Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Kbrin.a-bldg.louisville.edu
The Journal of Neuroscience, December 15, 2001,
21(24):9541–9548
A Role for the Cytoplasmic Polyadenylation Element in NMDA
Receptor-Regulated mRNA Translation in Neurons
David G. Wells,1
Xin Dong,1
Elizabeth M. Quinlan,1
Yi-Shuian Huang,3
Mark F. Bear,1,2
Joel D. Richter,3
and Justin R. Fallon1
1
Department of Neuroscience and 2
Howard Hughes Medical Institute, Brown University, Providence, Rhode Island02912, and 3
Department of Molecular Genetics and Microbiology, University of Massachusetts Medical School,Worcester, Massachusetts 01655
The ability of neurons to modify synaptic connections based on
neurons. Cultured hippocampal neurons were transfected with
activity is essential for information processing and storage in
constructs encoding green fluorescent protein (GFP). At 6 hr
the brain. The induction of long-lasting changes in synaptic
after transfection, ⬃35% of the transfected neurons (as deter-
strength requires new protein synthesis and is often mediated
mined by
in situ hybridization) expressed detectable GFP pro-
by NMDA-type glutamate receptors (NMDARs). We used a
tein. Glutamate stimulation of the cultures at this time induced
dark-rearing paradigm to examine mRNA translational regula-
an increase in the number of neurons expressing GFP protein
tion in the visual cortex after visual experience-induced synap-
that was NMDAR dependent. Importantly, the glutamate-
tic plasticity. In this model system, we demonstrate that visual
induced increase was only detected when the 3⬘-untranslated
experience induces the translation of mRNA encoding the
region of the GFP constructs contained intact cytoplasmic
␣-subunit of calcium/calmodulin-dependent kinase II in the
polyadenylation elements (CPEs). Together, these findings de-
visual cortex. Furthermore, this increase in translation is
fine a molecular mechanism for activity-dependent synaptic
NMDAR dependent. One potential source for newly synthe-
plasticity that is mediated by the NMDA receptor and requires
sized proteins is the translational activation of dormant cyto-
the CPE-dependent translation of an identified mRNA.
plasmic mRNAs. To examine this possibility, we developed a
Key words: protein synthesis; synaptic plasticity; CPEB;
culture-based assay system to study translational regulation in
NMDA receptor; dendrites; visual cortex; hippocampus
Neural information is transmitted and processed by synapses.
protein kinase A), the cognate
trans-acting DNA-binding protein
Synaptic plasticity, the bidirectional modification of synaptic
(cAMP response element-binding protein), and the structure of
strength based on activation history, is thought to play a key role
the gene promoter region (cAMP response element) have been
in development, learning, and memory. The induction of long-
identified (Shaywitz and Greenberg, 1999). In sharp contrast,
lasting synaptic changes and memory formation both require
little is known about the molecular pathways underlying transla-
tightly regulated, activity-driven protein synthesis (Davis and
tional regulation in neurons. In particular, in no case have defined
Squire, 1984; Bailey et al., 1996). The mRNAs encoding these
aspects of synaptic activation been functionally linked to specific
newly synthesized proteins can have two distinct histories: some
structural elements in an identified, translationally activated
are transcribed in direct response to neural activity, whereas
others are stored in the cytoplasm and are regulated at the
One approach to this problem is to draw on mechanisms that
translational level (Wells et al., 2000; Aakalu et al., 2001). Char-
have been elucidated in non-neural systems. Oocyte maturation
acterization of the molecular mechanisms regulating activity-
and early embryonic development require the translational acti-
induced transcription and translation is essential for understand-
vation of maternal mRNAs that are stored in the cytoplasm. A
ing how experience and neural activity can be transformed into
process known as cytoplasmic polyadenylation regulates one key
discrete, stable synaptic changes. Our knowledge of transcription-
set of maternal messages. These mRNAs harbor a specific
cis-
ally based mechanisms is relatively advanced. In the best studied
element in their 3⬘-untranslated regions (UTRs), the cytoplasmic
case of activity-induced transcriptional activation, the relevant
polyadenylation element (CPE) that binds a
trans-acting binding
synaptic stimuli [NMDA-type glutamate receptors (NMDARs)],
protein [CPE-binding protein (CPEB)]. CPEB in turn is part of
several putative intracellular signaling molecules (cAMP and
a protein complex that regulates the translational state of CPE-containing mRNAs (Richter, 2000).
Received Aug. 1, 2001; revised Sept. 25, 2001; accepted Sept. 26, 2001.
This mechanism not only regulates the activation of mRNA
This work was supported by National Institutes of Health Grants NS39321 and
translation but is directly involved in keeping CPE-containing
RR15578 (J.F.) and by National Research Scientist postdoctoral Award NS10919
(D.W.) Y.-S.H. was supported by the Charles A. King trust postdoctoral fellowship.
mRNA translationally dormant before progesterone stimulation
D.G.W. and X.D. contributed equally to this work.
in the oocyte (de Moor and Richter, 1999). A CPEB-associated
Correspondence should be addressed to Justin R. Fallon at the above address.
protein, maskin, binds to the translation initiation factor 4E
D. G. Wells's present address: Department of Molecular, Cellular, and Develop-
(eIF-4E). The maskin complex precludes the activation of trans-
mental Biology, Yale University, New Haven, CT 06511.
lation by prohibiting the binding of eIF-4G to eIF-4E. Progester-
E. M. Quinlan's present address: Department of Biology, University of Maryland,
College Park, MD 20742.
one stimulation of the oocyte leads to the phosphorylation of
Copyright 2001 Society for Neuroscience 0270-6474/01/219541-08$15.00/0
CPEB by the Aurora serine–threonine kinase (also known as Eg2
9542 J. Neurosci., December 15, 2001,
21(24):9541–9548
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
or IAK1 kinase) (Bischoff and Plowman, 1999; Mendez et al.,
The cultures were washed and remained with the glial feeder layer for an
2000). CPEB phosphorylation is required for cytoplasmic poly-
additional 1.5 hr before fixation with 4% paraformaldehyde. In
adenylation and the subsequent disassociation of maskin and
D-aminophosphonovalerate (APV)-treated cultures, the drug was applied
immediately after transfection and remained in the media for the dura-
eIF-4E, which in turn permits translational initiation. In the
tion the stimulation and through the remainder of the experiment. In
rodent brain, both CPEB and Aurora have been localized to
actinomycin D-, cycloheximide-, and cordycepin-treated cultures, the
synapses (Wu et al., 1998) (Y.-S. Huang, M.-Y. Jung, M. Sarkiss-
drugs were applied 30 min before stimulation and remained in the media
ian, and J. D. Richter, unpublished observations), suggesting that
for the duration of the experiment.
In situ
hybridization. At indicated times after transfection, the cover-
this mechanism could play an important role in local synaptic
slips were washed once in 1⫻ PBS, fixed in 4% formaldehyde–PBS for 10
protein synthesis (Wells et al., 2000).
min at room temperature, and washed three more times in PBS. The
In a recent study, we showed that CPEB is expressed in the
coverslips were then incubated in 1⫻ SSC for 5 min at room temperature
visual cortex and that the CPE-containing the ␣-subunit of
and permeabilized by 1% Triton X-100 –1⫻ SSC for 30 min at room
calcium/calmodulin-dependent kinase II (␣-CaMKII) mRNA is
temperature. The coverslips were placed cell-side down on Parafilm and
hybridized overnight at 37°C in 40 l of hybridization mix (50% form-
polyadenylated and translated in this brain region in response to
amide, 2⫻ SSC, 10% dextran sulfate, 1 mg/ml tRNA, 0.02% RNase-free
visual experience (Wu et al., 1998). In this report, we demon-
BSA, and 2 mM vanadyl–ribonucleoside complex) plus 30 ng of digoxy-
strate that NMDAR activation is essential for ␣-CaMKII protein
genin (DIG)-labeled DNA oligonucleotide probe against green fluores-
synthesis in the visual cortex and that this synthesis is also
cent protein (GFP) coding region having the following sequence: 5⬘-
sensitive to inhibitors of cytoplasmic polyadenylation. We also
(X indicates the DIG-modified base) (Oligos Etc., Wilsonville, OR).
introduce a new cell culture-based assay for studying translational
After hybridization, the coverslips were washed twice with 50% form-
regulation in neurons. Using this system, we show that NMDAR-
amide–2⫻ SSC for 30 min at 37°C and then incubated for 1 hr at 37°C in
stimulated translation requires the CPEs in the 3⬘-UTR of
blocking solution (2⫻ SSC, 8% formamide, 2 mM vanadyl–ribonucleo-
␣-CaMKII mRNA. These findings link a specific mechanism of
side complex, and 0.2% RNase-free BSA) and washed four times for 5
min each in 8% formamide–2⫻ SSC at room temperature. The DIG-
translational regulation to many of the key molecular elements
labeled oligo probes were detected with monoclonal mouse anti-
thought to play critical roles in synaptic plasticity, learning, and
digoxygenin antibody (1:250; Boehringer Mannheim) overnight at 4°C,
memory formation.
followed by biotinylated anti-mouse IgG (1:100; Vector Laboratories,
Burlingame, CA) for 45 min and Cy3 streptavidin (1:500; Jackson Im-
MATERIALS AND METHODS
munoResearch, West Grove, PA) for 30 min. Finally, the coverslips were
incubated with 4⬘,6⬘-diamidino-2-phenylindole (DAPI) (Sigma, St.
Analysis of ␣
-CaMKII in synaptoneurosomes. Long–Evans rats were born
Louis, MO) for 5 min at room temperature to stain the nuclei.
in a room specifically designed for rearing of animals in a light-free
Scoring of GFP-fluorescent neurons and GFP-
in situ hybridization
environment. They were raised in this room between 4 and 6 weeks
(ISH)-positive neurons cell counts was performed on a Nikon (Tokyo,
before use in these experiments. Dark-reared rats were either anesthe-
Japan) E800 fluorescent microscope. Cultures derived from E18 rat
tized in the dark (DR) or anesthetized after 30 min of light exposure
embryos (as above) consist of two types of neurons. The majority
(DR ⫹ 30⬘). Treated DR rats were injected intraperitoneally in the dark
(⬃94%) are glutamatergic pyramidal neurons, and the remaining 6% are
and either kept in the dark for 1 hr or brought into the light for 30 min,
GABAergic interneurons (Benson et al., 1994). Non-neural cells com-
0.5 hr after injection. The primary visual cortex was rapidly dissected in
prise a small proportion (⬃1%) of the total number of cells in these
cold, sterile PBS and immediately homogenized in ice-cold buffer (10 mM
cultures and were distinguished from neurons based on their distinctive
HEPES, 2.0 mM EDTA, 2.0 mM EGTA, 0.5 mM DTT, 0.1 mM PMSF, 10
cellular morphology. No effort was made to distinguish between neuro-
mg/l leupeptin, 50 mg/l soybean trypsin inhibitor, and 100 nM microcys-
nal cell types. For each coverslip, the total number of neurons and the
tin). Synaptoneurosome fractions were isolated as by Quinlan et al.
total number of GFP-fluorescent neurons was counted. Neurons were
(1999). Briefly, the tissues were homogenized and passed through two
scored as GFP-expressing if they exhibited intense fluorescence through-
100 m nylon mesh filters, followed by a 5 m pore filter. The filtrate was
out the entire cell. Intermediate levels of GFP expression and non-
then centrifuged at 1000 ⫻
g for 10 min.
uniform distribution of fluorescence were rare. GFP-ISH-positive neu-
Equal amounts of total protein (25 g) from the synaptoneurosome
rons were counted from 10 random fields per coverslip using a 40⫻
fractions were resolved on a 5–15% polyacrylamide gel, blotted, and
objective. The scoring was performed blind to the stimulation history of
probed simultaneously with monoclonal antibodies to ␣-CaMKII (clone
the cultures. The absolute numbers of transfected neurons was the same
#6G9; Boehringer Mannheim, Indianapolis, IN) and NMDAR1
at 24 hr under all conditions, indicating that viability was not influenced
(PharMingen, San Diego, CA), followed by an alkaline phosphatase-
by these transfections (mean number of GFP-CPE WT, 169 ⫾ 7.5 per
conjugated secondary antibody. Digital images of the ␣-CaMKII West-
1800 total neurons; mean number of GFP-CPE MUT, 193 ⫾ 26.9 per 2000
ern blots were obtained using a ScanJet IIcx (Hewlett-Packard, Palo Alto,
total neurons).
CA) with DeskScan II (Hewlett-Packard) software, and quantitative
Images were recorded with a PhotoMetrics Inc. (Huntington Beach,
densitometry was performed with NIH Image 1.60 software.
CA) CCD camera using IP Lab Systems software and imported into an
Hippocampal neuron cultures. Cultures of rat hippocampal neurons
Adobe Photoshop (Adobe Systems, San Jose, CA) file.
were made as described previously (Goslin and Banker, 1991). Briefly,
Construction of GFP–␣
-CaMKII–3⬘
-UTR plasmids. pEGFP-C1 vector
the hippocampus was removed from embryonic day 18 (E18) rat em-
(Clontech, Cambridge, UK) was digested with
EcoRI and blunt-ended
bryos, trypsinized (0.25%), dissociated by trituration, and plated onto
with Klenow to generate the stop codon TAA and self-ligated. The
poly-L-lysine (1 mg/ml)-coated glass coverslips (240,000 cells/ml) for 3 hr.
resulting plasmid was digested with
SalI and
XbaI, and a partial
The coverslips were then transferred to dishes containing a monolayer of
␣-CaMKII 3⬘-UTR (⬃170 bp) with wild-type (WT) CPEs or mutant
glial cells in growth medium. After 7–10 d
in vitro, individual coverslips
(MUT) CPEs (Wu et al., 1998) were ligated into this vector between
were transferred to 12 well plates for transfection with 0.5 g of DNA per
these sites.
coverslip for 1 hr using Effectene (Qiagen, Hilden, Germany) or 1 g of
DNA per coverslip for 5 hr using Lipofectamine 2000 (Invitrogen, San
Diego, CA). In preliminary experiments, we attempted transfection with
calcium phosphate and an earlier version of Lipofectamine (Lipo-
fectamine Plus), but transfection efficiencies were low and cell viability
Experience-induced protein synthesis in the visual
after transfection was often compromised. The highest efficiency and
cortex is NMDAR dependent
greatest viability 24 hr after transfection was obtained with Lipo-
We used the visual cortex of dark-reared rats exposed to light for
fectamine 2000. After transfection, the coverslips were then washed and
brief periods as a model for robust, experience-driven synaptic
placed back into the dishes containing the glial feeder layer. Removal
from the transfection media was considered time 0 for all experiments.
reorganization (Carmignoto and Vicini, 1992; Kirkwood et al.,
Cultures were stimulated at indicated times after transfection with bath
1996; Quinlan et al., 1999). In this paradigm, ␣-CaMKII mRNA
application of either 100 M glutamate (30 sec) or 35 mM KCl (5 min).
is polyadenylated after 30 min of visual experience (Wu et al.,
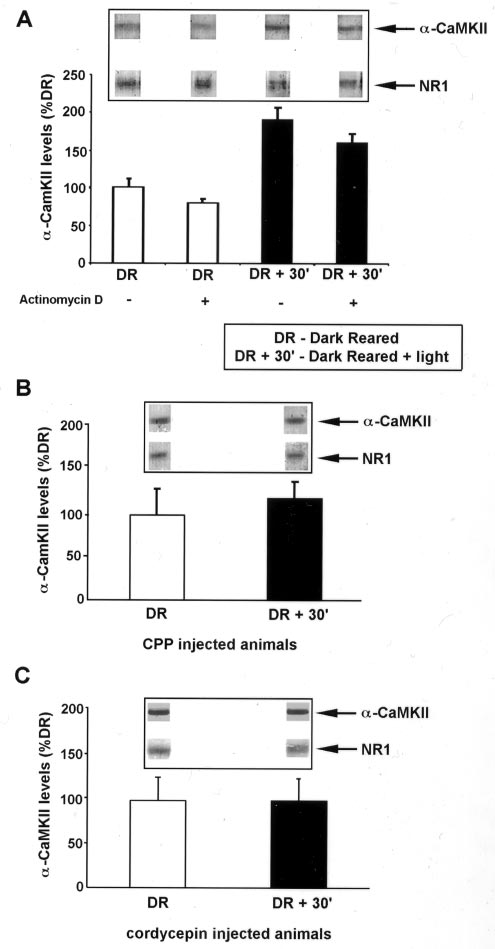
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
J. Neurosci., December 15, 2001, 21(24):9541–9548 9543
1998). This polyadenylation is accompanied by an increase in
␣-CaMKII protein in the synaptic fraction isolated from the
visual cortex (Fig. 1A) (Wu et al., 1998). In contrast, the level of
NR1 (NMDA receptor subunit-1) protein in the same fractions
remains unchanged after light exposure. This elevation in
␣-CaMKII protein is sensitive to the protein synthesis inhibitor
cycloheximide (Wu et al., 1998). The increased production of
␣-CaMKII protein could be attributable to newly synthesized
message or to the enhanced translation of existing mRNA. To
distinguish between these possibilities, rats were injected with the
transcription inhibitor actinomycin D before light exposure. As
shown in Figure 1, this treatment failed to block the increase in
␣-CaMKII protein in the synaptoneurosome fraction of dark-
reared, light-exposed animals. Therefore, the activation or en-
hancement of mRNA translation is required for the visual
experience-induced increase in synaptic ␣-CaMKII.
The activation of NMDARs is thought to drive experience-
induced synaptic plasticity during postnatal development of the
visual cortex (Bear et al., 1990; Daw et al., 1999). To examine
whether NMDAR activation triggers this new protein synthesis,
rats were injected with the NMDAR antagonist 3-(2
carboxypiperazin-4yl) propyl-1-phosphonic acid (CPP) just be-
fore visual experience. As shown in Figure 1B, NMDAR block-
ade of dark-reared, light-exposed rats inhibited the increase in
␣-CaMKII protein in synaptic fractions. Thus, NMDAR activa-
tion is essential for experience-induced translation of ␣-CaMKII
mRNA in the visual cortex.
As one test of whether mRNA polyadenylation is required for
this new ␣-CaMKII protein synthesis in the visual cortex, we
injected the dark-reared animals with 3⬘-deoxyadenosine
(cordycepin), an adenosine analog that inhibits mRNA polyade-
nylation (Beach and Ross, 1978; Ulibarri and Yahr, 1987;
McGrew et al., 1989; Groisman et al., 2000). Cordycepin treat-
ment blocked the light-induced increase in ␣-CaMKII protein in
the synaptic fraction of visual cortex (Fig. 1C). This finding
suggests that visual experience-induced NMDAR activation trig-
gers ␣-CaMKII protein synthesis via a mechanism that requires
A role for cytoplasmic polyadenylation elements in
activity-dependent mRNA translation in neurons
To elucidate the mechanistic basis of this process in more detail,
we next developed a cell culture model to directly assess the role
Figure 1. Experience-induced increase in ␣-CaMKII protein in the visual
of the ␣-CaMKII CPEs in translational regulation. We based this
cortex mediated by NMDAR activation and mRNA polyadenylation. A,
assay on the well defined low-density hippocampal neuron culture
Quantification of ␣-CaMKII levels in synaptoneurosome (SN) fractions
system (Bartlett and Banker, 1984; Fletcher et al., 1991, 1994;
isolated from the visual cortex of animals reared in complete darkness (DR)
Goslin and Banker, 1991). These cultures are comprised of ⬃99%
and animals reared in the dark and exposed to light for 30 min (DR ⫹ 30⬘).
Western blots for ␣-CaMKII and NMDAR subunit NR1 were performed
neurons and ⬃1% glial cells. In all of our experiments, we scored
from PAGE loaded with equal total protein of SN samples isolated from
only neurons, which were identified based on morphology using
DR and DR ⫹ 30⬘ visual cortex. Quantitative densitometry was performed
phase contrast microscopy and/or fluorescence imaging of GFP-
on the ␣-CaMKII bands, and these were normalized to the level of NR1 in
transfected cells. Control experiments with anti-microtubule-
the same lane [the amount of NR1 subunit in SN fraction does not change
associated protein 2, synaptic markers, and anti-GFAP confirmed
with visual experience (Quinlan et al., 1999)]. Where indicated, actinomy-
cin D (1 mg/kg) was injected (intraperitoneally) 30 min before light
the reliability of these identification methods (data not shown).
exposure. This dose of actinomycin D is effective in blocking protein
Neurons cultured for 7–10 d were transfected with plasmids
synthesis in the brain (Jackson, 1972; Pickering and Fink, 1976). Each
containing the GFP coding sequence linked to a fragment of the
experiment consisted of two to four rats per treatment group, and results
␣-CaMKII 3⬘-UTR that harbored either intact or mutated CPEs
shown are the mean ⫾ SEM of three experiments. Insets show represen-
tative bands from one experiment. B, Quantification of ␣-CaMKII expres-
(GFP-CPEWT and GFP-CPEMUT, respectively) (Fig. 2). We
sion as in A, in animals injected with the NMDAR antagonist CPP (10
then monitored the expression of the GFP-encoding mRNA and
mg/kg) 30 min before light exposure. Each experiment consisted of two to
GFP protein in the transfected neurons by ISH and GFP fluores-
four rats per treatment group, and results shown are the mean ⫾ SEM of
three experiments. C, Quantification of ␣-CaMKII performed as in A, in
To enable reliable quantification, we established conditions in
animals injected with cordycepin (6 mg/kg) 30 min before light exposure.
Each experiment consisted of two to four rats per treatment group, and
which ⬃10% of the neurons (⬃200 per coverslip) were trans-
results shown are the mean ⫾ SEM of three experiments.
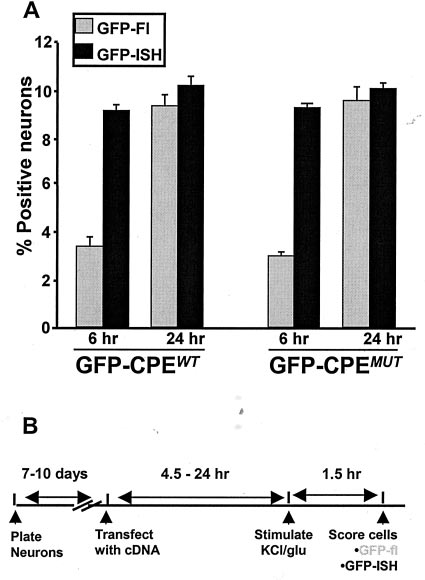
9544 J. Neurosci., December 15, 2001, 21(24):9541–9548
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
Figure 2. Transfection of hippocampal cells in culture with reporter GFP
constructs. A, Schematic of ␣-CaMKII mRNA and the GFP constructs
used for transfections. GFP constructs were modified to contain the last
⬃160 nucleotides of ␣-CaMKII 3⬘-UTR with either intact CPE se-
quences (GFP-CPE WT; top) or mutated CPEs (GFP-CPE MUT; bottom).
B, Hippocampal neurons grown in culture for 7 d, transfected with
Quantification of GFP expression in cultured hippocampal
. This culture was processed for GFP fluorescence 8 hr after
transfection. GFP-fluorescing neurons are readily distinguished from
neurons and experimental design. A, GFP fluorescence is not correlated
non-GFP-fluorescing neurons, and GFP is detected throughout the entire
with GFP mRNA expression at early times after transfection. In cultures
neuron (right). Scale bar, 20 m.
transfected with GFP-CPE WT, 9.23 ⫾ 0.002% of all neurons expressed
GFP mRNA at 6 hr. However, significantly fewer neurons (3.4 ⫾ 0.002%)
expressed GFP protein at detectable levels ( p ⱕ 0.005). In contrast, at 24
hr after transfection, 10.27 ⫾ 0.003% of the total neuronal population
contained GFP mRNA, and 9.41 ⫾ 0.004% exhibited GFP fluorescence
( p ⫽ 0.09). Similar results were obtained when cultures were transfected
with GFP-CPE MUT constructs: at 6 hr after transfection, 9.3 ⫾ 0.002%
contained GFP mRNA, with only 3.07 ⫾ 0.002% expressing detectable
protein ( p ⱕ 0.01). This difference was not present at 24 hr after
transfection (10.2 ⫾ 0.002% contained GFP mRNA, and 9.7 ⫾ 0.005%
contained GFP fluorescence; p ⫽ 0.4). Data represent mean ⫾ SEM. B,
Experimental design. Seven- to 10-d-old cultures were transfected and
then stimulated with either glutamate or KCl at time points between 4.5
and 24 hr after transfection. GFP fluorescence and GFP mRNA presence
(using fluorescent in situ hybridization) was scored 1.5 hr after stimula-
tion. GFP-Fl, GFP fluorescence; GFP-ISH, GFP-fluorescent in situ
expression at these time points showed that the same percentage
Figure 3. GFP mRNA and GFP protein expression in transfected neu-
of neurons were transfected; however, at 6 hr after transfection,
rons. Neurons were processed for GFP fluorescence (GFP-Fl ) and fluo-
only ⬃35% of the neurons containing GFP mRNA expressed
rescent in situ hybridization (GFP-ISH ) at either 6 or 24 hr after trans-
fection. Neurons at 6 hr can contain GFP mRNA without expressing
detectable GFP protein (Fig. 4A). Importantly, the transfection
detectable GFP fluorescence (top panel ). In contrast, at 24 hr after
efficiencies observed using the unmodified GFP construct, GFP-
transfection, all GFP mRNA-containing neurons also express the fluores-
CPEWT and GFP-CPEMUT were indistinguishable (Fig. 4A and
cent GFP protein (middle panel ). In the bottom panel, the anti-DIG
data not shown).
primary antibody was replaced with a nonspecific normal mouse IgG
antibody (control IgG). DAPI staining reveals the nuclei of cells within
The presence of neurons containing GFP mRNA without de-
tectable levels of GFP protein suggested a time window in which
activity-regulated translation might be readily revealed. Because
fected (see Materials and Methods). As shown in Figure 3,
the number of cells transfected was the same under all conditions
GFP-encoding mRNA was detected in transfected neurons. We
tested, we predicted that, at early times after transfection, new
then compared the presence of GFP-encoding mRNA to the
translation would manifest as an increase in the number of neu-
expression of GFP protein in neurons as detected by intrinsic
rons expressing detectable GFP protein.
GFP fluorescence. At 24 hr, all neurons containing GFP mRNA
To test this hypothesis, we designed experiments in which
also expressed GFP protein. In contrast, at 6 hr after transfection,
neurons were stimulated by either direct glutamate application
only a fraction of the GFP mRNA-containing neurons expressed
or KCl depolarization at varying times after transfection (Fig.
detectable GFP protein (Fig. 3). Scoring GFP protein and mRNA
4 B). Figure 5A shows that glutamate stimulation (100 M for
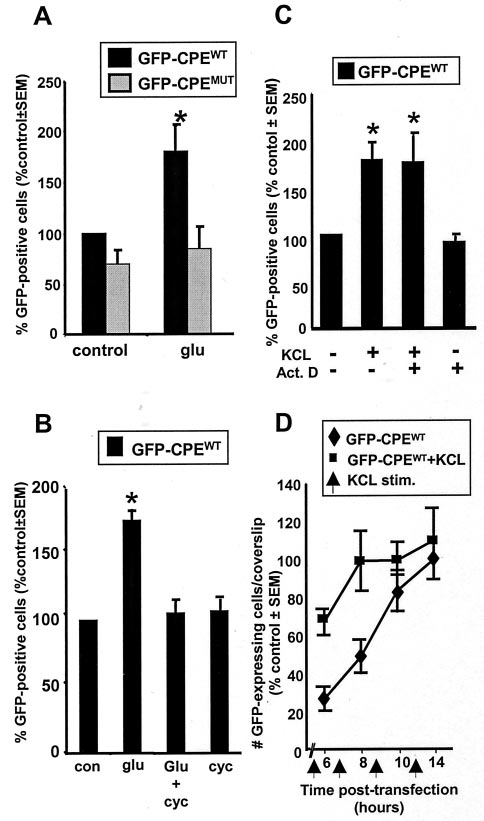
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
J. Neurosci., December 15, 2001, 21(24):9541–9548 9545
30 sec) at an early time after transfection triggered an increase
in the number of GFP protein-expressing neurons. This in-
crease was completely blocked by the translation inhibitor
cycloheximide, indicating that the GFP is newly synthesized
(Fig. 5B). This increase in GFP-expressing neurons could also
be induced by KCl depolarization (35 mM for 5 min) (Fig. 5C)
and was insensitive to treatment with actinomycin D (Fig. 5C).
An examination of the time course showed that the number of
GFP protein-expressing neurons in unstimulated cultures in-
creased gradually over the first 10 hr after transfection, reach-
ing a plateau at ⬃14 hr (Fig. 5D). KCl treatment at early times
after transfection resulted in a significant increase in the num-
ber of neurons expressing detectable GFP protein compared
with unstimulated control cultures. On the other hand, such
increases were not observed when neurons were treated with
KCl at later times after transfection. This result is completely
consistent with our observations of GFP mRNA and protein
expression at 6 and 24 hr (Fig. 4). We thus conclude that
activity-induced stimulation of mRNA translation is mani-
fested by an increase in the number of neurons with detectable
GFP protein expression.
The experiments described above show that glutamate stimu-
lation activates the translation of GFP reporter constructs that
contain the wild-type CPE elements from the 3⬘-UTR of the
␣-CaMKII mRNA (GFP-CPEWT). To determine whether these
CPEs are necessary for the observed translational activation, we
tested neurons transfected with a construct in which these ele-
ments were mutated (GFP-CPEMUT) (Fig. 2). Glutamate stimu-
lation of these cultures at 6 hr after transfection failed to elicit a
significant increase in the number of GFP protein-expressing
neurons ( p ⬎ 0.7) (Fig. 5A). Glutamate stimulation also failed to
stimulate translation in cells transfected with an unmodified GFP
reporter construct that lacks CPEs (Clontech). We conclude that
the CPE is required for an activity-driven increase in translation
in hippocampal neurons.
NMDAR-dependent protein synthesis mediated
Figure 5. Activity induces an increase in translation of CPE-containing
mRNA at early times after transfection. A, Hippocampal neuron cultures
Our in vivo experiments indicated that NMDAR activation is
transfected with either GFP-CPE WT (black bars) or GFP-CPE MUT ( gray
necessary for the experience-induced translation of ␣-CaMKII
bars) were stimulated 6 hr after transfection by a 30 sec application of
glutamate ( glu; 100 M) and processed for GFP fluorescence 1.5 hr later.
mRNA (Fig. 1). However, because the entire animal was treated
A significant increase in the number of GFP-expressing neurons was
with the antagonist, it was impossible to determine whether the
detected only in the cultures transfected with the CPE-containing con-
NMDAR activation and the protein synthesis occurred in the
struct (n ⫽ 3). B, The glutamate-induced increase in GFP-expressing
same neuron. Therefore, we examined NMDAR-driven protein
neurons is dependent on protein synthesis. Cultures transfected with
synthesis in our in vitro model system using the receptor-specific
GFP-CPE WT and stimulated with glutamate ( glu) as above were treated
with cycloheximide 30 min before glutamate stimulation. Cycloheximide
antagonist APV to determine whether the NMDAR mediates
(cyc) treatment blocked the increase in the number of GFP-expressing
activity-induced translation in neurons. As shown in Figure 6A,
neurons. Cycloheximide treatment alone for the duration of the post-
APV treatment completely inhibited the glutamate-induced in-
stimulation period (1.5 hr) had no effect on the number of GFP-
crease in GFP-expressing neurons transfected with GFP-CPEWT.
expressing neurons (n ⫽ 3). con, Control. C, Depolarization induces an
increase in GFP-expressing neurons that is not dependent on new gene
The KCl-induced increase in GFP synthesis was also APV sen-
transcription. Where indicated, cultures transfected with GFP-CPE WT
sitive (Fig. 6B). These results place the NMDAR in the signal
were depolarized with KCl (35 mM, 5 min) 1.5 hr before fixation. KCl
transduction pathway leading from synaptic stimulation to the
depolarization induced a significant increase in the number of GFP-
translational activation of CPE-containing mRNA.
expressing neurons. Addition of actinomycin D (Act. D; 25 M) 30 min
In the visual cortex, ␣-CaMKII mRNA was polyadenylated in
before KCl application did not alter the response to KCl depolarization
(n ⫽ 3). D, Time course of GFP expression in neurons transfected with
response to visual experience, a process reminiscent of CPE-
GFP-CPE WT. Hippocampal neurons were transfected with GFP-CPE WT
dependent translation activation during Xenopus oocyte matura-
and then processed for GFP fluorescence at 6, 8, 10, and 14 hr after
tion (Richter, 1996; Wu et al., 1998). To test whether the
transfection (⽧). In parallel experiments, cultures were stimulated with
NMDAR-mediated, CPE-dependent increase in GFP expression
35 mM KCl for 5 min (arrows at 4.5, 6.5, 8.5, and 10.5 hr) 1.5 hr before
fixation (f). Three coverslips were counted at each time point in each
in neurons requires polyadenylation, we incubated the cultures in
experiment, and results are the mean ⫾ SEM of two experiments. All
cordycepin for 30 min before and during glutamate stimulation.
coverslips were counted blind to treatment protocol (control is unstimu-
Cordycepin blocked the increase in GFP expression observed
lated; *p ⱕ 0.05).
after glutamate stimulation (Fig. 6C). Note that, in these exper-
9546 J. Neurosci., December 15, 2001, 21(24):9541–9548
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
iments, direct activation of glutamate receptors bypasses the need
for synaptic release. Thus, possible presynaptic effects of cordyce-
pin can be ruled out. Together, both the in vivo and in vitro data
indicate that NMDAR activation can stimulate cytoplasmic poly-
adenylation and translation of CPE-containing mRNA.
DISCUSSION
New protein synthesis triggered by neural activity is required for
invoking long-lasting changes in synaptic strength and for mem-
ory formation. Although some of these polypeptides arise as a
consequence of increased transcription, recent evidence suggests
that the synthesis of others is regulated at the translational level.
Here, we used both in vivo and cell culture systems to demonstrate
a molecular mechanism for the activity-driven translation of a
specific mRNA.
We used the rat visual cortex as a model system to examine
the changes in protein synthesis during experience-induced
synaptic plasticity. Dark-rearing rats from birth results in a
relatively immature visual cortex that maintains the high de-
gree of synaptic plasticity characteristic of the critical period
(Kirkwood et al., 1995). Exposure of dark-reared rats to light
results in a rapid, robust and coordinated burst of experience-
driven synaptic plasticity that can be readily monitored at the
biochemical and electrophysiological level (Quinlan et al.,
1999). In previous work, we showed that visual experience
evokes the polyadenylation of ␣-CaMKII mRNA in visual
cortex and the elevation of ␣-CaMKII protein in synaptic
fractions from this brain region. Moreover, this increase was a
direct result of new synthesis because it was sensitive to the
translation inhibitor cycloheximide (Wu et al., 1998). Here we
show that the experience-induced increase of ␣-CaMKII pro-
tein does not require new transcription. Thus, the source of
newly synthesized ␣-CaMKII protein is derived from the
translational activation of already existing mRNA. This pro-
cess of translational activation was blocked by an NMDAR
antagonist, indicating that NMDAR signaling is necessary for
this experience-evoked increase in synaptic ␣-CaMKII pro-
tein. Finally, the increase in synaptic ␣-CaMKII protein was
blocked by the polyadenylation inhibitor cordycepin. Together,
this data suggests that neural activity, transduced by the
NMDAR, activates mRNA translation mediated by mRNA
We developed a novel cell culture assay to elucidate both the
cellular signaling mechanisms and the mRNA regulatory se-
quences that underlie this activity-dependent translation. In
this system, hippocampal neurons are transfected with con-
structs encoding GFP and either wild-type or mutated
␣-CaMKII 3⬘-UTR sequences. To quantify the results, we
took advantage of two observations. First, the transfection
efficiency was the same regardless of which construct was used
Figure 6. Activity-dependent translation in cultured hippocampal neurons
regulated by NMDAR activation and mediated by polyadenylation. A,
(Fig. 4 A). Second, the number of cells expressing GFP protein
Neurons cultured and transfected as in Figure 5 were treated with the
at early times after transfection was only ⬃35% of the neurons
NMDAR antagonist APV (300 M) starting immediately after transfection
containing GFP mRNA, with detectable GFP fluorescence
and continuing through the end of the stimulation protocol (total of 7.5 hr).
increasing slowly during the first 14 hr after transfection.
The glutamate ( glu)-induced increase in the number of neurons expressing
GFP in cultures transfected with GFP-CPE WT was inhibited by APV. APV
Accordingly, we reasoned that stimulating translation of a
treatment alone for the entire post-transfection interval (7.5 hr) caused a
given GFP-encoding mRNA during these early times after
small but significant ( p ⬍ 0.05) decrease in GFP expression. B, The KCl
transfection would result in more of the transfected cells ex-
depolarization-induced increase in GFP-expressing neurons was similarly
hibiting detectable GFP expression. Our findings with both
inhibited by APV (n ⫽ 4). C, The glutamate-induced stimulation of GFP
KCl depolarization and glutamate stimulation at 5– 8 hr after
translation is blocked by the treatment of cordycepin (cordy; 200 M) for 30
min before glutamate stimulation (n ⫽ 3). Cordycepin alone did not affect
transfection supported this interpretation (Fig. 5). Moreover,
GFP expression in these neurons (control is unstimulated; *p ⱕ 0.05).
the increase in the number of GFP-expressing cells was a
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
J. Neurosci., December 15, 2001, 21(24):9541–9548 9547
consequence of mRNA translation because it was sensitive to
cycloheximide but resistant to actinomycin D. Furthermore, as
Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic
predicted by this model, stimulation of cultures ⬎14 hr after
visualization of local protein synthesis in hippocampal neurons. Neu-
transfection did not result in an increase in the number of cells
ron 30:489–502.
Bailey CH, Bartsch D, Kandel ER (1996) Toward a molecular definition
with detectable fluorescence. We exploited the disparity be-
of long-term memory storage. Proc Natl Acad Sci USA 93:13445–13452.
tween the presence of transfected mRNA and protein expres-
Bartlett WP, Banker GA (1984) An electron microscopic study of the
development of axons and dendrites by hippocampal neurons in cul-
sion at the early times after transfection to investigate the
ture. II. Synaptic relationships. J Neurosci 4:1954–1965.
signaling mechanisms and mRNA sequence elements that
Beach LR, Ross J (1978) Cordycepin. An inhibitor of newly synthesized
function in activity-regulated translation in neurons. Guided
globin messenger RNA. J Biol Chem 253:2628–2633.
Bear MF, Kleinschmidt A, Gu Q, Singer W (1990) Disruption of
by our in vivo data, we stimulated cultured neurons with
experience-dependent synaptic modifications in striate cortex by infu-
glutamate and demonstrated that translation was only en-
sion of NMDA receptor antagonist. J Neurosci 10:909–925.
Benson DL, Watkins FH, Steward O, Banker G (1994) Characterization
hanced if the reporter contained the intact CPEs from the
of GABAergic neurons in hippocampal cell cultures. J Neurocytol
3⬘-UTR of ␣-CaMKII. Furthermore, consistent with our ob-
servations in the visual cortex, NMDAR activation is required
Bischoff JR, Plowman GD (1999) The aurora/Ipl1p kinase family: reg-
ulators of chromosome segregation and cytokinesis. Trends Cell Biol
for this increase in translation. It should be noted that, in the
current study, the mRNA encoding the GFP reporter con-
Carmignoto G, Vicini S (1992) Activity-dependent decrease in NMDA
receptor responses during development of the visual cortex. Science
structs are expressed in cell bodies, as well as in dendrites (Fig.
3). Therefore, it is not possible to determine the localization of
Davis HP, Squire LR (1984) Protein synthesis and memory: a review.
the translation detected. Studies to address this issue are
Psychol Bull 96:518–559.
Daw NW, Gordon B, Fox KD, Flavin HJ, Kirsch JD, Beaver CJ, Ji QH,
currently in progress.
Reid SNM, Czepita D (1999) Injection of MK-801 affects ocular dom-
Our results in vitro and in vivo suggest that cytoplasmic poly-
inance shifts more than visual activity. J Neurophysiol 81:204–215.
de Moor CH, Richter JD (1999) Cytoplasmic polyadenylation elements
adenylation is a mechanism for regulating activity-induced
mediate masking and unmasking of cyclin B1 mRNA. EMBO J
mRNA translation in neurons. When activity is induced by visual
experience, NMDAR activation induces the translation of
Fletcher TL, Cameron P, De Camilli P, Banker G (1991) The distribu-
tion of synapsin I and synaptophysin in hippocampal neurons develop-
␣-CaMKII, a message that contains two CPE sequences in its
ing in culture. J Neurosci 11:1617–1626.
3⬘-UTR (Wu et al., 1998). In a previous study, we demonstrated
Fletcher TL, De Camilli P, Banker G (1994) Synaptogenesis in hip-
pocampal cultures: evidence indicating that axons and dendrites be-
that the CPE-binding protein CPEB is localized to synapses in
come competent to form synapses at different stages of neuronal de-
the brain (Wu et al., 1998). CPEB is likely to be a key regulator
velopment. J Neurosci 14:6695–6706.
of CPE-dependent cytoplasmic polyadenylation (Richter, 2000;
Goslin K, Banker G (1991) Rat hippocampal neurons in low-density
culture. In: Culturing Nerve Cells (Banker G, Goslin K, eds), pp
Wells et al., 2000), suggesting that CPE-mediated translation
251–282. Cambridge, MA: MIT.
could be occurring synaptically. Furthermore, inhibiting polyad-
Groisman I, Huang Y-S, Mendez R, Cao Q, Theurkauf W, Richter JD
(2000) CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus:
enylation, by injecting cordycepin, blocks the increase in synaptic
implications for local translational control of cell division. Cell
␣-CaMKII after light exposure. In vitro, GFP-CPEWT-
transfected neurons stimulated by direct glutamate receptor ac-
Jackson GL (1972) Effect of actinomycin D on estrogen-induced release
of luteinizing hormone in ovariectomised rats. Endocrinology
tivation in the presence of cordycepin produces a complete inhi-
bition of the increase in GFP-expressing neurons. In addition,
Kirkwood A, Lee HK, Bear MF (1995) Co-regulation of long-term
potentiation and experience-dependent synaptic plasticity in visual
translation was not induced in hippocampal neurons transfected
cortex by age and experience. Nature 375:328–331.
with either unmodified constructs or constructs that contained
Kirkwood A, Rioult MC, Bear MF (1996) Experience-dependent mod-
mutated CPEs (GFP-CPEMUT).
ification of synaptic plasticity in visual cortex. Nature 381:526–528.
Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH,
NMDAR activation and ␣-CaMKII are critical for synaptic
Kandel ER (1997) Synapse-specific, long-term facilitation of aplysia
plasticity and memory formation (Morris et al., 1986; Bear et
sensory to motor synapses: a function for local protein synthesis in
memory storage. Cell 91:927–938.
al., 1990; Silva et al., 1992; Tsien et al., 1996; Shimizu et al.,
McGrew LL, Dworkin-Rastl E, Dworkin MB, Richter JD (1989)
2000). The data presented here suggests that one signal trans-
Poly(A) elongation during Xenopus oocyte maturation is required for
duction pathway leading from NMDAR activation to an in-
translational recruitment and is mediated by a short sequence element.
Genes Dev 3:803–815.
crease in protein by a mechanism that is CPE-dependent and
Mendez R, Murthy KGK, Manley JL, Richter JD (2000) Phosphoryla-
involves cytoplasmic polyadenylation. This proposal is sup-
tion of CPEB by Eg2 mediates the recruitment of CPSF into an active
cytoplasmic polyadenylation complex. Mol Cell 6:1253–1259.
ported by recent results demonstrating NMDAR-dependent
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impair-
cytoplasmic polyadenylation in synaptic fractions and the ac-
ment of learning and blockade of long-term potentiation by an
companying activation of an Aurora kinase (Huang, Jung,
-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774–776.
Pickering AJ, Fink G (1976) Priming effect of luteinizing hormone re-
Sarkissian, and Richter, unpublished observations). Together,
leasing factor: in-vitro and in-vivo evidence consistent with its depen-
these findings link many of the key molecular elements in
dence upon protein and RNA synthesis. J Endocrinol 69:373–379.
Quinlan EM, Philpot BD, Huganir RL, Bear MF (1999) Rapid,
synaptic plasticity and establish a pathway whereby experi-
experience-dependent expression of synaptic NMDA receptors in vi-
ence-induced synaptic activation can generate new synaptic
sual cortex in vivo. Nat Neurosci 2:352–357.
protein synthesis. Knowledge of this pathway, combined with
Richter JD (1996) Dynamics of poly(A) addition and removal during
development. In: Translational control (Hershey J, Mathews M, Sonen-
other recently described translational mechanisms in dendrites
burg S, eds), pp 481–503. Cold Spring Harbor, NY: Cold Spring Harbor
(Martin et al., 1997; Scheetz et al., 2000; Aakalu et al., 2001),
Richter JD (2000) Influence of polyadenylation-induced translation on
opens the way for elucidating the molecular basis of long-term
metazoan development and neuronal synaptic function. In: Transla-
changes in synaptic efficacy. In turn, such understanding could
tional control of gene expression (Sonenberg N, Hershey J, Mathews M,
provide the insights and tools for determining the role of how
eds), pp 785–805. Cold Spring Harbor, NY: Cold Spring Harbor
synaptic modification contributes to learning and memory.
Scheetz AJ, Nairn AC, Constantine-Paton M (2000) NMDA receptor-
9548 J. Neurosci., December 15, 2001, 21(24):9541–9548
Wells et al. • NMDAR-Mediated Regulation of mRNA Translation in Neurons
-mediated control of protein synthesis at developing synapses. Nat
pocampal CA1 NMDA receptor-dependent synaptic plasticity in spa-
tial memory. Cell 87:1327–1338.
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced tran-
Ulibarri C, Yahr P (1987) Poly-A ⫹ mRNA and defeminization of sexual
scription factor activated by a diverse array of extracellular signals.
behaviour and gonadotripin secretion in rats. Physiol Behav
Annu Rev Biochem 68:821–861.
Shimizu E, Tang YP, Rampon C, Tsien JZ (2000) NMDA receptor-
Wells DG, Richter JD, Fallon JR (2000) Molecular mechanisms for
dependent synaptic reinforcement as a crucial process for memory
activity-regulated protein synthesis in the synapto-dendritic compart-
consolidation. Science 290:1170–1174.
ment. Curr Opin Neurobiol 10:132–137.
Silva AJ, Stevens CF, Tonegawa S, Wang Y (1992) Deficient hippocam-
Wu L, Wells D, Tay J, Mendis D, Abbott MA, Barnitt A, Quinlan E,
pal long-term potentiation in alpha-calcium/calmodulin kinase II mu-
Heynen A, Fallon JR, Richter JD (1998) CPEB-mediated cytoplasmic
tant mice. Science 257:201–206.
polyadenylation and the regulation of experience-dependent transla-
Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hip-
tion of alpha-CaMKII mRNA at synapses. Neuron 21:1129–1139.
Source: http://kbrin.a-bldg.louisville.edu/JournalClub2006/Pingle5.pdf
Innovative Solutions for the Energy Utility Israel Company Directory Category: Renewable EnergySub Category: Solar Thermalwww.aora-solar.com Company profileAORA (formerly EDIG Solar) has developed an advanced solar-hybrid gas-turbine engine (IP protected). Our system is the world's first commercial application of such technology, capable of producing power and heat
om Suter – Fotolia.com Pitta – der Hitzkopf und das Leben auf der Überholspur «Auf die Dauer hilft nur Power!» Die- strahlung feurig. Die oftmals rötliche Gesichtsfarbe neigt ser Spruch stammt sicherlich von einem zu Sommersprossen und Leberfl ecken. Ihr Verdauungs- feuer, Agni, ist hoch, sie haben stets Hunger und eine gute klassischen Pitta-Typ. Sein Lebensprinzip Verdauung – und können darum sehr ungehalten werden,






