Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Klpr.ibcas.ac.cn


This article appeared in a journal published by Elsevier. The attached
copy is furnished to the author for internal non-commercial research
and education use, including for instruction at the authors institution
and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier's archiving and manuscript policies are
encouraged to visit:


Author's personal copy
Journal of Plant Physiology 170 (2013) 1434–1441
Contents lists available at ScienceDirect
Journal of Plant Physiology
Molecular Biology
The ER luminal binding protein (BiP) alleviates Cd2+-induced
programmed cell death through endoplasmic reticulum stress–cell
death signaling pathway in tobacco cells
Hua Xu a,b,1, Wenzhong Xu a,1, Hongmei Xi a,b, Wenwen Ma a, Zhenyan He a, Mi Ma a,∗
a Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, PR China
b University of Chinese Academy of Sciences, Beijing 100049, PR China
Cadmium (Cd) is very toxic to plant cells and Cd2+ stress induces programmed cell death (PCD) in Nicotiana
Received 8 March 2013
tabacum L. cv. bright yellow-2 (BY-2) cells. In plants, PCD can be regulated through the endoplasmic reti-
Received in revised form 22 May 2013
culum (ER) stress–cell death signaling pathway. However, the mechanism of Cd2+-induced PCD remains
Accepted 24 May 2013
unclear. In this study, we found that Cd2+ treatment induced ER stress in tobacco BY-2 cells. The expres-
Available online 16 July 2013
sion of two ER stress markers NtBLP4 and NtPDI and an unfolded protein response related transcription
factor NtbZIP60 were upregulated with Cd2+ stress. Meanwhile, the PCD triggered by prolonged Cd2+
stress could be relieved by two ER chemical chaperones, 4-phenylbutyric acid and tauroursodeoxycholic
acid. These results demonstrate that the ER stress–cell death signaling pathway participates in the medi-
Bright yellow-2 cells
ation of Cd2+-induced PCD. Furthermore, the ER chaperone AtBiP2 protein alleviated Cd2+-induced ER
stress and PCD in BY-2 cells based on the fact that heterologous expression of AtBiP2 in tobacco BY-2
Programmed cell death
cells reduced the expression of NtBLP4 and a PCD-related gene NtHsr203J under Cd2+ stress conditions. In
summary, these results suggest that the ER stress–cell death signaling pathway regulates Cd2+-induced
PCD in tobacco BY-2 cells, and that the AtBiP2 protein act as a negative regulator in this process.
2013 Elsevier GmbH. All rights reserved.
The ER is a very important organelle for protein synthesis, signal
transduction, and Ca homeostasis. ER stress is generally caused by
Cadmium (Cd), a toxic heavy metal, has been classified as a
an overload of unfolded proteins in the ER, which activates signal
human carcinogen. Thus Cd is a tremendous danger to plants and
transduction of unfolded protein response (UPR) (Ron and Walter,
animals (Bernard, 2008; DalCorso et al., 2010). The damage caused
2007; Zhang and Kaufman, 2004). Inositol-requiring enzyme-1
by Cd2+ in plant cells mainly includes reactive oxygen species
(IRE1), activating transcription factor 6 (ATF6), and PKR-like ER
(ROS) production, disturbances in photosynthesis, disorder of cal-
kinase (PERK) act as transducers in the UPR signaling pathway in
cium (Ca) signaling, and induction of programmed cell death (PCD)
mammals (Calfon et al., 2002; Harding et al., 1999, 2000; Sidrauski
(Chaffei et al., 2004; Ma et al., 2010; Rodriguez-Serrano et al., 2009;
and Walter, 1997; Yamamoto et al., 2007; Ye et al., 2000). Then,
Zhang et al., 2005). Cd2+-induced endoplasmic reticulum stress (ER
the ER luminal binding protein (BiP) and glucose-regulated pro-
stress) has been demonstrated in yeasts and mammals (Gardarin
tein 94 (GRP94) are induced by the UPR (Yoshida et al., 1998),
et al., 2010; Liu et al., 2006), but it has not been reported in plants
and they enhance ER protein-folding capacity and maintain stor-
age of ER Ca2+ (Lievremont et al., 1997). BiP is a central regulator of
the UPR and a classical marker of UPR activation. When misfolded
proteins accumulate in the ER, UPR is activated by BiP released
from those three ER trans-membrane sensors IRE1, ATF6, and PERK
Abbreviations: ATF6, activating transcription factor 6; BiP, luminal binding
(Bertolotti et al., 2000). Several UPR-related genes have been iden-
protein; BY-2, bright yellow-2; bZIP, basic-leucine zipper; Ca, calcium; Cd, cad-
mium; ER, endoplasmic reticulum; GRP94, glucose-regulated protein 94; Hac1,
tified in plants in recent years. Two orthologs of IRE1 in Arabidopsis
ATF/CREB homolog 1; IRE1, inositol-requiring enzyme-1; NRP, N-rich protein; PCD,
(AtIRE1a and AtIRE1b) are expressed in various organs and local-
programmed cell death; PDI, protein disulfide isomerase; PERK, PKR-like ER kinase;
ized to the perinuclear ER membrane (Koizumi et al., 2001). Moreno
UPR, unfolded protein response; Xbp1, X-box binding protein 1.
and Hayashi found that AtIRE1b modulates activation of some
Corresponding author at: Institute of Botany, Chinese Academy of Sciences,
basic-leucine zipper (bZIP) transcription factors (Hayashi et al.,
Xiangshan, Beijing 100093, PR China. Tel.: +86 10 62836255; fax: +86 10 62836690.
E-mail address: [email protected] (M. Ma).
2012; Moreno et al., 2012). Moreover, two groups of membrane-
1 These authors contributed equally to this work.
associated bZIP transcription factors play very important roles in
0176-1617/$ – see front matter
2013 Elsevier GmbH. All rights reserved.
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
transducing ER stress signals in plant cells. One group, including
promoter and the 3� un-translated end of the nopaline synthase
AtbZIP60 and OsbZIP50, is activated by IRE1-mediated mRNA splic-
gene present on pSN1301, resulting in pSN1301-BiP2. Using this
ing, which is similar to activation of ATF/CREB homolog 1 (Hac1)
same process, the AtBiP2 coding region was amplified by PCR with
in yeast and X-box binding protein 1 (Xbp1) in animals (Cox and
another two primer pairs (BiP2F2 and BiP2R2, BiP2F2 and BiP2R3),
Walter, 1996; Shen et al., 2001). The new proteins encoded by the
particularly the BiP2R3 primer containing the 6-His-tag codes
spliced mRNA are trans-located to the nucleus where they activate
(5�-GTGGTGGTGGTGGTGGTG-3�) before the termination codon
transcription of some ER function-related genes (Deng et al., 2011;
(Table S1). Those fragments were separately inserted into pER8
Hayashi et al., 2012; Iwata and Koizumi, 2005a; Nagashima et al.,
(Zuo et al., 2000), which is an estrogen-dependent expression
2011). Activation of the other group of proteins such as AtbZIP28
vector in plants, and were respectively named pER8-BiP2, and
and OsbZIP39 depends on regulated intra-membrane proteolysis
pER8-BiP2-6H. All plasmids obtained were identified by DNA
and resembles the ATF6 process in animals in which site-1 and
sequencing before they were transformed into Agrobacterium C58.
site-2 proteases are cleaved in response to ER stress (Liu et al.,
2007; Tajima et al., 2008; Takahashi et al., 2012). As one of the most
Cell culture conditions
important ER chaperone proteins, BiP alleviates ER stress in tobacco
(Alvim et al., 2001; Leborgne-Castel et al., 1999). However, PCD
Tobacco cell (Nicotiana tabacum L. cv bright yellow BY-2)
is the result of prolonged ER stress triggered by multiple stimuli,
(Nagata et al., 1992) lines were grown in Murashige and Skoog
although the UPR alleviates ER stress damage.
liquid medium supplemented with 256 mg/L KH2PO4, 1 mg/L thi-
The main apoptotic cell death signaling pathways demonstrated
amine, 100 mg/L myo-inositol, 0.2 mg/L 2,4-dichlorophenylacetic
in mammalian cells are dependent on plasma membrane recep-
acid, and 30 g/L sucrose. The cells were maintained on a gyratory
tors, mitochondria, and the ER (Earnshaw et al., 1999; Nakagawa
shaker (120 rpm) in a dark, temperature-controlled room at 25 ◦C
et al., 2000). ER-dependent apoptotic pathways have two differ-
and sub-cultured weekly with 2% inoculum.
ent modes. The cleavage of procaspase 12 depends on calpain or
caspase 7, which is released from ER membranes into the cyto-
BY-2 cell transformations
sol during prolonged ER stress and forms active caspase 12 to
activate downstream apoptotic factors (Rao et al., 2002; Yoneda
Each expression vector was transformed into the C58 Agrobac-
et al., 2001). BiP acts as an anti-apoptotic moderator and prevents
terium rifampicin-resistant strain by electroporation. Two- or
activation of procaspase-7 and procaspase-12 by binding them at
three-week-old tobacco BY-2 cell clusters were infused into a sus-
the ER membrane (Reddy et al., 2003). The mode of the other
pension of respective Agrobacterium stains with a BY-2 cell culture
ER-dependent pathway is based on the lack of Ca2+ homeostasis.
medium for 20 min. After the clusters grew on the normal medium
Whenever prolonged ER stress is triggered by depletion of Ca2+ in
for 2 days, they were washed three times, and then dropped into
the ER, mitochondria overloaded with Ca2+ release cytochrome c
water containing 400 g/mL cefotaxime for 45 min. Finally, the
into the cytosol, which causes activation of caspase-9 (Hacki et al.,
transformants were selected on tobacco BY-2 cell culture medium
2000). Similarly, PCD triggered by ER stress in plants has also been
containing 20 g/mL hygromycin B and 400 g/mL cefotaxime.
reported. In soybean cells, cyclopiazonic acid treatment induces ER
stress, increases cytoplasmic Ca2+, generates hydrogen peroxide,
Semi-quantitative reverse transcription (RT)-PCR
induces release of cytochrome c from mitochondria, and activates
caspase-like proteases causing PCD (Zuppini et al., 2004). In addi-
RNA isolation and the generation of cDNA were performed as
tion, tobacco BY-2 cells, treated with a UPR inducer tunicamycin,
described above. The primers for each target gene are shown in
may also lead to PCD along with up-regulation of NtHsr203J, a
Table S2, which also includes the annealing temperature and PCR
marker of PCD (Iwata and Koizumi, 2005b).
cycles. The amplified fragments of the target genes, such as NtBLP4
As studies on UPR and PCD become more in-depth, these
(Accession No: X60057), NtHsr203J (Accession No: AF212184), and
signaling pathways have been revealed in plants as well as ani-
NtEF1-˛ (Accession No: D63396), were segregated by agarose gel
mals. BiP plays a very important role in stress-induced plant cell
electrophoresis under 6 V/cm pressure for 10 min. Gel Doc-ItTM 300
death, such as ER stress, osmotic stress, and water stress (Alvim
(Ultra-Violet Products Ltd., Cambridge, UK) was used to scan the
et al., 2001; Leborgne-Castel et al., 1999; Reis et al., 2011). More-
over, BiP over-expression enhances tolerance to drought stress and
delays leaf senescence induced by drought in soybean (Valente
Real-time quantitative RT-PCR
et al., 2009). The primary focus of the present study was on the
pathway through which Cd2+ triggers PCD in plant cells and the
RNA isolation and the generation of cDNA were performed as
mode of BiP action during this process.
described above. For real-time quantitative RT-PCR, the final primer
concentration was 0.2 M in a total 20 L reaction volume, and
all primers were listed in Table S3. Real-time quantitative RT-PCR
Materials and methods
was performed with the StepOneTM Real-Time PCR System (Applied
Biosystems, CA, USA) and prepared with the SYBR Premix Ex TaqTM
Plant expression vector construction
GC Kit (DRR071A; TaKaRa Biotechnology Ltd, Dalian, China). The
efficiencies of all cDNAs amplification were between 90 and 100%.
The reagent of TRIzol (15596026; Life Technologies Corp.,
Each RNA sample was assayed in triplicate. Expression levels of
Carlsbad, CA, USA) was used to extract Arabidopsis total RNA,
NtBLP4, NtPDI (Accession No: Y11209), and NtbZIP60 (Accession
and full length cDNA was produced using the SuperScript® II
No: AB281271) were calculated relative to the standard sample for
Reverse Transcriptase kit (18064-014; Life Technologies Corp.).
calibration and then normalized to the NtEF1-˛ gene.
The BiP coding the isoform AtBiP2 (at5g42020) region was ampli-
fied by polymerase chain reaction (PCR) with the BiP2F1 and
Protein gel blot hybridization
BiP2R1 primers (Table S1) to create two KpnI (E.C.3.1.23.26)
sites located in front of the translation initiation codon and after
Each treated cell line was collected and quickly frozen in liquid
the stop codon, respectively. After KpnI digestion, this fragment
nitrogen. Frozen samples were ground and transferred to tubes.
was inserted between the cauliflower mosaic virus (CaMV) 35S
Protein extraction buffer (100 mM HEPES, pH 7.5, 5 mM EDTA, pH
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
8.0, 5 mM EGTA, pH 7.0, 50 mM glycerol-p, 10 mM DTT, 5% glyc-
erol, 1 mM PMSF) was added to each tube, shaken well, kept in
ice-water for 40 min, and then centrifuged at 13,000 rpm for 40 min.
Cell lysates containing proteins were transferred to new tubes. Pro-
tein concentrations were determined using the CWBIO Bradford
assay reagent.
Proteins in 12% sodium dodecyl sulfate–polyacrylamide gels
were transferred to polyvinylidene fluoride membranes and
blocked with Tris-buffered saline (TBST: 50 mM Tris, pH 7.5,
150 mM NaCl, and 0.05% Tween 20) containing 5% nonfat dry milk
at 4 ◦C overnight. The membranes were then incubated in TBST con-
taining 0.5% nonfat dry milk BIP goat polyclonal antibody (1:3000;
aC-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a dilu-
tion of 1:4000 for mouse tubulin and His-tag monoclonal antibody
(AT819 and AH367; Beyotime Institute of Biotechnology, Shanghai,
China). After 1 h, three 10-min washes were performed with TBST,
and then the membranes were incubated at 1:4000 in horseradish
peroxidase-conjugated anti-goat or anti-mouse secondary anti-
body (A0181 or A0216; Beyotime Institute of Biotechnology) in
TBST containing 0.5% nonfat dry milk for 45 min. Three 10-min
washes were performed in TBST, the antigen–antibody complexes
were detected using an Enhanced Chemiluminescence-Plus Detec-
tion kit (P0018; Beyotime Institute of Biotechnology), and the
images were recorded on film.
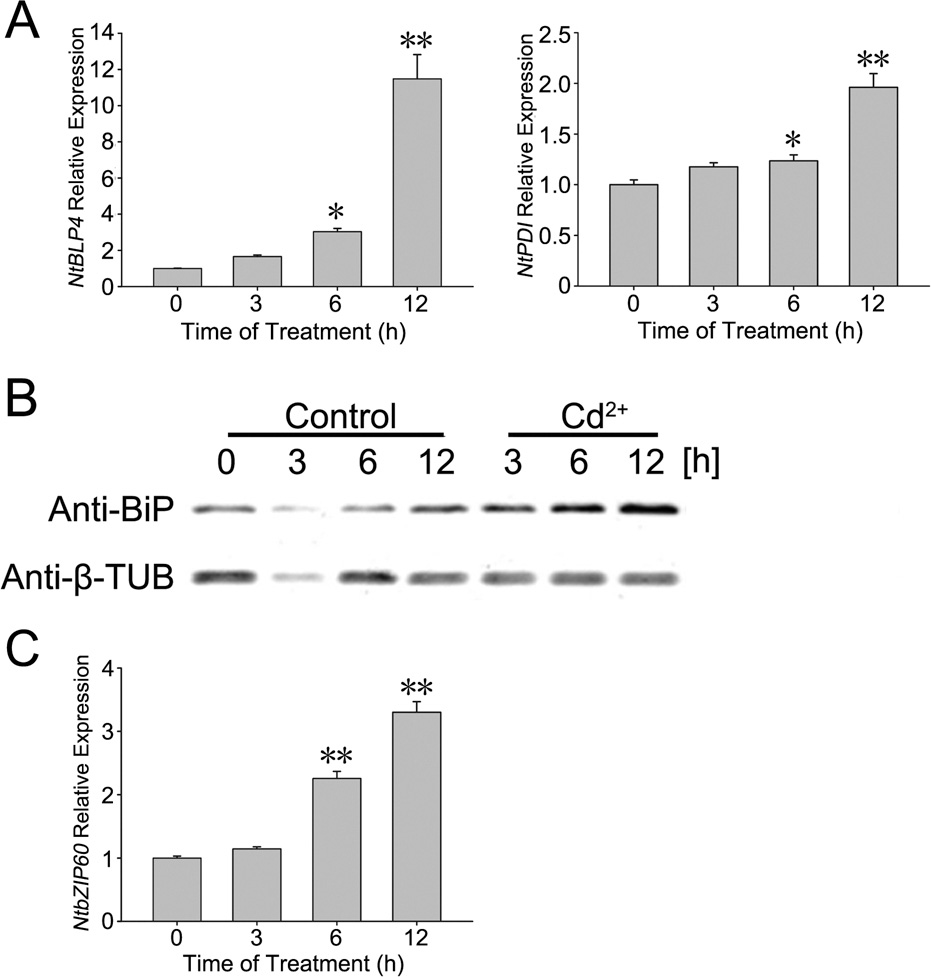
Fig. 1. (A) The amounts of NtBLP4 and NtPDI transcripts were detected by real-time
quantitative RT-PCR at each time point with the treatment of 100 M CdCl2. NtBLP4
and NtPDI expression were separately normalized to NtEF1-˛. The values at 0 h were
further normalized to 1. Error bars represent SE for three independent replicates.
The significant differences between 0 h and the other time points were marked by
PCD using Hoechst 33342 and propidium iodide (PI) co-staining
asterisks (*: p < 0.05; **: p < 0.01). (B) BLP protein was inducible expressed in BY-2
was detected based on a method described previously (Ormerod
cells treated with CdCl2. Cells were harvested at 3, 6 and 12 h after treatment with
et al., 1993; Yuan et al., 2002). The Apoptosis and Necrosis Assay
100 M CdCl2 (Cd2+) or not (control). Total protein was separated by SDS-PAGE
and analyzed by protein gel blot hybridization using anti-BiP and anti--tubulin
kit (C1056; Beyotime Institute of Biotechnology) was used as a
antibodies. The amount of -tubulin protein was used as a loading control. (C) The
method to detect cell death. Samples were collected from each
amount of NtbZIP60 transcripts was detected by real-time quantitative RT-PCR at
treatment, washed twice with BY-2 cell culture medium, incu-
each time point with the treatment of 100 M CdCl2. The data normalization and
bated in buffer-containing Hoechst 33342 and PI at 4 ◦C for 30 min,
significant analysis were the same as above.
and then washed twice with phosphate-buffered saline (137 mM
NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4). All
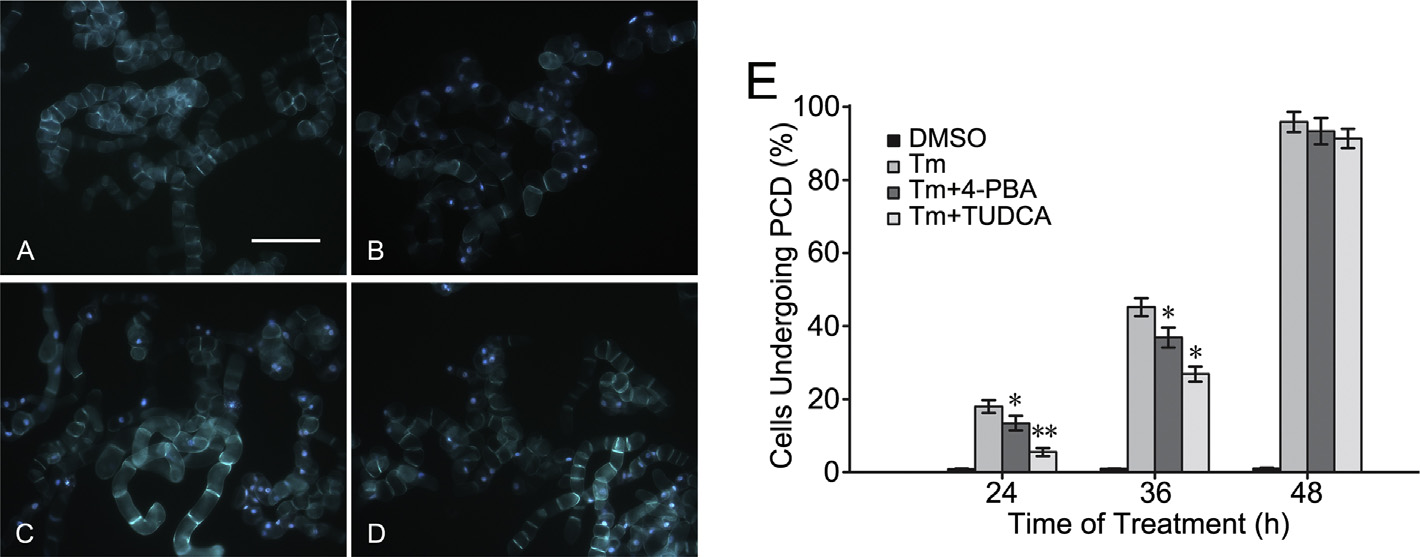
Cd2+ induces PCD in BY-2 tobacco cells
stained cells were observed under a fluorescence microscope (Zeiss
Axioskop 40; Carl Zeiss, Oberkochen, Germany) with a 353–377-
BY-2 cells treated with 150 M CdCl2 (Fig. 2C) or 0.2 mg/L tuni-
nm excitation filter. The percentages of cells undergoing PCD were
camycin (Fig. 2D) were observed to undergo PCD, whereas bright
calculated by randomly observing >750 cells in each treatment.
blue fluorescence did not appear in BY-2 cells under normal cul-
ture (Fig. 2A and B). Furthermore, the number of cells undergoing
Statistical analysis
PCD increased continuously from 24 h to 48 h under treatment with
150 M CdCl2 or 0.2 mg/L tunicamycin (Fig. 2E). The percentages of
SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical
BY-2 cells undergoing PCD exceeded 80% when they were treated
analyses, and p values were calculated using one-way analysis of
with 150 M CdCl
0.2 mg/L tunicamycin for 48 h.
variance. A p < 0.05 was considered significant.
ER chemical chaperones relieve the PCD induced by Cd2+
Two ER chemical chaperones, 4-phenylbutyric acid and tau-
Cd2+ leads to ER stress in BY-2 tobacco cells
roursodeoxycholic acid, which have the ability to relieve ER
stress (Oezcan et al., 2006), were used to investigate whether
The UPR pathway is activated during ER stress, and then induces
Cd2+ induces PCD by triggering ER stress. In this experiment,
the expression of ER chaperone proteins, such as BiP and pro-
both 4-phenylbutyric acid and tauroursodeoxycholic acid mod-
tein disulfide isomerase (PDI). Consequently, the up-regulations
estly reduced the number of tobacco cells treated with 0.2 mg/L
of BiP and PDI are considered as the occurrence of ER stress in
tunicamycin undergoing PCD (Fig. 3). The PCD percentages of cells
cells. NtBLP4 and NtPDI were homologous genes of BiP and PDI
pre-treated with 500 M 4-phenylbutyric acid or 100 M tau-
in N. tabacum, and the amounts of NtBLP4 and NtPDI transcripts
roursodeoxycholic acid for 12 h respectively decreased to 4.56%
were examined by real-time quantitative RT-PCR (Fig. 1A). Both
(13.41 ± 2.03%) and 12.45% (5.52 ± 1.10%) at 24 h, compared with
NtBLP4 and NtPDI transcripts gradually increased with 100 M
17.97 ± 1.77% of the cells with 0.2 mg/L tunicamycin treatment.
CdCl2 treatment. Moreover, BLP proteins also accumulated grad-
A similar situation was observed after 36 h of treatment, and the
ually following the 100 M CdCl2 treatment (Fig. 1B). Additionally,
percentages of cells undergoing PCD were 45.16 ± 2.48% (tuni-
the NtbZIP60 was considered as the UPR-related transcription fac-
camycin alone), 36.88 ± 2.76% (pre-treated with 4-phenylbutyric
tor in N. tabacum, and its transcript was induced to increase with
acid) and 26.87 ± 2.05% (pre-treated with tauroursodeoxycholic
100 M CdCl2 treatment (Fig. 1C). These results indicate that Cd2+-
acid), respectively, at 36 h. Almost all tobacco BY-2 cell under-
induced ER stress in BY-2 tobacco cells.
went PCD after being treated for 48 h. These results indicate

Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
Fig. 2. The cells with bright blue fluorescence in their nucleus were considered as undergoing programmed cell death (PCD), whereas bright red fluorescence in cells indicated
that cells had been already dead. However, no bright blue and red fluorescence could be detected in the cells alive. The images exhibited the fluorescence of BY-2 cells treated
for 36 h under the conditions of normal culture (control) (A), dimethyl sulfoxide (DMSO) (B), 150 M CdCl2 (Cd2+) (C) or 0.2 mg/L tunicamycin (Tm) (D). Bar 200 m. (E) The
statistical analysis of cells undergoing PCD was based on the examinations of more than 750 cells in each experiment. Each value and its standard error was calculated from
three independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3. The images exhibited the fluorescence of BY-2 cells treated for 36 h under the conditions of dimethyl sulfoxide (DMSO) (A), 0.2 mg/L tunicamycin (Tm) (B), 0.2 mg/L
tunicamycin (Tm) besides having been pretreated for 12 h with 500 M 4-phenylbutyric acid (4-PBA) (C), or 100 M tauroursodeoxycholic acid (TUDCA) (D). Bar 200 m.
(E) At three different time points, the ratios of each treatment cell lines undergoing programmed cell death (PCD) were shown and the calculation of data was operated just
as above. The significant differences between pretreated cells and non-pretreated cells were marked by asterisks (*: p < 0.05; **: p < 0.01).
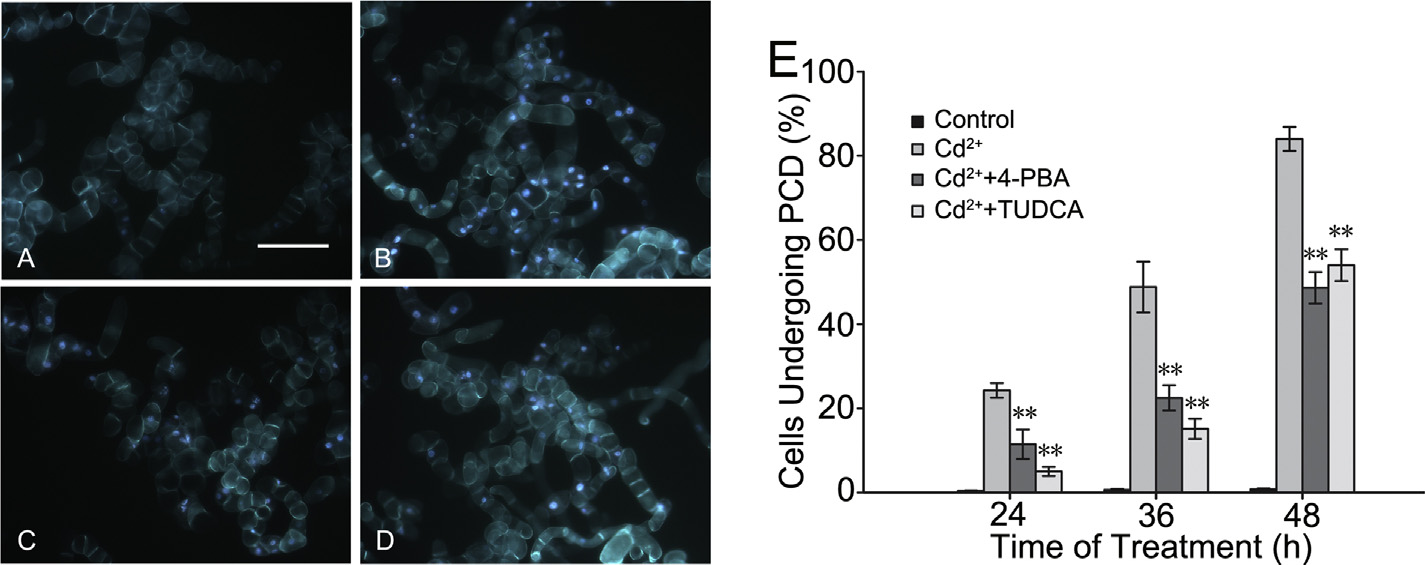
that 4-phenylbutyric acid and tauroursodeoxycholic acid partially
48 h. However, the number of pretreated cells undergoing PCD was
relieved ER stress-induced PCD in tobacco BY-2 cells. BY-2 cells
less than half of the non-pretreated cells at 36 h, and it was slightly
treated with 150 M CdCl2 instead of 0.2 mg/L tunicamycin were
more than a half of the non-pretreated cells at 48 h. As a result, ER
used to observe PCD (Fig. 4). The percentages of cells undergoing
chemical chaperones had a great effect on relieving PCD in BY-2
PCD decreased by 12.81% (11.47 ± 3.48%) and 19.26% (5.02 ± 1.10%)
cells induced by Cd2+.
in the 4-phenylbutyric acid and tauroursodeoxycholic acid pre-
treated groups compared with that in the non-pretreated group
BiP is capable of downregulating Cd2+-induced ER stress
(24.28 ± 1.74%) when 150 M CdCl2 was added to the culture
medium for 24 h. Then, the number of cells undergoing PCD
Constitutively expressing cell lines BiP2-Ox were used in which
increased in both non-pretreated and pretreated groups at 36 and
AtBiP2 was driven by the CaMV 35S promoter to identify the role
Fig. 4. The images exhibited the fluorescence of BY-2 cells treated for 36 h under the conditions of normal culture (control) (A), 150 M CdCl2 (Cd2+) (B), 150 M CdCl2 (Cd2+)
besides having been pretreated for 12 h with 500 M 4-phenylbutyric acid (4-PBA) (C), or 100 M tauroursodeoxycholic acid (TUDCA) (D). Bar 200 m. (E) At three different
time points, the conditions of each treatment cell lines undergoing programmed cell death (PCD) were shown, and the method of statistical analysis was the same as before.
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
Fig. 6. The transformed cells named BiP2-In-6H were able to express 6-His tagged
AtBiP2 protein under the induction of 17--estradiol. The 6-His tag was located
just in front of C-terminal four peptides (HDEL) in AtBiP2 protein. (A) The BiP2-In-
6H cells were divided into an untreated group named control and a 150 M CdCl2
treated group named Cd2+. Both groups of cells were collected at each time point,
and then their proteins were prepared for protein gel blot hybridization. Anti-BiP
antibody could detect both NtBLP and AtBiP2 protein, and Anti-His antibody was
used to immunize 6-His tagged AtBiP2 alone. The amount of -tubulin protein was
Fig. 5. Two transgenic cell lines were used in the experiment. The cell line trans-
used as a loading control. (B) All procedures were the same as the description in (A)
formed by an empty plant expression vector SN1301 was named EV, and the other
except that transgenic cells had been pretreated with 5 M 17--estradiol for 2 h.
cell line named BiP2-Ox was able to express AtBiP2 driven by a CaMV 35S promoter.
(A) The expression of NtBLP4 was detected by semi-quantitative RT-PCR at each time
point with treatment of 150 M CdCl2 in both cell lines. The detection of AtBiP2 tran-
script was used to distinguish two cell lines. The amount of NtEF1-˛ transcript was
Cd2+-induced ER stress seemed somewhat impaired in the BiP2-
considered as an internal control. (B) NtBLP4 transcripts were detected by real-time
Ox cell lines. However, constitutively expressing AtBiP2 partially
quantitative RT-PCR at each time point with the treatment of 150 M CdCl2. NtBLP4
repressed the transcription of NtBLP4 even under normal culture
expression was normalized to NtEF1-˛. The values in EV cell lines at 0 h were fur-
condition (Fig. 5A and B). To eliminate such impact, a chemically
ther normalized to 1. Error bars represent SE for three independent replicates. The
inducible expression vector pER8 was introduced. The expression
significant differences between two cell lines were marked by asterisks (*: p < 0.05;
**: p < 0.01). (C) The quantity of BiP protein in both transgenic cell lines treated with
of AtBiP2 in tobacco cells was induced by the chemical inducer (17-
150 M CdCl2 was measured by protein gel blot hybridization. Anti-BiP antibody
-estradiol). The AtBiP2 protein was 6-His tagged at its C terminus
was used to detect NtBLP alone in EV cell line and totality of AtBiP2 and NtBLP pro-
to detect the amount of AtBiP2 protein. After being treated with
tein in BiP2-Ox cell line. The same procedure was used just as the description in
150 M CdCl AtBiP2 and NtBLP were detected with anti-BiP and
AtBiP2 was also detected with anti-His antibodies. As shown in
Fig. 6A, NtBLP expression increased gradually under the 150 M
CdCl2 treatment, and 6-His tagged AtBiP2 was not expressed with-
out 17--estradiol. This result suggests that ER stress occurred
of AtBiP2 in Cd2+-induced ER stress, while the control cell lines
acutely without 6-His tagged AtBiP2 expression in cells cultured
transformed empty vector were named EV cell lines. Furthermore,
with 150 M CdCl 6-His tagged AtBiP2 protein accumulated along
NtBLP4 is considered as an ER stress marker in N. tabacum, and
with 17--estradiol induction in the control group (Fig. 6B) and
the detection of its expressing is used to report the occurrence
NtBLP expression did not increase. Then, 6-His tagged AtBiP2 in the
of ER stress. The amounts of AtBiP2 and NtBLP4 transcripts in the
Cd2+ group (Fig. 6B) was almost the same as that the former group.
transgenic cell lines were detected by RT-PCR (Fig. 5A). The amount
However, levels of the AtBiP2 and NtBLP proteins were no obvi-
of AtBiP2 transcript driven by the CaMV 35S promoter was stable
ous differences between the control and Cd2+ groups, although the
with or without treatment with 150 M CdCl
2 (Fig. 5A). However,
NtBLP protein should be induced to increase under CdCl
the amount of NtBLP4 transcript in the BiP2-Ox cell lines was dis-
(Fig. 6B). Based on these results, the 6-His tagged AtBiP2 protein
tinctly lower than that in the EV cell lines at each time point (except
was demonstrated to be functional for suppressing Cd2+-induced
36 h). Moreover, the data of real-time quantitative RT-PCR further
confirmed the results of RT-PCR (Fig. 5B). At each time point, the
differences of NtBLP4 transcript between two cell lines were signif-
icant (p < 0.01). Additionally, anti-BiP antibody was used to detect
BiP is efficient at alleviating PCD triggered by Cd2+
the target protein in two cell lines. The blots detected by anti-BiP
antibody indicated the amount of NtBLP protein in the EV cell line;
The constitutively expressing cell lines BiP2-Ox were used to
while the blots detected by anti-BiP antibody contained both AtBiP2
examine the function of BiP for alleviating PCD induced by Cd2+
and NtBLP protein in the BiP2-Ox line, since anti-BiP antibody can-
stress. The percentage (16.63 ± 1.95%) of cells undergoing PCD
not distinguish the NtBLP from AtBiP2. As shown in Fig. 5C, the
in EV lines was more than twice higher than the BiP2-Ox lines
intensities of the blots detected by anti-BiP antibody had no obvi-
(7.49 ± 1.65%) when BY-2 cells were treated with 150 M CdCl2
ous difference between two cell lines through the entire treatment
for 24 h. Furthermore, the number of cells undergoing PCD contin-
(Fig. 5C). We therefore inferred that the amount of NtBLP protein in
ued to increase and exceeded one-third of all cells in the EV group,
the BiP2-Ox line was less than that in the EV line at each time point.
whereas the percentage in BiP2-Ox lines was only 21.21 ± 1.48%
Based on the transcriptional and translational detections, NtBLP
at 36 h. About 90% of cells underwent PCD in EV cell lines after a
expression was less induced in transformed AtBiP2 cells under the
48 h treatment, and the percentage of BiP2-Ox lines was approxi-
150 M CdCl2 treatment. In other words, the overexpressing cells
mately 10% lower (Fig. 7A). As shown in Fig. 7B, Cd2+ was capable of
appeared to have less capacity to induce ER stress in response to
inducing NtHsr203J transcription, which was considered as a PCD-
related gene in BY-2 cells. In addition, the amount of NtHsr203J
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
Fig. 7. (A) Cells were treated with 150 M CdCl2 for 24, 36, or 48 h, and then
observed through a fluorescence microscope. The percentages of cells undergoing
programmed cell death (PCD) in EV and BiP2-Ox cell lines at each time point were
shown. The statistical analysis of cells undergoing PCD was based on the examina-
tions of more than 750 cells in each experiment. Each value and its standard error
were calculated from three independent experiments. The significant differences
between two cell lines were marked by asterisks (*: p < 0.05; **: p < 0.01). (B) Both
kinds of transgenic cells were treated with 150 M CdCl2. The changes of NtHsr203J
expression were detected by semi-quantitative RT-PCR at five different time points.
The amount of NtEF1-˛ transcript was considered as an internal control.
transcript increased gradually in the two kinds of transformed
cell lines under the 150 M CdCl2 treatment. However, clear dif-
ferences were observed between the EV and BiP2-Ox lines. The
amount of NtHsr203J transcript increased distinctly at 12 h in EV
cell lines, whereas the increase appeared at 24 h in the transformed
AtBiP2 cells. Thus, the BiP2-Ox cell lines had more capacity to resist
Fig. 8. (A) BY-2 cells named BiP2-In were able to express AtBiP2 protein under the
Cd2+-induced PCD than that of the EV cell lines.
induction of 17--estradiol. The cells without pre-treatment were signed as BiP2-In.
The cells pre-treated with 5 M 17--estradiol for 2 h were signed as BiP2-In (5) and
Next, the inducible expressing AtBiP2 cell line BiP2-In was used.
20 M 17--estradiol as BiP2-In (20). The chart exhibited the percentages of BiP2-
After being treated with 150 M CdCl2 for 36 h, the percentage of
In cells undergoing programmed cell death (PCD) with treatment of 150 M CdCl2
cells undergoing PCD was 47.14 ± 2.64% in the non-17--estradiol
at 36 h. The statistical analysis of cells undergoing PCD was based on the exam-
treated group, whereas it decreased about 10% (37.24 ± 3.75%)
inations of more than 750 cells in each experiment. Each value and its standard
in the 5 M 17--estradiol-treated group and by more than
error were calculated from three independent experiments. The significant differ-
ences between pretreated cells and non-pretreated cells were marked by asterisks
half (15.78 ± 4.71%) in the 20 M 17--estradiol-treated group
(*: p < 0.05; **: p < 0.01). (B) In the same way, the percentages of cells undergoing
(Fig. 8A). These results suggest that inducible expression of AtBiP2
PCD in BiP2-In-6H were shown in (B). The same statistical analysis was used just as
enabled BY-2 cells have higher resistance to PCD triggered by CdCl2.
Furthermore, the more chemical inducer added, the stronger resis-
tance tobacco cells got. The same experiment was carried out
et al., 2012; Shen et al., 2001). Tateda et al. cloned a UPR-related
to examine whether the 6-His tagged AtBiP2 protein remained
transcription factor NtbZIP60, which was the AtbZIP60 homolog in
functional in BiP2-In-6H cell lines. The results showed that the
N. tabacum. Although the NtbZIP60 mRNA splicing just as AtbZIP60
non-17--estradiol-treated group was 41.17 ± 3.03%, the 5 M
has not been verified, NtbZIP60 transcript was induced by ER stress
17--estradiol-treated group was 23.63 ± 2.49%, and the 20 M
inducer such as tunicamycin and dithiothreitol (Deng et al., 2011;
17--estradiol-treated group was 13.20 ± 2.69% (Fig. 8B), indicat-
Tateda et al., 2008). In this paper, CdCl
also triggered the
ing that the tagged protein was effective in reducing the number
accumulation of NtbZIP60 mRNA, and two ER stress marker genes
of cells undergoing PCD and that the 6-His tag had no deleterious
NtBLP4 and NtPDI were upregulated by Cd2+ stress as well (Fig. 1).
effect on AtBiP2 function. Thus, BiP had a good effect on alleviating
Together with those results, it indicated that Cd2+ stress led to ER
PCD triggered by Cd2+ stress.
stress in BY-2 tobacco cells. Based on current works, however, it was
still uncertain that Cd2+ stress directly activated ER stress or not.
Prolonged ER stress determines the occurrence of PCD in both
animals and plants. In this study, the existence of the ER stress–PCD
Several studies reported that Cd2+ leads to ER stress in yeast and
pathway was supported by new evidence. Tunicamycin, an inducer
mammals (Gardarin et al., 2010; Liu et al., 2006; Yokouchi et al.,
of ER stress, led to PCD in BY-2 tobacco cells (Fig. 2). Moreover, PCD
2007, 2008). BiP, a major marker of ER stress in mammalian cells,
triggered by tunicamycin was markedly attenuated when BY-2 cells
is up-regulated following Cd2+ treatment (Liu et al., 2006). Three
were pretreated with 4-phenylbutyric acid and tauroursodeoxy-
main sensors of the UPR such as PERK, ATF6, and IRE1 are acti-
cholic acid, which are two types of ER chemical chaperones (Fig. 3).
vated by CdCl2 (Yokouchi et al., 2007). AtbZIP60 is the first reported
Consequently, BY-2 cells responded with ER stress–cell death
UPR-related transcription factor in Arabidopsis, and the amount of
signaling. Our examination of Cd2+-induced PCD in BY-2 cells was
its transcript was in response to ER stress (Iwata and Koizumi,
the same as that of some other investigation, such as those using
2005a). Furthermore, AtbZIP60 was activated by IRE1-mediated
DNA laddering and terminal deoxynucleotidyl transferase dUTP
mRNA splicing, which was similar to activation of Hac1 in yeast and
nick end labeling assays (Kuthanova et al., 2008; Ma et al., 2010). As
Xbp1 in animals (Cox and Walter, 1996; Deng et al., 2011; Moreno
expected, if either 4-phenylbutyric acid or tauroursodeoxycholic
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
acid were added to the culture medium, the number of cells under-
After analyzing the BY-2 cell phenotype following Cd2+ treat-
going PCD clearly decreased (Fig. 4). However, the 4-phenylbutyric
ment, we proposed that the over-expressing cell lines had greater
acid and tauroursodeoxycholic acid mechanisms were different to
resistance to PCD than that of the control (Fig. 7A). Furthermore, the
maintain ER function. Tauroursodeoxycholic acid treatment led to
AtBiP2 protein inhibited PCD, and this ability was positively corre-
increased stability of the mutant protein, whereas 4-phenylbutyric
lated with AtBiP2 protein quantity (Fig. 8). The results of NtBLP
acid facilitated degradation of misfolded proteins (de Almeida
expression indicated that exogenous BiP proteins were able to
et al., 2007). Based on these results, we predicted that Cd2+-
inhibit the UPR activation in constitutively expressing and instantly
triggered ER stress was probably the result of an accumulation
expressing cell lines. So, it is reasonable to assume that the Cd2+-
of misfolded proteins in the ER, and 4-phenylbutyric acid and
induced ER stress–cell death pathway depended on UPR signaling
tauroursodeoxycholic acid treatment delayed PCD. In other words,
together with the 4-phenylbutyric acid and tauroursodeoxycholic
the UPR-dependent signaling of ER stress-induced cell death
acid results. In addition, the amount of NtHsr203J transcript as a
clearly participated in the regulation of Cd2+-induced PCD.
PCD-related marker gene in tobacco increased more quickly in
The BiP is one of the most important chaperone proteins
the control cell lines compared with that in the over-expressing
that assist in maintaining physiological function of the ER. The
cell lines. However, the PCD-related NtHsr203J may be induced
BiP protein not only protects peptide folding and preserves Ca2+
by ER stress through a pathway independent of the UPR (Iwata
homeostasis but also acts as a modulator of the UPR and ER
and Koizumi, 2005b). Therefore, determining whether the Cd2+-
stress–cell death signaling pathways. The amount of NtBLP4 tran-
induced ER stress–cell death occurs through another signaling
script was less than that in control cells under CdCl2 treatment
pathway requires further research. Some researchers have found
when AtBiP2 was constitutively expressed in BY-2 cells (Fig. 5A and
that signaling of plant-specific N-rich protein (NRP) mediates ER
B). Therefore, the exogenous BiP protein reduced ER damage caused
stress- and osmotic stress-induced cell death pathways, which
by Cd2+ stress. The reason might be that the exogenous BiP pro-
diverged from a branch of the UPR (Costa et al., 2008). Furthermore,
tein partly took the place of the endogenous BLP protein, which
BiP acts as a negative regulator of the NRP-mediated cell death
would maintain the UPR in a less activated state. Above all, this
response (Reis et al., 2011). The senescence-associated marker
result indicated that exogenous BiP protein expression regulated
gene GmCystP, DNA fragmentation, and stress-triggered cell death
activation of the UPR to alleviate Cd2+-triggered ER stress. Addi-
were all less induced in over-expressing BiP lines. These discov-
tionally, the amount of NtBLP4 transcript in the AtBiP2 expressed
eries have provided a new direction to research the action of BiP
cell lines was clearly less than that in the control even under
in Cd2+-induced PCD. The BiP protein relieved Cd2+-induced ER
normal growth conditions (Fig. 5A and B). This interesting phe-
stress, which was reasonable considering that the anti-PCD action
nomenon was quite similar with a previous study showing that
on Cd2+ stress originated from alleviating ER stress by the BiP pro-
BiP over-expression leads to down-regulation of endogenous BiP
tein. Nevertheless, the BiP protein also acted as an ER apoptotic
mRNA and a reduction in the UPR in tobacco (Leborgne-Castel
modulator by inhibiting activation of the BIK pro-apoptotic fac-
et al., 1999). Thus, a transcriptional regulatory mechanism might
tor, which is a member of the Bcl-2 family in mammals. Moreover,
maintain the quantity of BiP in the ER. However, feedback regu-
some researchers have reported that several homologs in the Bcl-
lation at the translational level in HeLa cells was more efficient
2-associated athanogene (BAG) family affect ER stress and PCD in
at controlling BiP expression (Gulow et al., 2002). The efficiency
Arabidopsis, particularly AtBAG7 interacting with AtBiP2 in the UPR
of translational regulation represented rapid control of the total
(Williams et al., 2010). Consequently, it is quite probable that the
BiP balance when it was disturbed by transient expression of
BiP protein might use this mechanism as well, although a homolo-
exogenous BiP protein. The mechanism of transcriptional regula-
gous pro-apoptotic factor has not been discovered in plants.
tion seemed to be a long-term adjustment compared with that
In summary, Cd2+ treatment induced ER stress and activated
of translational control. However, both transcriptional and trans-
the UPR. Cd2+-induced PCD was dependent on the ER stress–cell
lational regulation lost control when ER stress was triggered by
death signaling pathway in BY-2 tobacco cells. Furthermore, the BiP
a stimulator. As shown in Fig. 5C, total BiP expression accumu-
protein as a Ca2+ binding chaperone and regulator of both the UPR
lated gradually in tobacco BY-2 cells treated with CdCl2. Although
and ER stress–cell death signaling played an extremely important
constitutive expression of AtBiP2 led to transcriptional regulation
role in maintenance of the ER. Thus, BiP alleviated ER stress and
of NtBLP4 expression in tobacco BY-2 cells, the expressed AtBiP2
delayed the appearance of Cd2+-induced PCD.
cell lines actually had stronger resistance to ER stress than that
of control cells before CdCl2 treatment. An estrogen-dependent
inducible expression vector pER8 (Zuo et al., 2000) was introduced
to eliminate the difference between the two cell lines, which also
The authors thank Prof. Xuejun Hua (Key Laboratory of Plant
enabled us to control the timing and quantity of AtBiP2 expres-
Resources, Institute of Botany Chinese Academy of Sciences, Beijing
sion. It was useful to help examine whether BiP directly alleviated
100093, PR China) for providing the tobacco BY-2 cells. Prof. Nam-
Cd2+-induced ER stress. AtBiP2 reduced the quantity of endoge-
Hai Chua (Laboratory of Plant Molecular Biology, The Rockefeller
nous BLP induced by Cd2+ stress when comparing AtBiP2 and
University, New York, New York 10065) gave us the expression vec-
NtBLP expression at two levels of estrogen-dependent induction
tor of pER8 as a gift, which was very helpful in our research. We also
(Fig. 6). Based on this result, BiP probably functioned by eliminat-
appreciate the suggestions on paper writing that Prof. Jinxing Lin
ing damage to the ER environment before the Cd2+-induced UPR.
(Key Laboratory of Plant Molecular Physiology, Institute of Botany
Thus, rapid expression of the AtBiP2 protein relieved Cd2+-induced
Chinese Academy of Sciences, Beijing 100093, PR China) advised us.
ER stress through another mechanism. In addition, translational
This work was supported by the National Natural Science Founda-
regulation mentioned above lost control of total BiP expression
tion of China (31170164 and 90713030).
under the induction of 17--estradiol in the control cell lines
(Fig. 6B). It was probably because the induction was so strong that
the translational regulation was overridden. Although excess and
Appendix A. Supplementary data
unnecessary BiP proteins are induced to degrade under normal
growth conditions (Gulow et al., 2002), BiP proteins make a con-
Supplementary data associated with this article can be found,
tribution to maintaining the ER environment at the initiation of
in the online version, at http://dx.doi.org/10.1016/j.jplph.2013.
Author's personal copy
H. Xu et al. / Journal of Plant Physiology 170 (2013) 1434–1441
Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the hela cell in the cell
biology of higher plants. Int Rev Cytol 1992;132:1–30.
Alvim FC, Carolino SMB, Cascardo JCM, Nunes CC, Martinez CA, Otoni WC, et al.
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates
Enhanced accumulation of BiP in transgenic plants confers tolerance to water
endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta.
stress. Plant Physiol 2001;126:1042–54.
Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res
Oezcan U, Yilmaz E, Oezcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical
chaperones reduce ER stress and restore glucose homeostasis in a mouse model
Bertolotti A, Zhang YH, Hendershot LM, Harding HP, Ron D. Dynamic interaction of
of type 2 diabetes. Science 2006;313:1137–40.
BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol
Ormerod MG, Sun XM, Brown D, Snowden RT, Cohen GM. Quantification of apoptosis
and necrosis by flow cytometry. Acta Oncol 1993;32:417–24.
Calfon M, Zeng HQ, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endo-
Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, et al. Coupling
plasmic reticulum load to secretory capacity by processing the XBP-1 mRNA.
endoplasmic reticulum stress to the cell death program—an Apaf-1-independent
intrinsic pathway. J Biol Chem 2002;277:21836–42.
Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Masclaux-Daubresse C.
Reddy RK, Mao CH, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic
Cadmium toxicity induced changes in nitrogen management in Lycopersicon
reticulum chaperone protein GRP78 protects cells from apoptosis induced by
esculentum leading to a metabolic safeguard through an amino acid storage
topoisomerase inhibitors—role of ATP binding site in suppression of caspase-7
strategy. Plant Cell Physiol 2004;45:1681–93.
activation. J Biol Chem 2003;278:20915–24.
Costa MDL, Reis PAB, Valente MAS, Irsigler AST, Carvalho CM, Loureiro ME, et al. A
Reis PAA, Rosado GL, Silva LAC, Oliveira LC, Oliveira LB, Costa MDL, et al. The
new branch of endoplasmic reticulum stress signaling and the osmotic signal
binding protein BiP attenuates stress-induced cell death in soybean via modula-
converge on plant-specific asparagine-rich proteins to promote cell death. J Biol
tion of the N-rich protein-mediated signaling pathway. Plant Physiol 2011;157:
Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor
Rodriguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueno MC,
that controls the unfolded protein response. Cell 1996;87:391–404.
del Rio LA, et al. Cellular response of pea plants to cadmium toxicity: cross
DalCorso G, Farinati S, Furini A. Regulatory networks of cadmium stress in plants.
talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol
Plant Signal Behav 2010;5:663–7.
de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein
Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant
response. Nat Rev Mol Cell Biol 2007;8:519–29.
HFE aggregate formation. J Biol Chem 2007;282:27905–12.
Shen XH, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, et al. Complementary signaling
Deng Y, Humbert S, Liu J-X, Srivastava R, Rothstein SJ, Howell SH. Heat induces
pathways regulate the unfolded protein response and are required for C-elegans
the splicing by IRE1 of a mRNA encoding a transcription factor involved
development. Cell 2001;107:893–903.
in the unfolded protein response in Arabidopsis. Proc Nat Acad Sci USA
Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonu-
clease that initiates mRNA splicing in the unfolded protein response. Cell
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure,
activation, substrates, and functions during apoptosis. Annu Rev Biochem
Tajima H, Iwata Y, Iwano M, Takayama S, Koizumi N. Identification of an Arabidopsis
transmembrane bZIP transcription factor involved in the endoplasmic reticulum
Gardarin A, Chedin S, Lagniel G, Aude J-C, Godat E, Catty P, et al. Endoplas-
stress response. Biochem Biophys Res Commun 2008;374:242–7.
mic reticulum is a major target of cadmium toxicity in yeast. Mol Microbiol
Takahashi H, Kawakatsu T, Wakasa Y, Hayashi S, Takaiwa F. A rice transmembrane
bZIP transcription factor, OsbZIP39, regulates the endoplasmic reticulum stress
Gulow K, Bienert D, Haas IG. BiP is feed-back regulated by control of protein trans-
response. Plant Cell Physiol 2012;53:144–53.
lation efficiency. J Cell Sci 2002;115:2443–52.
Tateda C, Ozaki R, Onodera Y, Takahashi Y, Yamaguchi K, Berberich T, et al. NtbZIP60,
Hacki J, Egger L, Monney L, Conus S, Rosse T, Fellay I, et al. Apoptotic crosstalk
an endoplasmic reticulum-localized transcription factor, plays a role in the
between the endoplasmic reticulum and mitochondria controlled by Bcl-2.
defense response against bacterial pathogens in Nicotiana tabacum. J Plant Res
Harding HP, Zhang YH, Bertolotti A, Zeng HQ, Ron D. Perk is essential for transla-
Valente MAS, Faria JAQA, Soares-Ramos JRL, Reis PAB, Pinheiro GL, Piovesan ND, et al.
tional regulation and cell survival during the unfolded protein response. Mol
The ER luminal binding protein (BiP) mediates an increase in drought tolerance
in soybean and delays drought-induced leaf senescence in soybean and tobacco.
Harding HP, Zhang YH, Ron D. Protein translation and folding are coupled by an
J Exp Bot 2009;60:533–46.
endoplasmic-reticulum-resident kinase. Nature 1999;397:271–4.
Williams B, Kabbage M, Britt R, AtB. A.G7 Dickman MB. an Arabidopsis
Hayashi S, Wakasa Y, Takahashi H, Kawakatsu T, Takaiwa F. Signal transduction by
Bcl-2-associated athanogene, resides in the endoplasmic reticulum and is
IRE1-mediated splicing of bZIP50 and other stress sensors in the endoplasmic
involved in the unfolded protein response. Proc Nat Acad Sci USA 2010;107:
reticulum stress response of rice. Plant J 2012;69:946–56.
Iwata Y, Koizumi N. An Arabidopsis transcription factor, AtbZIP60, regulates the
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional
endoplasmic reticulum stress response in a manner unique to plants. Proc Nat
induction of mammalian ER quality control proteins is mediated by single or
Acad Sci USA 2005a;102:5280–5.
combined action of ATF6alpha and XBP1. Dev Cell 2007;13:365–76.
Iwata Y, Koizumi N. Unfolded protein response followed by induction of cell
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, et al. ER stress induces
death in cultured tobacco cells treated with tunicamycin. Planta 2005b;220:
cleavage of membrane-bound ATF6 by the same proteases that process SREBPs.
Mol Cell 2000;6:1355–64.
Koizumi N, Martinez IM, Kimata Y, Kohno K, Sano H, Chrispeels MJ. Molecular char-
Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, et al. Atypical,
acterization of two arabidopsis Ire1 homologs, endoplasmic reticulum-located
bidirectional regulation of cadmium-induced apoptosis via distinct signaling of
transmembrane protein kinases. Plant Physiol 2001;127:949–62.
unfolded protein response. Cell Death Differ 2007;14:1467–74.
Kuthanova A, Fischer L, Nick P, Opatrny Z. Cell cycle phase-specific death
Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, et al. Involve-
response of tobacco BY-2 cell line to cadmium treatment. Plant Cell Environ
ment of selective reactive oxygen species upstream of proapoptotic branches of
unfolded protein response. J Biol Chem 2008;283:4252–60.
Leborgne-Castel N, Jelitto-Van Dooren E, Crofts AJ, Denecke J. Overexpression of BiP
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, et al. Activation of caspase-
in tobacco alleviates endoplasmic reticulum stress. Plant Cell 1999;11:459–69.
12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis
Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein
factor receptor-associated factor 2-dependent mechanism in response to the
of the endoplasmic reticulum lumen, plays a direct and important role in the
ER stress. J Biol Chem 2001;276:13935–40.
storage of the rapidly exchanging pool of Ca2+. J Biol Chem 1997;272:30873–9.
Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplas-
Liu F, Inageda K, Nishitai G, Matsuoka M. Cadmium induces the expression of Grp78,
mic reticulum stress response element responsible for transcriptional induction
an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial
of mammalian glucose-regulated proteins—involvement of basic leucine zipper
cells. Environ Health Perspect 2006;114:859–64.
transcription factors. J Biol Chem 1998;273:33741–9.
Liu J-X, Srivastava R, Che P, Howell SH. An endoplasmic reticulum stress response
Yuan YJ, Ge ZQ, Li JC, Wu JC, Hu ZD. Differentiation of apoptotic and necrotic cells in
in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a
suspension cultures of Taxus cuspidata by the combined use of fluorescent dying
membrane-associated transcription factor, bZIP28. Plant Cell 2007;19:4111–9.
and histochemical staining methods. Biotechnol Lett 2002;24:71–6.
Ma W, Xu W, Xu H, Chen Y, He Z, Ma M. Nitric oxide modulates cadmium influx
Zhang HY, Jiang YN, He ZY, Ma M. Cadmium accumulation and oxidative burst in
during cadmium-induced programmed cell death in tobacco BY-2 cells. Planta
garlic (Allium sativum). J Plant Physiol 2005;162:977–84.
Zhang KZ, Kaufman RJ. Signaling the unfolded protein response from the endoplas-
Moreno AA, Mukhtar MS, Blanco F, Boatwright JL, Moreno I, Jordan MR, et al.
mic reticulum. J Biol Chem 2004;279:25935–8.
IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant
Zuo JR, Niu QW, Chua NH. An estrogen receptor-based transactivator XVE medi-
immunity and abiotic stress responses. PLoS One 2012:7.
ates highly inducible gene expression in transgenic plants. Plant J 2000;24:
Nagashima Y, Mishiba K-i Suzuki E, Shimada Y, Iwata Y, Koizumi N. Arabidopsis
IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active
Zuppini A, Navazio L, Mariani P. Endoplasmic reticulum stress-induced programmed
transcription factor. Sci Rep 2011:1.
cell death in soybean cells. J Cell Sci 2004;117:2591–8.
Source: http://klpr.ibcas.ac.cn/upLoad/news/month_1309/201309221357326804.pdf
Handerwärmungstraining bei Morbus Raynaud-Syndrom Das Raynaud-Syndrom (Morbus Raynaud) ist eindie durch anfallsweises Erblassen der Hände oder Füße aufgrund von gekennzeichnet ist. Unter Umständen können auch Nase und Ohren betroffen sein. Etwa 3% der Bevölkerung leiden an einem primären M. Raynaud (siehe weiter unten). Frauen sind fünfmal häufiger betroffen als Männer. Bei stillenden Frauen können auch die Brustwarzen betroffen sein, während des Stillens verfärbt sich die jeweilige Brustwarze weiß. Manifestationsalter des primären M. Raynaud meist zwischen dem 20-40 Lj. Die Erkrankung ist nach ihrem Entdecker – dem französischen Arzt(1834–1881) – benannt. Umgangssprachlich wird sie auch als Weißfingerkrankheit oder Leichenfinger bezeichnet, andere Bezeichnungen hinsichtlich der Symptome sind Digitus mortuus (Totenfinger) oder Reilscher Finger.
2012 Drug Plan rePort b e n e f i t s plan costs. In particular, Brilinta and Eliquis join new agents such as Pradax and Xarelto to provide a wide range of new innovations in the oral blood clot treatment market—at a cost premium to existing therapies. Byetta and Trajenta are examples of the significant research and development focused on treating diabetes.











