukopoulos, P., O'Brien, T., Ghoddusi, M., Mungall, B.A., Robinson, W.F., 2004.
Veterinary Surgery 33, 301–308.
Characterization of three novel canine osteosarcoma cell lines producing high
Powers, B.E., LaRue, S.M., Withrow, S.J., Straw, R.C., Richter, S.L., 1988. Jamshidi
level of matrix metalloproteinases. Research in Veterinary Science 77, 131–141.
needle biopsy for diagnosis of bone lesions in small animals. Journal of the
American Veterinary Medical Association 193, 205–210.
histopathological diagnosis in canine osteosarcoma. Veterinary Record 157, 784.
Powers, B.E., Gillette, E.L., McChesney, S.L., LeCouteur, R.A., Withrow, S.J., 1989. Bone
MacEwen, E.G., Kurzman, I.D., Helfand, S., Vail, D., London, C., Kisseberth, W.,
Rosenthal, R.C., Fox, L.E., Keller, E.T., Obradovich, J., Madewell, Rodriguez, C.,
irradiation. International Journal Radiation Oncology, Biology and Physics 17,
Kitchell, B., Fidel, J., Susaneck, S., Rosenberg, M., 1994. Current studies of
liposome muramyl tripeptide (CGP 19835A lipid) therapy for metastasis in
Powers, B.E., Withrow, S.J., Thrall, D.E., 1991. Percent tumor necrosis as a predictor
spontaneous tumors: a progress review. Journal of Drug Targeting 2, 391–396.
of treatment response in canine osteosarcoma. Cancer 67, 126–134.
MacEwen, E.G., Pastor, J., Kutzeke, J., Tsan, R., Kurzman, I.D., Thamm, D.H., Wilson,
Ramirez III, O., Dodge, R.K., Page, R.L., Price, G.S., Hauck, M.L., LaDue, T.A., Nutter, F.,
M., Radinsky, R., 2004. IGF-1 receptor contributes to the malignant phenotype
Thrall, D.E., 1999. Palliative radiotherapy of appendicular osteosarcoma in 95
in human and canine osteosarcoma. Journal of Cellular Biochemistry 92, 77–91.
dogs. Veterinary Radiology and Ultrasound 40, 517–522.
Malawer, M.M., Helman, L.J., O'Sullivan, B., 2008. Sarcomas of bone. In: DeVita, V.T.,
Reinhardt, S., Stockhaus, C., Teske, E., Rudolph, R., Brunnberg, L., 2005. Assessment
Lawrence, T.S., Rosenberg, S.A. (Eds.), DeVita. Hellman and Rosenberg's Cancer
of cytological criteria for diagnosing osteosarcoma in dogs. Journal of Small
Principles and Practice of Oncology. Lippincott Williams and Wilkins,
Animal Practice 46, 65–70.
Philadelphia, PA, USA, pp. 1794–1833.
Riser, W.H., Brodey, R.S., Biery, D.N., 1972. Bone infarctions associated with
Marcellin-Little, D.J., DeYoung, D.J., Thrall, D.E., Merrill, C.L., 1999. Osteosarcoma at
malignant bone tumors in dogs. Journal of the American Veterinary Medical
the site of bone infarction associated with total hip arthroplasty in a dog.
Association 160, 414–421.
Veterinary Surgery 28, 54–60.
Rodriguez, C.O., Crabbs, T.A., Wilson, D.W., Cannan, V.A., Skorupski, K.A., Gordon, N.,
Massin, P., Bocquet, L., Huten, D., Badelon, O., Duparc, J., 1995. Radiographic and
Koshkina, N., Kleinerman, E., Anderson, P.M., 2010. Aerosol gemcitabine:
histologic observations of autoclaved and non autoclaved allografts in the distal
preclinical safety and in vivo antitumor activity in osteosarcoma bearing
femoral metaphysis in dogs. Revue de chirurgie orthopedique et reparatrice del
dogs. Journal of Aerosol Medicine and Pulmonary Drug Delivery 23, 197–206.
l'appareil moteur 81, 189–197.
Rosenberger, J.A., Pablo, N.V., Crawford, P.C., 2007. Prevalence of and intrinsic risk
Mauldin, G.N., Matus, R.E., Withrow, S.J., Patnaik, A.K., 1988. Canine osteosarcoma
factors for appendicular osteosarcoma in dogs: 179 cases (1996–2005). Journal
treatment by amputation versus amputation and adjuvant chemotherapy using
of the American Veterinary Medical Association 23, 1076–1080.
doxorubicin and cisplatin. Journal of Veterinary Internal Medicine 2, 177–180.
Rovesti, G.L., Bascucci, M., Schmidt, K., Marcellin-Little, D.J., 2002. Limb sparing
Mehl, M.L., Seguin, B., Dernell, W.S., Lafferty, M., Kass, P.H., Withrow, S.J., 2005.
using a double bone-transport technique for treatment of a distal tibial
Survival analysis of one versus two treatments of local delivery cisplatin in a
osteosarcoma in a dog. Veterinary Surgery 31, 70–77.
biodegradable polymer for canine osteosarcoma. Veterinary and Comparative
Ru, G., Terracini, B., Glickman, L.T., 1998. Host related risk factors for canine
Oncology 3, 81–86.
osteosarcoma. The Veterinary Journal 156, 31–39.
Mendoza, S., Konishi, T., Dernell, W.S., Withrow, S.J., Miller, C.W., 1998. Status of the
Sagartz, J.E., Bodley, W.L., Gamblin, R.M., Couto, C.G., Tierney, L.A., Capen, C.C., 1996.
p53, Rb and MDM2 genes in canine osteosarcoma. Anticancer Research 18,
P53 tumor suppressor protein overexpression in osteogenic tumors of dogs.
Veterinary Pathology 33, 213–221.
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010),
E. Morello et al. / The Veterinary Journal xxx (2010) xxx–xxx
Seguin, B., Walsh, P.J., Mason, D.R., Wisner, E.R., Parmenter, J.L., Dernell, W.S., 2003.
Ta, H.T., Dass, C.R., Choong, P.F.M., Dunstan, D.E., 2009. Osteosarcoma treatment:
Use of an ipsilateral vascularized ulnar transposition autograft for limb-sparing
state of the art. Cancer Metastasis Reviews 28, 247–263.
surgery of the distal radius in dogs: an anatomic and clinical study. Veterinary
Thompson, J.P., Fugent, M.J., 1991. Evaluation of survival times after limb
Surgery 32, 69–79.
amputation, with and without subsequent administration of cisplatin, for
Shapiro, W., Fossum, T.W., Kitchell, B.E., Couto, C.G., Theilen, G.H., 1988. Use of
treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990). Journal
cisplatin for treatment of appendicular osteosarcoma in dogs. Journal of the
of the American Veterinary Medical Association 200, 531–533.
American Veterinary Medical Association 192, 507–511.
Thrall, D.E., 1984. Orthovoltage radiotherapy of acanthomatous epulides in 39 dogs.
Shi, F., MacEwen, E.G., Kurzman, I.D., 1993. In vitro and in vivo effect of doxorubicin
Journal of the American Veterinary Medical Association 184, 826–829.
Tomlin, J.L., Sturgeon, C., Pead, M.J., Muir, P., 2000. Use of the bisphosphonate drug
monocyte activation. Cancer Research 53, 3986–3991.
alendronate for palliative management of osteosarcoma in two dogs. Veterinary
Sinibaldi, K.R., Rosen, H., Liu, S.K., DeAngelis, M., 1976. Tumors associated with
Record 29, 129–132.
metallic implants in animals. Clinical Orthopaedics and Related Research 118,
Tommasini-Degna, M., Ehrhart, N., Ferretti, A., Buracco, P., 2000. Bone transport
osteogenesis for limb salvage following resection of primary bone tumors:
Sinibaldi, K.R., Pugh, J., Rosen, H., Liu, S.K., 1982. Osteomyelitis and neoplasia
experience with six cases (1991–1996). Veterinary Comparative Orthopaedic
associated with use of the Jonas intramedullary splint in small animals. Journal
and Traumatology 13, 18–22.
of American Veterinary Medical Association 181, 885–890.
van Leeuwen, I.S., Cornelisse, C.J., Misdorp, W., Goedegebuure, S.A., Kirpensteijn, J.,
Spodnick, G.J., Berg, J., Rand, W.M., Schelling, S.H., Couto, C.G., Harvey, H.J.,
Rutteman, G.R., 1997. P53 gene mutations in osteosarcomas in the dog. Cancer
Henderson, R.A., MacEwen, G., Mauldin, N., MacCaw, D.L., Moore, A.S.,
Letters 111, 173–178.
Morrison, W., Norris, A.M., O'Bradovich, J., O'Keefe, D.A., Page, R., Ruslander,
Vignoli, M., Ohlerth, S., Rossi, F., Pozzi, L., Terragni, R., Corlazzoli, D., Kaser-Hotz, B.,
D., Klausner, J., Straw, R.C., Thompson, J.P., 1992. Prognosis for dogs with
2004. Computed tomography-guided fine-needle aspiration and tissue-core
appendicular osteosarcoma treated by amputation alone: 162 cases (1978–
biopsy of bone lesions in small animals. Veterinary Radiology and Ultrasound
1988). Journal of the American Veterinary Medical Association 200, 995–999.
45, 125–130.
Spugnini, E.P., Vincenzi, B., Caruso, G., Baldi, A., Citro, G., Santini, D., Tonini, G., 2009.
Yamamoto, T., Hitora, T., Marui, T., Akisue, T., Nagira, K., Kawamoto, T., Yoshiya, S.,
Zoledronic acid for the treatment of appendicular osteosarcoma in a dog.
Kurosaka, M., 2002. Reimplantation of autoclaved or irradiated cortical bones
Journal of Small Animal Practice 50, 44–46.
invaded by soft tissue sarcomas. Anticancer Research 22, 3685–3690.
Stevenson, S., 1991. Fracture-associated sarcomas. Veterinary Clinics of North
Wallach, S.T., Wisner, E.R., Werner, J.A., Walsh, P.J., Kent, M.S., Fairley, R.A., Hornof,
America Small Animal Practice 21, 859–872.
Straw, R.C., Withrow, S.J., 1996. Limb-sparing surgery versus amputation for dogs
intramedullary osteosarcoma extent in pre-operative planning of canine limb-
with bone tumors. Veterinary Clinics of North America Small Animal Practice
salvage procedures. Veterinary Radiology and Ultrasound 43, 432–441.
26, 135–143.
Walter, C.U., Dernell, W.S., Larue, S.M., Lana, S.E., Lafferty, M.H., Ladue, T.A.,
Straw, R.C., Withrow, S.J., Powers, B.E., 1990. Management of canine appendicular
Withrow, S.J., 2005. Curative-intent radiation therapy as a treatment modality
osteosarcoma. Veterinary Clinics of North America Small Animal Practice 20,
for appendicular and axial osteosarcoma: a preliminary retrospective
evaluation of 14 dogs with the disease. Veterinary and Comparative Oncology
Straw, R.C., Withrow, S.J., Richter, S.L., Powers, B.E., Klein, M.K., Postorino, N.C.,
LaRue, S.M., Ogilvie, G.K., Vail, D.M., Morrison, W.B., McGee, M., Dickinson, K.,
White, R.A., Jefferies, A.R., Gorman, N.T., 1986. Sarcoma development following
1991. Amputation and cisplatin for treatment of canine osteosarcoma. Journal
irradiation of acanthomatous epulis in two dogs. Veterinary Record 118, 668.
of Veterinary Internal Medicine 5, 205–210.
Withrow, S.J., Liptak, J.M., Straw, R.C., Dernell, W.S., Jameson, V.J., Powers, B.E.,
Straw, R.C., Withrow, S.J., Doulple, E.B., Brekke, J.H., Cooper, M.F., Schwarz, P.D.,
Johnson, J.L., Brekke, J.H., Douple, E.B., 2004. Biodegradable cisplatin polymer in
Greco, D.S., Powers, B.E., 1994. The effect of cis-diamminedichloroplatinum II
limb-sparing surgery for canine osteosarcoma. Annals of Surgical Oncology 11,
released from D,L,-polylactic acid implanted adjacent to cortical allografts in
dogs. Journal of Orthopaedic Research 12, 871–877.
Please cite this article in press as: Morello, E., et al. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differenceswith human osteosarcoma. The Veterinary Journal (2010), doi:
Source: http://www.ad-domus.it/app/download/5790307835/OSA+REVIEW+VJ+2011.pdf
PROCESSUS STRATEGIQUES : DES ELEMENTS CLES POUR COMPRENDRE L'APRES FUSION : LE CAS SANOFI AVENTIS Philippe REBIERE Professeur Associé (ICN Business School) Université Nancy 2 CEREFIGE Cahier de Recherche n°2010-02 Université Nancy 2 13 rue Maréchal Ney Téléphone : 03 54 50 35 80
Cardiovascular Formulary for the Hypertensive Cat Lusitrope, Vasodilator, Negative chronotrope 180, 240 mg caps. 180, 240 mg caps. Enacard (Vasotec) 1, 2.5, & 5 mg tablets ACE-I (CHF, Hypertension) Lotensin (Foretkor) 5 & 10 mg tablets .25-.5 mg/kg PO qd-bid Negative chronotrope, 6.25-12.5 mg PO qd Antiarrhythmic, Lusi-trope, Antihypertensive 1/8–¼ inch topically tid








