Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Proteinad1.ro
Scientific report
regarding the implementation of the project
" Functional diversity of D1 proteins in photosystem II in cyanobacteria" code PN-II-ID-PCE-2011-
3-0765 during January – December 2015
Following the previous stages of the project, the initial phase in 2011, when the project was
started, the one in 2012 when the studies on the functional characterization of D1 protein were done, the one in 2013 when studies regarding the functional characterization of D1 protein in different cyanobacteria communities were started and the phase from 2014 when the previous studies have been continued, this year we have done the following activities:
- continued the studies regarding the characterization of photosystem II functionality in model
cyanobacteria cultures which have a completely sequenced genome; in this respect we looked for new aspects of the electron transport chain in
Synechococcus sp. PCC 7002 and we've also done the oxygen evolution measurements for the functional characterization of D1 protein from
Cyanothece sp. ATCC 5114 model strain.
- the study for functional characterization of D1 proteins from
Synechocystis PCC 6803 using
the immunodetection method with specific antibodies
- continued the studies regarding the functional characterization of D1 protein forms from
different cyanobacteria communities.
As a result of these experiments, a large volume of experimental data was collected, which
allowed us to elaborate 3 articles, 2 of them are published in ISI journals and one is in press. A synthesis of these data is presented below.
1. Results of the expression of psbA genes which codify different isoforms of D1 protein
Using the Real-time q-RT-PCR we compared the expression level of two D1 protein isoforms,
which are codified by
psbA genes from
Synechococcus PCC 7002 model cyanobacteria strain. The cells have been UVB stressed for 60min, then recovered in visible light for another 60min. The results show a significant difference between relative quantities of the two studied isoforms. In all the samples, the D1 isoform is predominant and it represents more than 99,9% of total analyzed transcripts (table 1). The D1' isoform is lightly induced during UVB stress, while the gene expression that codify for the other D1 protein isoforms is lightly downregulated under the same treatment conditions (fig. 1).
psbA isoform
Table 1. The different D1 isoforms relative quantity during the control, UVB treatment and
visible light recovery.
2. Fluoresecence measurements of D1 protein in different experimental conditions
The fluorescence measurements were done on
Synechococcus sp. PCC 7002 cells during UV-B
treatment; the samples were harvested at 0 time (control), after 60min of treatment and after 60min visible light recovery. The samples were either treated or not with DCMU.
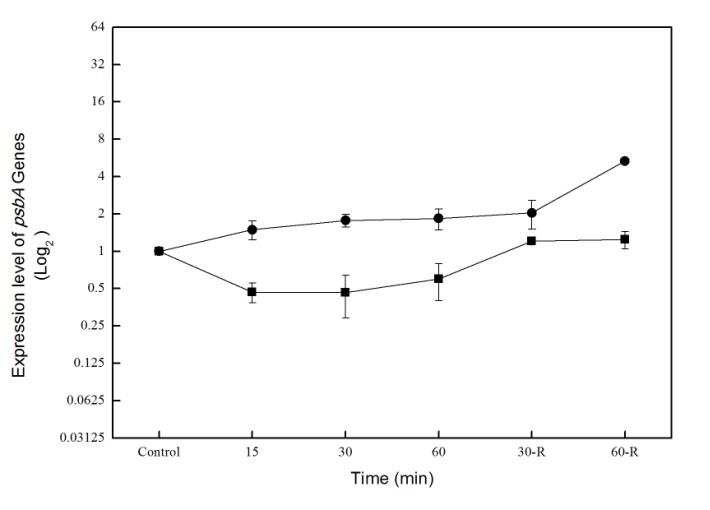
Figure. 1. The analysis of psbA-D1' (square)
and other D1 isoforms (circle) during UV-B
stress and visible light recovery time at
Synechococcus PCC7002
The amplitude of the fluoresecnce signal offers a good indication relative to the number of
photosystem II (PSII) active centers at the time of the actinic flash. Under normal conditions, the population of centers in the membrane is heterogeneous, with a fraction of the centers in a physiological inactive state at the time of the flash (fig. 2a). Adding DCMU to the cells brings all centers into the active state, allowing us to measure the total potential active centers (fig. 2b). During the UVB treatment, we observed a reduction in the number of PSII active centers (with 35%) that recovers to about 90% of initial state during recovery time (fig 2a and b).
The fluorescence measurements have been made using a fluorometer with double modulation
(PSI, Czech Republic). The decay of flash-induced fluorescence was monitored by measuring flashes on a logarithmic time scale. We studied the cells treated and not treated with DCMU. In the presence of DCMU, the acceptor side of PSII is blocked and QA- is forced into recombination with the water oxidation complex resulting in preservation of a high fluorescence level for a significantly longer time (up to about 100 ms) (fig. 2d) compared to untreated samples (fig. 2c). In the presence of UVB, a specific fast phase appears at short time ranges. We observed that UVB also induces a modification of the donor side of PSII forcing QA- into recombination with TyrZ or P680. This modification is quick and fully recovered upon returning the cells to growth light conditions. This could be linked to specific mechanism of UVB affecting the photosynthetic machinery in Synechococcus PCC 7002 or particular characteristic of PSII electron flow in this species that remains to be investigated.
These data were published in Romanian Biotechnology Letters as "UV-B stress changes the
electron flow on photosynthesis II complex in Synechococcus PCC 7002." Chis C., Druga B., Chis I., Ardelean A., Sicora C. I.
3. Oxygen evolving measurements at model cyanobacteria for the functional characterization of
D1 protein isoforms
We performed experiments of oxygen evolution measurements on two parallel cyanobacteria
cultures, in the presence or absence of lincomycin (300µg/ml), a "de novo" protein synthesis inhibitor. The apparatus used in these experiments was Oxylab 1.15 from Hansatech Instruments.
The culture of mutant Synechococcus sp. PCC 7002 was grown in BG11 medium supplemented
with Turks Island Salts and vitamin B12, with continuous illumination at 50µE/m2 s, at 38⁰C, till the chlorophyll concentration reached 14μg chl/ml. The UVB treatment was applied using a potassium dichromate filter. We performed parallel experiments on treated and non-treated cultures. In the first step, we measured the oxygen evolution of the control culture in the presence of 2,6-dimethoxy benzoquinone (DMBQ) (1µl/mL); after 10min in visible light (50µE) lincomycin was added and after another 15min the UVB treatment is started using the filter. The samples were harvested after 15, 30, 60 and 90 min respectively after UVB treatment. During the recovery time, in the visible light, the samples were harvested at 30, 60 and 90 min respectively. The results are shown in Fig. 3.
During the recovery time, we observed a smaller rate of oxygen evolution at the culture treated
with lincomycin as compared to the control culture. This suggests the blocking of protein synthesis with lincomycin, impairing the synthesis of some PSII proteins involved in the reaction of cells to stress.
We performed oxygen evolution measurements on a Cyanothece sp. ATCC 51142 culture in a
volume of 250ml, grown in the light-dark cycles till the chlorophyll concentration reached 10μg chl/ml.
Figure 2. The effect of UV-B on
PSII function at Synechococcus PCC
7002. Changes in the number of
actual active centers (panel a) and
potential active centers (panel b) at
control,
recovery in the presence (panel b) or absence (panel a) of DCMU. The decay of flash-induced fluorescence was monitored on control cells, cells treated with UV-B and on cells during recovery period, in the absence (panel c) or presence (panel d) of DCMU.
In order to observe the variation of oxygen evolution during a light-dark cycle, the samples have
been harvested at 700, 900, 1400, 1900, 2100 and 200respectivly and measured with Oxylab. The results are showed in Figure 4, panel a.
We observed there is a time during daylight when the respiration reaches a maximum, therefore,
we repeated the experiment on a 12 hours period, at light, with measurements performed every hour. The results shown in figure 3, point toward a modulation of PSII activity during the day, with a peak around 1 o'clock p.m. (fig. 4, panel b)
Figure 3. The variation of oxygen evolution on
mutant Synechococcus sp. PCC 7002 during
visible light and UV-B light treatment
4. Immunodetection of D1 protein using Western-Blot technique for functional characterization of
D1 protein
Synechocystis PCC 6803 cyanobacterial strain was used for this experiment. The culture was
treated with a mixture of gases (N2 95%, CO2 5%) in order to reach the microaerobic conditions. Previously, it was demonstrated that microaerobiosis leads to expression of genes that are repressed in normal growth conditions. Real-Time qRT-PCR studies on this cyanobacterial strain demonstrated a modulation of psbA-D1' gene in microaerobic conditions.
We wanted to see if microaerobiosis influences the protein expression. To this end, samples
were harvested at time 0 (control), after 15, 30 and 60 min of treatment with afore mentioned mixture of gases and after 30 and 60 min during recovery period. The samples were processed according to the protein isolation protocol, the proteins were quantified and then an equal amount of proteins were loaded onto the gel. The polyacrylamide gel was run in the running tank and then transferred onto the nitrocellulose membrane for immunodetection. For immunodetection we used as a primary antibody chick anti total D1 protein, C-terminal (Agrisera) diluted 1:10000 and rabbit anti-chick horseradish peroxidase (HRP) labeled as a secondary antibody, diluted 1:5000. Upon developing with ECL, the band pattern obtained (around 30KDa a specific band appears) indicates a slightly less D1 total protein at 60 min of microaerobiosis treatment that recovers totally during recovery period. This points towards
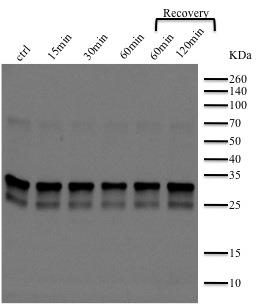
a slightly inhibitor effect of microaerobiosis on D1 protein expression at Synechocystis PCC 6803 but doesn't indicate which isoform is affected as does Real-Time qRT-PCR for the expression of different
Figure 4. Changes in the oxygen
evolution of Cyanothece sp. ATCC 51142
b grown in the light-dark cycles (panel a) or
during a 12 hours light period (panel b)
psbA gene isoforms. The lack of antibodies for these specific D1 protein isoforms, due to the similarity of their amino acid sequences, makes it difficult to interpret the immunoblot experiments.
Figure 5. Immunoblot showing the D1 total
protein expression at Synechocystis PCC 6803
during the microaerobiosis treatment. A specific
band at around 30KDa coresponds to D1 protein.
5. The detection of different PCR amplicons in environmental samples
We performed DNA isolation on environmental samples according to the protocol that has been
optimized in our laboratory. Using the Primer3 software (NCBI) we designed specific primers for different genes that codify different isoforms of D1 protein. After performing PCR with these primers, the expected amplicons were not obtained. The PCR optimization process is still running in the lab.
6. Chlorophyll fluorescence measurements on environmental samples
Bacterial crust are communities of living microorganisms distributed in overlapping layers that
are present at the interface of different substrates, especially wet ones. Microbial crusts contain photosynthetic prokaryote communities, bacteria and archaea. Cyanobacterial crust is a valuable experimental model for investigating the changes in photosynthesis with the environmental conditions.
The hot spring at Ciocaia, Bihor district, presents o variety of temperatures, from 65.50C at the
spring, 60.5°C 2 meters from the spring and between 32-38°C in the area where cyanobacteria communities form. It can be observed that very close to the spring, at the highest temperature, a white bacteria community developed, followed by a red and green one, at a lower temperature. The green community consists of a cyanobacterial crust. A big part of this crusts has been harvested together with water and brought to our laboratory. In the lab, the crust was placed in Ciocaia spring water in a growing tank with double walls that permits the water recirculation and is maintained at 370C. After 2 weeks, the Ciocaia spring water was replaced partially (2:1, 1:1, 1:2 mixture between Ciocaia spring water and BG11 medium) with BG11 medium.
The chlorophyll fluorescence measurements at this sample which was adapted to the lab
growing conditions shows some specific features: we observed a fast electron transfer between QA and QB which means that the acceptor part of PSII works normally. In the presence of DCMU that blocks the transfer between QA and QB and QA- combines with donor part of PSII, especially with water
reoxidation complex, we observed a longer time of fluorescence but without other specific changes. Comparing with the previously measured samples from Ciocaia, where we observed a delay in the electron transfer, our samples show a faster electron transfer which can be explained by the fact that cyanobacterial crusts are exposed to solar light and as a response to this the electron transport is delayed.
Figure 6. The chlorophyll fluorescence is
measured on the cyanobacterial culture adapted to
the laboratory conditions, untreated (full circle)
and treated (empty circle) with DCMU.
7.
Study of the taxonomic groups from the saline lake using qRT-PCR technique
We performed taxonomic studies of "metabarcoding" type on samples from two hypersaline
meromictic lakes from Transylvanian basin- Ursu Lake and Fara Fund Lake. Meromictic hypersaline lakes are extreme environments in which water stratification is associated with powerful physicochemical gradients and high salt concentrations. In order to analyze the composition and the structure of prokaryotes communities from these lakes, we collected samples that were used for "deep-coverage" sequencing of small subunit (16S) of ribosomal DNA (rDNA) amplicons. These amplicons were obtained upon quantitative PCR using specific primers for rDNA from Bacteria phylum or Archaea. To this end, the DNA was isolated from the samples harvested from the lakes from different stratification layers and qRT-PCR was performed for evaluation of relative abundance of phylum Bacteria or Archaea, using specific primers for SSU rDNA Archaea and Bacteria. After 16S rDNA amplicon sequencing a phylogenetic tree was built that indicates the prokaryotic abundance and composition of the studied lakes. It was observed that the diversity of Archaea and Bacteria phylum increases with the salinity level. This study is unique for Romania and is the first done in our country on the hypersaline lakes. This study identified some cyanobacterial strains, especially species from Synechococcus genera in Ursu Lake that will be further studied for psbA gene expression in these extreme environmental conditions from the studied lakes.
Source: http://www.proteinad1.ro/index_htm_files/raport_decembrie2015_PNII_english.pdf
Doro PhoneEasy® 624 15. Charging socket 16. Headset socket 17. Assistance button Left selection button 20. External display Input method/Silent 22. Green light = New message 10. Message shortcut 23. Red light = Battery level low 11. Volume control 24. Charging stand 12. End call/Power on/off 14. Right selection button The items supplied with your phone might vary depending on the soft-ware and accessories available in your region or offered by your serviceprovider. You can obtain additional accessories from your local Doro deal-er. The supplied accessories provide the best performance with yourphone.
Brown County Schools Brown County Schools prepares students to achieve success through quality instruction. New Student Enrollment Packet (preschool - grade 6) Please complete this New Student Enrollment Packet, print and bring it along with the following legal documents to the Brown County School Administration Office: 1) Birth Certificate 2) Immunization Records 3) Custody Papers (if applicable)





