Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Doi:10.1016/j.jaad.2006.04.084
Systematic review of rosacea treatments
Esther J. van Zuuren, MD,a Aditya K. Gupta, MD, PhD, FRCP(C),b,c
Melissa D. Gover, BSc,c Mark Graber, MD,d and Sally Hollis, MSce
Leiden, The Netherlands; Toronto and London, Ontario,
Canada; Iowa City, Iowa; and Lancaster, United Kingdom
Background: Rosacea is a common chronic skin and ocular condition. It is unclear which treatmentsare most effective. We have conducted a Cochrane review of rosacea therapies.This article is a distillationof that work.
Objective: We sought to assess the evidence for the efficacy and safety of rosacea therapies.
Methods: Multiple databases were systematically searched. Randomized controlled trials in people withmoderate to severe rosacea were included. Study selection, assessment of methodologic quality, dataextraction, and analysis were carried out by two independent researchers.
Results: In all, 29 studies met inclusion criteria. Topical metronidazole is more effective than placebo(odds ratio 5.96, 95% confidence interval 2.95-12.06). Azelaic acid is more effective than placebo (oddsratio 2.45, 95% confidence interval 1.82-3.28). Firm conclusions could not be drawn about other therapies.
Limitations: The quality of the studies was generally poor.
Conclusions: There is evidence that topical metronidazole and azelaic acid are effective. There is someevidence that oral metronidazole and tetracycline are effective. More well-designed, randomized controlledtrials are required to provide better evidence of the efficacy and safety of other rosacea therapies. ( J AmAcad Dermatol 2007;56:107-15.)
by recurrent episodes of facial flushing, ery-thema, papules, pustules, and telangiectasia
confidence interval
in a symmetrical, facial distribution.Several well-
RCT: randomized controlled trial
defined types of rosacea are described includingerythematotelangiectatic rosacea, papulopustularrosacea, phymatous rosacea, ocular rosacea, and
can develop without involvement of other areas of
the variant granulomatous rosacOcular rosacea
the skin and may wax and wane.Rosacea usuallypresents in the second or third decade of life andhas a prevalence of up to 10%.It is especially com-
From the Department of Dermatology B1-Q, Leiden University
mon in fair-skinned people of Celtic and northern
Medical Centera; Division of Dermatology, Department of
European heritage, with women more often affected
Medicine, Sunnybrook and Women's College Health Sciences
than However, men will more often progress
Center and the University of Torontob; Mediprobe Research Inc,
to the later stages.
Londonc; Emergency Medicine and Family Medicine, Universityof Iowa College of Medicined; and University of Lancaster.e
Traditionally, rosacea has been managed with a
Funding sources: None.
treatment tailored to the specific symptoms pre-
Conflicts of interest: None identified.
sented.A brief overview of these therapies is
This manuscript is based on an earlier publication by van Zuuren
presented in Other treatments tried
et al,copyright Cochrane Library, reproduced with permission.
include facial massage (for edema), spironolactone,
Reprint requests: Esther J. van Zuuren, MD, Department of Derma-
tology B1-Q, Leiden University Medical Center, PO Box 9600,
beta-blockers, dapsone, oral contraceptives, ben-
2300 RC Leiden, The Netherlands.
zoyl peroxide, bifonazole cream, and treatment of
Helicobacter pyloriUnfortunately, many of
Published online November 10, 2006.
these remain poorly studied. This review was per-
0190-9622/$32.00ª 2007 by the American Academy of Dermatology, Inc.
formed to systematically evaluate rosacea treatments
including the potential impact of nonpharmacologic
J AM ACAD DERMATOL
108 van Zuuren et al
Table I. Rosacea therapi
Topical therapies
Metronidazole (0.75%, 1%)
Clindamycin lotionPermethrin 5% creamTretinoin creamSulfacetamide 10%/sulfur 5%Azelaic acid (15% gel, 20% cream)
Proposed therapies
TacrolimusTopical NADH
Possible side effects
candidal vaginitis,reduction in oralcontraceptiveefficacy
Discontinue oral treatment once sufficient efficacy noted
Maintenance therapy with topical medications
intense pulsedlight
Oral isotretinoin
13-cis-retinoic acid
Possible side effects
persistent rosacea
include: dry sensitive
monitoring of liver
skin, dry mucosae,
dry eyes, pruritis,
dermatitis, myalgia,
enzymes, cholesteroland triglycerideelevation
abnormalities forwomen who becomepregnant
Oral hypotensives
Low-dose isotretinoin
Laser therapySurgical
MetronidazoleFusidic acid gel
NADH, reduced form of ß-nicotinamide adenine dinucleotide.
agents such as foods (eg, spicy food), certain cos-
of rosacea. As rosacea can cause shame, embarrass-
metics, and sunscreens.
ment, low self-esteem, anxiety, lack of confidence,
Unfortunately, there is no universally accepted
and depression, our primary outcome was the
clinical definition of rosacea, and there are no
patients' self-assessment of rosacea, and their per-
standard validated tools for assessing the severity
ception of their quality of life.
J AM ACAD DERMATOL
van Zuuren et al 109
VOLUME 56, NUMBER 1
Table II. Criteria used to assess the methodologic quality of randomized controlled trialsfor rosacea therapies
Quality assessment criteria:
Was the randomization procedure used appropriate?
Was the allocation concealment adequate?
Was an intention-to-treat analysis used?
Were health workers and study personnel blind to treatment?
Were participants blind to treatment?Aside from the intervention, were groups treated equally?
Was the study duration fixed/adequate (at least 4 weeks)?Were number and timing of assessment points fixed?
Was there an acceptable description or definition of rosacea?
Was the site of evaluation recorded?
y Were concomitant medications permitted and recorded?
Was previous oral and topical rosacea therapy stopped a minimum of 4 weeks before the initial assessment?Were the therapeutic interventions adequately described?Were adequate details about how to use/take the medication given to all participants?
Was the dropout rate less than 5%?
Modifiedand used with permission.
*All these criteria must be ‘‘yes'' to be high quality.
yStudy must not allow concomitant medications that might change outcome.
criteria were considered high quality, whereas stud-
A systematic review of randomized controlled
ies meeting some, but not all, were generally classi-
trials (RCTs) was performed according to a prespe-
fied as intermediate. Studies classified as low quality
were excluded from analysis. Supporting methodol-ogy descriptions for each criterion had to be present
Search strategies
in the published text to merit the grading. Details of
Two reviewers performed independent searches
eligible trials were extracted and summarized using
of the following 6 electronic databases: The
structured data collection forms.
Cochrane Skin Group Specialized Trials Register(February 2005), The Cochrane Central Register of
Controlled Trials (February 2005), MEDLINE (1966-
The primary outcome measures included impact
February 2005), EMBASE (1980-February 2005),
on quality of life and participant-assessed changes in
Biosis (1970-March 2002), and Science Citation
rosacea severity. Secondary outcome measures were
Index (1988-February 2005). In addition, the refer-
physician-assessed changes in rosacea severity, phy-
ence lists of all identified RCTs and key review
sician's global evaluation (improvement defined
articles were searched. Attempts were made to
as $ 50% change), lesion counts (treatment success
obtain details of unpublished and ongoing RCTs
defined as [50% reduction), time needed for
and grey literature through correspondence with
improvement, and duration of remission. Other
authors and pharmaceutical companies.
outcomes included dropout rates and incidence ofadverse events.
Selection criteria
We considered all RCTs evaluating any type of
intervention used to treat rosacea. Study participants
Quantitative pooling was performed using odds
had to be older than 19 years with moderate to severe
ratio (OR) for categorical measures or weighted
rosacea as assessed by a physician. Two reviewers
mean differences for continuous measures. Where
independently assessed these articles for eligibility.
study results were heterogeneous, the reasons for
Any disagreement was resolved by discussion.
this were explored (eg, treatment or participantfactors) and a random effects model was used to
Study design quality assessment
reflect the increased uncertainty. Investigation of
and data extraction
the robustness of the conclusions according to the
Study design was assessed by two reviewers as
methodologic quality of the contributing studies was
per the criteria in . Studies meeting all the
not practical because there were only a few studies
J AM ACAD DERMATOL
110 van Zuuren et al
contributing to each comparison; study quality was
within these therapeutic categories, making compar-
considered qualitatively when drawing conclusions.
isons and pooling of data was problematic because
Some studies used a split-face, within-patient
of heterogeneous study designs, skewed data, miss-
design, where two interventions were allocated
ing variability, and differences in comparators or
randomly to the left and right side of the face.
dosing regimens. Only data on outcome measures
Where possible, a conditional OR (based on the
from trials on topical metronidazole, topical azelaic
discordant cases only) was calculated; this can be
acid, and oral tetracycline could be pooled. Most
interpreted in the same way as the ORs from parallel
studies used numbers of papules or pustules as an
group studies.However, the paired data necessary
outcome measure rather than a more clinically rel-
for this were sometimes unavailable, in which case
evant measure, such as participant assessment of
marginal ORs (based on the overall rates for each
appearance. Below is a summary of the most impor-
treatment) were calculated and reported. These
tant conclusions. For details and full reporting of the
marginal ORs should be interpreted cautiously, be-
data, please refer to the complete Cochrane review
cause they differ from conditional ORs when there
as published in the Cochrane Li
is correlation between the outcomes of the twotreatments.
METRONIDAZOLETopical metronidazole versus placebo
Nine trials assessed the efficacy of topical metro-
Description of studies and methodologic
nidazole versus placebo.The
quality of included studies
treatment period ranged from 8 to 9 weeks in each
Searches identified 71 possible RCTs. A total of 29
trial, except for that of Dahl et al,which was 6
RCTs were included.Breneman et aland
months. Three studies addressed self-assessed im-
Leyden et aldescribed different outcome measures
provement of rosacea severitOnly data from
of the same study and Thiboutot et alreported
two studiescould be pooled , A) and there
two RCTs in one publication. Most of the participants
was clear evidence that metronidazole was more
in the included studies had papulopustular rosacea
effective than placebo. Bleicher et alconfirmed
and were between 40 and 50 years old; only two
these data (OR 7.0; 95% confidence interval [CI]
addressed ocular rosacea. Of the 71
2.5-20.0). Data on physician's global evaluation
studies, 41 were excluded because allocation con-
concerning improvement were similar to the pa-
cealment was inadequate, the study was not blinded,
tient-assessed measures in favor of metronidazole
the dropout rate was more than 10%, or other
(OR 7.01; 95% CI 3.56-13.81).The other stud-
major methodologic or because
ies showed comparable
they were awaiting assessment.Of the 29
Most of the adverse events mentioned were mild,
included studies, 8 were classified as high qual-
including pruritus, skin irritation, and dry skin. There
ity.The remaining 21 trials were
were no significant differences in the number of
of intermediate quaIn only 14
adverse events between groups.
of the 29 trialswas thereadequate blinding of treatment allocation. Blindingof outcome assessment was demonstrated in all
Topical azelaic acid versus topical
except two studies.Intention-to-treat analysis
was used in 17 of the 29 trials.
There was no statistically significant difference in
For 14 studies the variability (SD or SE) of contin-
the patient self-assessment between topical azelaic
uous measurements were completely or partially
acid and topical metronidazole.However, phy-
lacking, making these data unusable in a meta-
sicians rated the azelaic acid group more improved
(OR 1.84; 95% CI 1.10-3.09).The number ofadverse events was lower in the metronidazole
group (OR 4.56; 95% CI 2.07-1However,
the severity of adverse events in both groups was
reported as mild to moderate and mostly transient.
oral antibiotics (8
Topical metronidazole versus oral
trials),topical benzoyl peroxide com-
bined with topical antibiotics (2 and other
In two 8-week studiesno statistically signifi-
therapies (4 Five trials included com-
cant treatment difference was seen between metro-
parisons in more than one category.Even
nidazole cream and (oxy)-tetracycline.
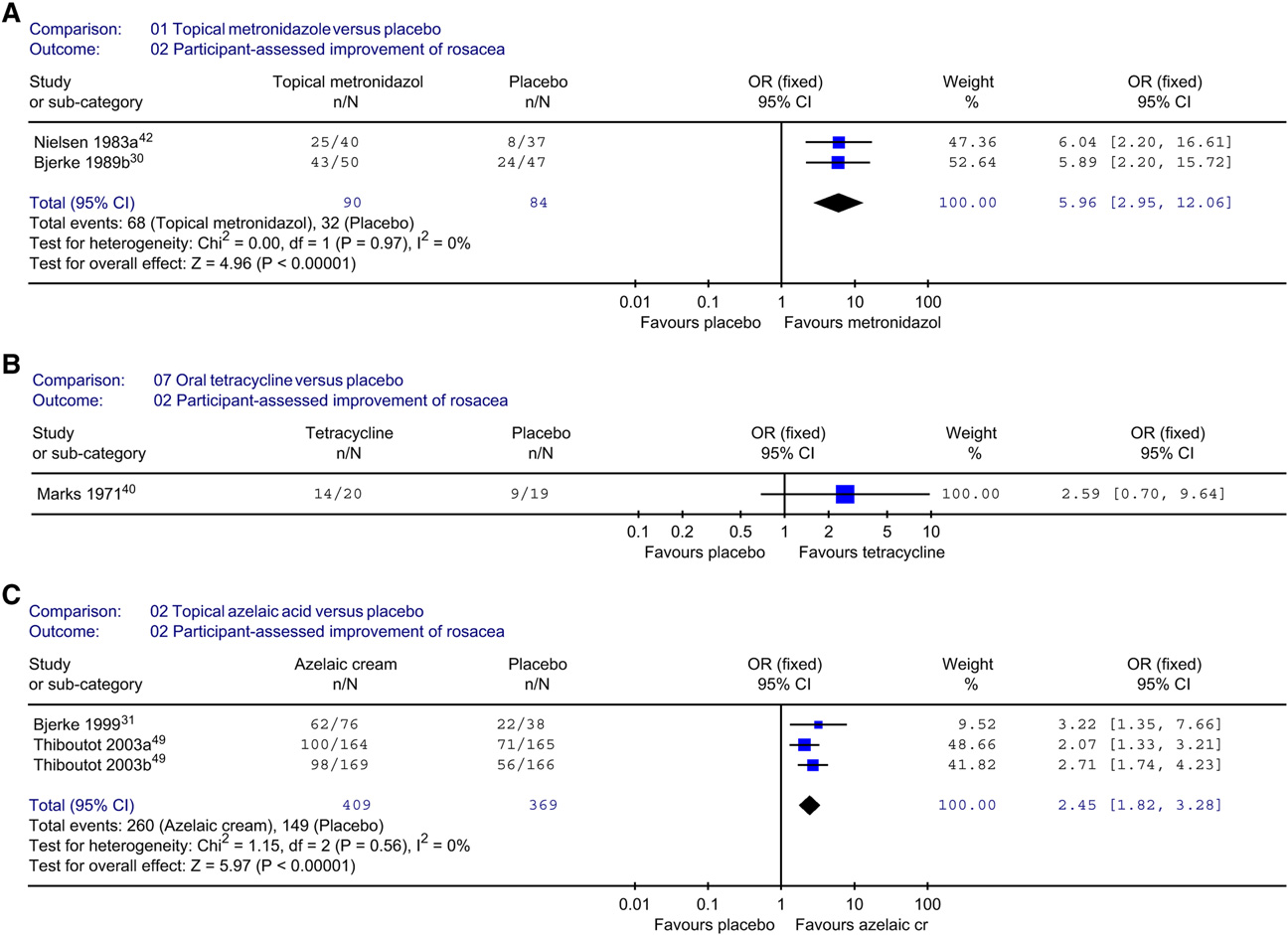
J AM ACAD DERMATOL
van Zuuren et al 111
VOLUME 56, NUMBER 1
Fig 1. Meta-analytic comparisons of participant-assessed improvement between topicalmetronidazole and placebo (A), oral tetracycline and placebo (B), and topical azelaic acidand placebo (C). Modifiedand used with permission. CI, Confidence interval; OR, odds ratio.
Metronidazole plus sunscreen
(sun protection factor 15) versus
Oral metronidazole versus oral
A poorly designed study favored metronidazole
In one study, oral metronidazole and oral oxytet-
plus sunscreen over place
racycline were not statistically different at 12 weeksby both physician and patient assessment.Noadverse events were reported in either group.
Topical metronidazole versus topicalpermethrin versus placebo
Koc¸ak et alinvestigated the efficacy and safety
Tetracycline versus placebo
One trialcompared oral oxytetracycline with
Permethrin was inferior to topical metronidazole
placebo, and in two oral tetracycline was
because it showed no effect on pustules.
compared with placebo. These are both (older)tetracyclines with a similar molecular structure andthe same pharmacokinetic and pharmacodynamic
Benzoyl peroxide 5%/erythromycin 3% gel
profile and so the results were pooled. Study dura-
versus metronidazole gel
tion ranged from 4 to 6 weeks. Bartholomew et
No significant difference was shown between
addressed treatment efficacy in ocular rosacea.
the two therapies in 4 weeks (OR 0.92; 95% CI
There was insufficient evidence of any advantage
of tetracycline over placebo according to patients'
J AM ACAD DERMATOL
112 van Zuuren et al
Table III. Data to be included in future rosacea studies
Well-designed RCT with reporting the Proper description of randomization procedure and allocation concealmentData presented with appropriate summaries and analysis (including variability)The number of participants who started in and dropped out from each groupOutcomes primarily based on: patient's opinion of treatment efficacy, quality of life, and patient-assessmentPhysician's opinion, reflected by global evaluation, lesion counts, and assessment of telangiectasiaUse of intention-to-treat analysis
RCT, Randomized controlled trial.
Modifiedand used with permission.
assessment (B).However, the dropout rate
The mean scores at 12 weeks for physician's global
was unclear and the data were skewed with large
assessment were 1.85 (marked to definite improve-
variability. By physician assessment, tetracyclines are
ment) versus 2.96 for placebo (minimal improve-
far more effective than placebo in the treatment
ment) (authors state P = .0026).
of rosacea (OR 6.06; 95% CI 2.96-12.42). Repeated
The data showed large variability and some data
courses of treatment with the same dose achieved
were missing. Most data were skewed. Treatment-
lasting remission 3 to 6 months after stopping
related adverse events included site burning and
itching, both well-known side effects of benzoylperoxiThe same study using photographic
Clarithromycin and omeprazole
assessments as outcomes came to similar same
These data were skewed with large variability
and, thus, it is impossible to draw conclusions about
Benzoyl peroxide acetone versus placebo
At 4 weeks, benzoyl peroxide showed an im-
provement on the physician's global evaluation
Azelaic acid versus placebo
score compared with placebo (OR 3.17; 95% CI
Four trials compared azelaic acid with pla-
1.08-9.31).The other measurements were also in
The treatment period ranged from 9
favor of benzoyl peroxide (P .05). Irritation and
to 12 weeks. Three studiesshowed a clear
burning were frequently reported in both groups.
improvement in the azelaic acid group as rated by
Oral metronidazole and topical
both physicians and patients , C ). A split-face,
hydrocortisone 1% cream versus oral placebo
and topical hydrocortisone 1% cream
(marginal OR 30.1; P .0003).
The physicians considered 10 of 14 participants
The data on lesion counts did not include varia-
treated with oral metronidazole improved versus
bility and the data in the study by Carmichael et al
only 2 of 13 participants on placebo (OR 13.75; 95%
were skewed.
CI 2.05-92.04).Only limited data were given in this
More side effects were reported in the azelaic
group (11.5%) versus the placebo group (5.7%) (OR1.61; 95% CI 0.89-2.92)The same holds true for
Rilmenidine versus placebo
the study of Carmichael et alSide effects were
Both the patients and the physicians believed that
considered mild and transient with burning, stinging,
there was no significant difference between rilmeni-
and irritation being reported most frequently.
dine and placebo; neither treatment was
BENZOYL PEROXIDE WITH ANTIBIOTICS
Sodium sulfacetamide/sulfur versus placebo
Benzoyl peroxide 5%/clindamycin 1% gel
The percentage of participants who considered
themselves improved was 90% in the sodium sulfa-
The mean scores at 12 weeks for patient's global
cetamide 10%/sulfur 5% group versus 58% in the
assessment in the study of Breneman et alwere
placebo group (authors state P The phy-
1.54 (much to slightly better) in the benzoyl peroxide
sicians shared this opinion. Adverse events were
and clindamycin group versus 2.50 (slightly better to
reported in 38% versus 29%, respectively. Applica-
same) in the placebo group (authors state P = .0002).
tion site reactions such as dryness, erythema, and
J AM ACAD DERMATOL
van Zuuren et al 113
VOLUME 56, NUMBER 1
Table IV. Questions for which evidence is lacking in the literature
1. What is the efficacy and safety of commonly used treatments for rosacea (eg, tetracycline, minocycline, doxycycline,
isotretinoin, and laser therapy)?
2. What is the efficacy and safety of treatments for ocular rosacea?3. Is there any efficacy of dietary measures and/or sun-protective measures in the treatment of rosacea?4. What is the efficacy and safety of benzoyl peroxide alone or in combination with topical antibiotics for rosacea?5. Is permethrin effective and safe for rosacea treatment?
Studies to answer these questions should meet the criteria mentioned in
pruritus were the most commonly reported adverse
the study with permethrin.Both benzoyl peroxide
events. It was unclear how many participants started
and permethrin are well-known drugs and further
in each group or how improvement was defined, and
investigation in the treatment of rosacea may be
for continuous measurements the variability was
large and the data
No studies could be included addressing dietary
or sun-protective measures; however, two studies
did combine treatment with a sun protection fac-
There were significant limitations in the quality of
tor.Although not really substantiated, most peo-
evidence available for most treatments. Although the
ple with rosacea are given the advice to avoid trigger
clinical design of the included studies was in theory
factors, (eg, spicy foods, alcohol, and sunlight).
adequate, closer examination revealed that the qual-
Only two trials could be included on treatment
ity of reported data was often low.
of ocular rosacea,even though almost 60%
summarize recommendations for future rosacea
of people with rosacea have ocular involve-
studiesIt should be noted that although split-face
ment.Although often mild, the ocular pre-
studies can be efficient, they are subject to potential
sentation can be both severe and debilitating. There
biases. Contamination may occur if active cream
was insufficient evidence for the efficacy of topical
is accidentally transferred onto the placebo side.
metronidazole.Oral oxytetracycline seems to be
Furthermore, a treatment may have systemic effects,
effective for ocular although only the
beneficial or harmful, which will affect both sides.
opinion of the physician was reported.
Our principal outcome measure, quality of life,
A very interesting treatment seems to be low-dose
was not assessed in any of the studies and only a few
doxycycline (20 mg twice a day),which is a
studies assessed the participant's own opinion. It is
subantimicrobial dose that reduces inflammation.
interesting to note that the investigators were more
Other potential advantages of this treatment include
satisfied at the end of the study than the partici-
lessening the risks of Propionibacterium acne's re-
pantFor other diseases it is often the reverse.
sistance to tetracyclines and lowering the incidence
This may have implications for clinicians, as a
of tetracycline-induced adverse events. Unfortu-
patient's perception of a lack of sufficient efficacy
nately, even though they are commonly used to treat
can impact compliance and may lead to the use of
rosacea, no RCTs evaluating doxycycline, minocy-
alternative therapies. Topical metronidazole and
cline, isotretinoin, or laser therapy could be included
azelaic acid appear to be effective and safe for
in this review (most often because of inadequate
short-term use, with the rate of adverse events in
study design). There is an urgent need for better
the placebo groups being similar to the active treat-
quality, adequately designed RCTs on the commonly
ment groups. With regard to tetracycline, only 3
used treatments for rosacea.
could be included in this review, onlyone of which assessed the opinion of the partici-
pants; however, this study failed to detect any
1. van Zuuren EJ, Graber MA, Hollis S, Chaudhry M, Gupta AK,
difference from placebo. It is possible that in this
Gover M. Interventions for rosacea. The Cochrane Database ofSystematic Reviews. 2005; issue 3. Available at:
case the study duration of 6 weeks was too short.
There were no studies evaluating other treatment
Accessed July 20, 2005.
options, such as erythromycin, dapsone, and topical
2. Crawford GH, Pelle MT, James WD. Rosacea, I: etiology,
tretinoin,that met the inclusion criteria.
pathogenesis, and subtype classification. J Am Acad Dermatol
Three studies were included using benzoyl per-
3. Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al.
oxide alone or in combination with topical antibi-
Standard classification of rosacea: report of the National
Unfortunately, the quality of these
Rosacea Society expert committee on the classification and
studies was suboptimal. The same holds true for
staging of rosacea. J Am Acad Dermatol 2002;46:584-7.
J AM ACAD DERMATOL
114 van Zuuren et al
4. Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, et al.
29. Bitar A, Bourgouin J, Dore´ N, Dubuc R, Giroux J, Landry M,
Standard grading system for rosacea: report of the National
et al. A double-blind randomized study of metronidazole
Rosacea Society expert committee on the classification and
(Flagyl) 1% cream in the treatment of acne rosacea, a placebo
staging of rosacea. J Am Acad Dermatol 2004;50:907-12.
controlled study. Drug Invest 1990;2:242-8.
5. Michel JL, Cabibel F. Frequency, severity and treatment of ocular
30. Bjerke J, Nyfors A, Austad J, Rajka G. Metronidazole (Elyzol)
rosacea during cutaneous rosacea [French]. Ann Dermatol
1% cream vs. placebo cream in the treatment of rosacea. Clin
Trials J 1989;26:187-94.
6. Berg M, Liden S. An epidemiological study of rosacea. Acta
31. Bjerke R, Fyrand O, Graupe K. Double-blind comparison of
Derm Venereol 1989;69:419-23.
azelaic acid 20% cream and its vehicle in treatment of papulo-
7. Jansen T, Plewig G. Rosacea: classification and treatment.
pustular rosacea. Acta Derm Venereol 1999;79:456-9.
J R Soc Med 1997;90:144-50.
32. Bleicher PA, Charles JH, Sober AJ. Topical metronidazole
8. Kligman AM. A personal critique on the state of knowledge of
therapy for rosacea. Arch Dermatol 1987;123:609-14.
rosacea. Dermatology 2004;208:191-7.
33. Breneman DL, Stewart D, Hevia O, Hino PD, Drake LA.
9. Landow K. Unraveling the mystery of rosacea: keys to getting
A double-blind, multicenter clinical trial comparing efficacy
the red out. Postgrad Med 2002;112:51-8, 82.
of once-daily metronidazole 1 percent cream to vehicle in
10. Bikowski JB, Goldman MP. Rosacea: where are we now?
patients with rosacea. Cutis 1998;61:44-7.
J Drugs Dermatol 2004;3:251-61.
34. Breneman D, Savin R, VandePol C, Vamvakias G, Levy S,
11. Del Rosso JQ. The use of topical azelaic acid 15% gel or
Leyden J. Double-blind, randomized, vehicle-controlled clini-
metronidazole 0.75% gel for the treatment of rosacea: eval-
cal trial of once-daily benzoyl peroxide/clindamycin topical gel
uation of a single-site comparative trial subset [abstract]. J Am
in the treatment of patients with moderate to severe rosacea.
Acad Dermatol 2004;50:P174.
Int J Dermatol 2004;43:381-7.
12. Ertl GA, Levine N, Kligman AM. A comparison of the efficacy
35. Carmichael AJ, Marks R, Graupe KA, Zaumseil RP. Topical
of topical tretinoin and low-dose oral isotretinoin in rosacea.
azelaic acid in the treatment of rosacea. J Dermatol Treat
Arch Dermatol 1994;130:319-24.
13. Espagne E, Guillaume JC, Archimbaud A, Baspeyras M, Boitier
36. Elewski BE, Fleischer AB Jr, Pariser DM. A comparison of 15%
F, Bussie re M, et al. Double-blind study versus excipient of
azelaic acid gel and 0.75% metronidazole gel in the topical
0.75% metronidazole gel in the treatment of rosacea [French].
treatment of papulopustular rosacea: results of a randomized
Ann Dermatol Venereol 1993;120:129-33.
trial. Arch Dermatol 2003;139:1444-50.
14. Odom RB. The subtypes of rosacea: implications for treatment.
37. Grosshans E, Michel C, Arcade B, Cribier B. Rilmenidine in
Cutis 2004;73:9-14.
rosacea: a double-blind study versus placebo [French]. Ann
15. Powell FC. The histopathology of rosacea: 'where's the beef?'
Dermatol Venereol 1997;124:687-91.
38. Jorizzo JL, Lebwohl M, Tobey RE. The efficacy of metronidazole
16. Rebora A. Rosacea. J Invest Dermatol 1987;88(Suppl):56s-60s.
1% cream once daily compared with metronidazole 1% cream
17. Shalita A, Leyden J. Mechanism-based selection of pharmaco-
twice daily and their vehicles in rosacea: a double-blind clinical
logic agents for rosacea. Cutis 2004;73:15-8.
trial. J Am Acad Dermatol 1998;39:502-4.
18. Signore RJ. A pilot study of 5 percent permethrin cream versus
39. Koc¸ak M, Yag˘li S, Vahapog˘lu G, Eks
xiog˘lu M. Permethrin 5%
0.75 percent metronidazole gel in acne rosacea. Cutis 1995;56:
cream versus metronidazole 0.75% gel for the treatment of
papulopustular rosacea: a randomized double-blind placebo-
19. Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin
controlled study. Dermatology 2002;205:265-70.
versus oral tetracycline. Int J Dermatol 1993;32:65-7.
40. Marks R, Ellis J. Comparative effectiveness of tetracycline
20. Wolf JE Jr. The role of topical metronidazole in the treatment
and ampicillin in rosacea: a controlled trial. Lancet 1971;2:
of rosacea. Cutis 2004;73:19-28.
21. Dahl MV, Katz HI, Krueger GG, Millikan LE, Odom RB, Parker F,
41. Montes LF, Cordero AA, Kriner J, Loder J, Flanagan AD. Topical
et al. Topical metronidazole maintains remissions of rosacea.
treatment of acne rosacea with benzoyl peroxide acetone gel.
Arch Dermatol 1998;134:679-83.
22. Maddin S. A comparison of topical azelaic acid 20% cream
42. Nielsen PG. Treatment of rosacea with 1% metronidazole
and topical metronidazole 0.75% cream in the treatment of
cream: a double-blind study. Br J Dermatol 1983;108:
patients with papulopustular rosacea. J Am Acad Dermatol
¨ zturkcan Sx, Ermertcan AT, Sahin MT, Afc¸ar FSx. Efficiency of
23. Nielsen PG. A double-blind study of 1% metronidazole cream
benzoyl peroxide-erythromycin gel in comparison with met-
versus systemic oxytetracycline therapy for rosacea. Br J
ronidazole gel in the treatment of acne rosacea. J Dermatol
24. Pelle MT, Crawford GH, James WD. Rosacea, II: therapy. J Am
44. Pye RJ, Burton JL. Treatment of rosacea by metronidazole.
Acad Dermatol 2004;51:499-512.
25. Curtin F, Elbourne D, Altman DG. Meta-analysis combining
45. Saihan EM, Burton JL. A double-blind trial of metronidazole
parallel and cross-over clinical trials, II: binary outcomes. Stat
versus oxytetracycline therapy for rosacea. Br J Dermatol
26. Bamford JT, Tilden RL, Blankush JL, Gangeness DE. Effect of
46. Sauder D, Miller R, Gratton D, Danby W, Griffiths C, Phillips S.
treatment of Helicobacter pylori infection on rosacea. Arch
The treatment of rosacea: the safety and efficacy of sodium
sulfacetamide 10% and sulfur 5% lotion (Novacet) is demon-
27. Barnhorst DA Jr, Foster JA, Chern KC, Meisler DM. The efficacy
strated in a double-blind study. J Dermatol Treat 1997;8:
of topical metronidazole in the treatment of ocular rosacea.
47. Sneddon IB. A clinical trial of tetracycline in rosacea. Br J
28. Bartholomew RS, Reid BJ, Cheesebrough MJ, MacDonald M,
Galloway NR. Oxytetracycline in the treatment of ocular
48. Tan JK, Girard C, Krol A, Murray HE, Papp KA, Poulin Y, et al.
rosacea: a double-blind trial. Br J Ophthalmol 1982;66:386-8.
Randomized placebo-controlled trial of metronidazole 1%
J AM ACAD DERMATOL
van Zuuren et al 115
VOLUME 56, NUMBER 1
cream with sunscreen SPF 15 in treatment of rosacea. J Cutan
71. Irvine C, Kumar P, Marks R. Acne and related disorderse
Med Surg 2002;6:529-34.
proceedings of an international symposium. London: Marks R,
49. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety
Plewig G, and Dunitz Ltd; 1988. pp. 301-5.
of azelaic acid (15%) gel as a new treatment for papulopus-
72. Koch R, Wilbrand G. Dark sulfonated shale oil versus placebo
tular rosacea: results from two vehicle-controlled, randomized
in the systemic treatment of rosacea. J Eur Acad Dermatol
phase III studies. J Am Acad Dermatol 2003;48:836-45.
50. Veien NK, Christiansen JV, Hjorth N, Schmidt H. Topical
73. Lebwohl M, Medansky R, Russo C, Plott R. The comparative
metronidazole in the treatment of rosacea. Cutis 1986;38:
efficacy of sodium sulfacetamide 10%/sulfur 5% (Sulfacet-R)
lotion and metronidazole 0.75% (metrogel) in the treatment of
51. Leyden JJ, Thiboutot D, Shalita A. Photographic review of
rosacea. J Geriatr Dermatol 1995;3:183-5.
results from a clinical study comparing benzoyl peroxide
74. Loo W, Ayyalaraju A, Chawla M, Finlay A, Coles E, Marks R.
5%/clindamycin 1% topical gel with vehicle in the treatment
Ivermectin cream in rosacea: comparison with metronidazole
of rosacea. Cutis 2004;73:11-7.
gel [abstract]. Br J Dermatol 2004;151:61.
52. Aitken G. Acne rosacea: efficacy of a metronidazole cream
75. Nasir MA. Treatment of rosacea with tetracycline and metro-
[French]. Presse Med 1983;12:1490-1.
nidazoleea comparative study. J Pak Med Assoc 1985;35:148-9.
53. Aizawa H, Niimura M. Oral spironolactone therapy in male
76. Nielsen PG. The relapse rate for rosacea after treatment with
patients with rosacea. J Dermatol 1992;19:293-7.
either oral tetracycline or metronidazole cream. Br J Dermatol
54. Aronson IK, Rumsfield JA, West DP, Alexander J, Fischer JH,
Paloucek FP. Evaluation of topical metronidazole gel in acne
77. Sanchez J, Somolino A, Webster G, Bradshaw M. Combined
rosacea. Drug Intell Clin Pharm 1987;21:346-51.
effect of doxycycline hyclate 20 mg tablets and metronidazole
55. Bernstein JE, Soltani K. Alcohol-induced rosacea flushing
0.75% topical lotion in the treatment of rosacea. J Am Acad
blocked by naloxone. Br J Dermatol 1982;107:59-61.
56. Bjerke JR. Rosacea: clinical features and treatment [Norwegian].
78. Schachter D, Schachter R, Long B, Shiffman N, Lester R, Miller
Tidsskr Nor Laegeforen 1989;109:2295-7.
S, et al. Comparison of metronidazole 1% cream versus oral
57. Blom I, Hornmark AM. Topical treatment with sulfur 10 per
tetracycline in patients with rosacea. Drug Invest 1991;3:
cent for rosacea. Acta Derm Venereol 1984;64:358-9.
58. Bukvic´-Mokos Z, Basta-Juzbasˇic´ A, Barasˇic´-Drusˇko V. Treatment
79. Seal DV, Wright P, Ficker L, Hagan K, Troski M, Menday P.
of Helicobacter pylori infection in the management rosacea.
Placebo controlled trial of fusidic acid gel and oxytetracycline
Acta Dermatovenerol Croat 1998;6:185-8.
for recurrent blepharitis and rosacea. Br J Ophthalmol 1995;
59. Cunliffe WJ, Dodman B, Binner JG. Clonidine and facial
flushing in rosacea. Br Med J 1977;1:105.
80. Torresani C, Pavesi A, Manara GC. Clarithromycin versus
60. Dahl MV, Jarratt M, Kaplan D, Tuley MR, Baker MD. Once-daily
doxycycline in the treatment of rosacea. Int J Dermatol 1997;
topical metronidazole cream formulations in the treatment
of the papules and pustules of rosacea. J Am Acad Dermatol
x S, U¨nver U¨. Treatment of rosacea with ketoconazole.
J Eur Acad Dermatol Venereol 1997;8:69-70.
61. De Witt S, Wilkin J. Double blind, parallel study of efficacy and
82. Van Landuyt H, Joubert-Lequain I, Humbert P, Lucas A,
safety of clindamycine lotion in the treatment of rosacea. Clin
Drobacheff C, Mercier M, et al. Treatment of rosacea: clonidine
Pharmacol Ther 1987;41:176.
(0.075 mg per day) versus placebo (initial results) [French].
62. Dreno B, Dubertret L, Naeyaert J, De La Brassine M, Marks R,
Ann Dermatol Venereol 1997;124:729.
Powell F, et al. Comparison of the clinical efficacy and safety of
83. Veien NK. Metronidazole cream for the local treatment of
metronidazole 0.75% cream with metronidazole 0.75% gel in
rosacea [Danish]. Ugeskr Laeger 1988;150:381-2.
the treatment of rosacea. P373. J Eur Acad Dermatol Venereol
84. Veraldi S, Scarabelli G, Rizzitelli G, Caputo R. Treatment of
rosacea fulminans with isotretinoin and topical aclometasone
63. Erdogan FG, Yurtsever P, Aksoy D, Eskioglu F. Efficacy of low-
dipropionate. Eur J Dermatol 1996;6:94-6.
dose isotretinoin in patients with treatment-resistant rosacea.
85. Wilkin JK. Effect of nadolol on flushing reactions in rosacea.
Arch Dermatol 1998;134:884-5.
J Am Acad Dermatol 1989;20:202-5.
64. Fernandez-Obregon A. Oral use of azithromycin for the
86. Monk B, Logan R, Cook J, White J, Mason R. Topical metro-
treatment of acne rosacea. Arch Dermatol 2004;140:489-90.
nidazole in the treatment of rosacea. J Dermatol Treat 1991;2:
65. Frigerio G, Mazzoni P, Ferrari V. Dimensions of the sample and
power of the clinical experimentation in a study of antibiotic
87. Verea Hernando M, Margusino Framin˜a´n L, Seco Vilarin˜o C,
therapy [Italian]. Boll Chim Farm 1969;108:506-14.
Feal Cortizas B, Cun˜a Este´vez B. Comparative study of topical
66. Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P. Efficacy of
erythromycin and topical metronidazole in the treatment of
doxycycline and tetracycline in ocular rosacea. Am J Oph-
rosacea [Spanish]. Farm Clin 1992;9:472-9.
88. Altman D, Schulz K, Moher D, Egger M, Davidoff F, Elbourne D,
67. Go MJ, Wuite J. Comparative study of triamcinolone acetonide
et al. The revised CONSORT statement for reporting random-
and hydrocortisone 17-butyrate in rosacea with special regard
ized trials: explanation and elaboration. Ann Intern Med
to the rebound phenomenon. Dermatologica 1976;152:239-46.
68. Goldsmith MF. New topical therapy for acne rosacea offers
89. de Groot A. De behandeling of rosacea [Treatment of rosacea].
conspicuous improvement, no systemic effects. JAMA 1989;
Gebu 1998;32:101-5.
90. Thiboutot DM. Acne and rosacea: new and emerging thera-
69. Guillet B, Rostain E, Powell F, Gimenez Camarasa C, Dahan E,
pies. Dermatol Clin 2000;18:63-71, viii.
Pie´rard G, et al. Metronidazole 0.75% gel and lotion are both
91. Wilkin JK. Rosacea. Int J Dermatol 1983;22:393-400.
effective in the treatment of rosacea. J Eur Acad Dermatol
92. Wilkin JK. Rosacea: pathophysiology and treatment. Arch
70. Hofer T. Continuous 'microdose' isotretinoin in adult recalci-
93. Bikowski JB. Subantimicrobial dose doxycycline for acne and
trant rosacea. Clin Exp Dermatol 2004;29:204-5.
rosacea. Skinmed 2003;2:234-45.
Source: http://www.oxforddaysurgery.com.au/Files/Articles/2009-03-23_17-44-45_Rosacea_Treatments.aspx
Presorted StandardU.S. POSTAGE PAID 330 West 42nd Street New York, NY 10036-6977 Your Key to the 1199SEIU Benefi t Funds Cost Savings Initiatives As you know, the 1199SEIU Benefi t Funds provide our health care workers – your patients - with some of the most comprehensive coverage in the nation. As health care costs escalate, we
¿RESPONSABILIDAD DEL ESTADO POR ACTOS LÍCITOS? Alvaro Quintanilla Pérez I. INTRODUCCIÓN Problemas de responsabilidad civil asociados a actividades públicas, impensables hace unas décadas en Chile, no sólo son hoy verosímiles, acusan un inquietante y progresivo aumento. Explican y favorecen esta situación nociones jurídicas en boga, como el personalismo ético,




