Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Jocdp.jp


Contents lists available at
Brain, Behavior, and Immunity
Invited Minireview
The role of immune dysfunction in the pathophysiology of autism
Charity Onore, Milo Careaga, Paul Ashwood
Department of Medical Microbiology and Immunology, University of California, Davis, CA, USAThe Medical Investigation of Neurodevelopmental Disorders (M.I.N.D) Institute, UC Davis Health System, Sacramento, CA, USA
Autism spectrum disorders (ASD) are a complex group of neurodevelopmental disorders encompassing
Received 1 June 2011
impairments in communication, social interactions and restricted stereotypical behaviors. Although a
Received in revised form 19 August 2011
link between altered immune responses and ASD was first recognized nearly 40 years ago, only recently
Accepted 22 August 2011
has new evidence started to shed light on the complex multifaceted relationship between immune dys-
Available online 28 August 2011
function and behavior in ASD. Neurobiological research in ASD has highlighted pathways involved inneural development, synapse plasticity, structural brain abnormalities, cognition and behavior. At the
same time, several lines of evidence point to altered immune dysfunction in ASD that directly impacts
some or all these neurological processes. Extensive alterations in immune function have now been
described in both children and adults with ASD, including ongoing inflammation in brain specimens,
elevated pro-inflammatory cytokine profiles in the CSF and blood, increased presence of brain-specific
Social interactions
auto-antibodies and altered immune cell function. Furthermore, these dysfunctional immune responses
Maternal immune activation
are associated with increased impairments in behaviors characteristic of core features of ASD, in partic-ular, deficits in social interactions and communication. This accumulating evidence suggests thatimmune processes play a key role in the pathophysiology of ASD. This review will discuss the currentstate of our knowledge of immune dysfunction in ASD, how these findings may impact on underlyingneuro-immune mechanisms and implicate potential areas where the manipulation of the immuneresponse could have an impact on behavior and immunity in ASD.
Ó 2011 Elsevier Inc. All rights reserved.
increased risk of developing ASD. In addition to the heritabilityobserved in twin-pairs, the risk of developing ASD in non-twin sib-
Autism spectrum disorders (ASD) are a series of pervasive devel-
lings is increased 25-fold in comparison to the general population
opment disorders which include autistic disorder, Rett's disorder,
(). While the heritability of ASD suggests a genetic
childhood disintegrative disorder, Asperger's syndrome or pervasive
component in the disorders etiology, the genes involved vary greatly
developmental disorder not otherwise specified (PDD-NOS). Autism
among individuals and family clusters.
spectrum disorders are characterized by severe and pervasive
Whole-genome linkage studies, gene association studies, copy
impairment in several areas of development: reciprocal social inter-
number variation screening and SNP analyses have uncovered a
action skills, communication skills, or the presence of stereotyped
large number of ASD candidate genes (
behavior, interests and activities (). According to the most
Associations with ASD have been demonstrated for genes
recent estimates calculated by the US Center of Disease Control, ASD
involved in a diverse range of functions including RELN (
affects 1 in 110 children under the age of eight
), SHANK3 (), NLGN3, NLGN4X
Although current research suggests there may be no single genetic
cause for ASD, there are several lines of evidence to suggest that
(), OXTR ), and SLC6A4 (
the disorder is highly heritable. There is a concordance rate for
Furthermore, in several syndromic disorders with sin-
ASD of 0–37% reported for dizygotic twins, while concordance rates
gle gene mutations, including Rett's syndrome (MeCP2) (
of 44–91% are reported for monozygotic twins
), Fragile X (FMR1) (
tuberous sclerosis (either TSC1 or TSC2) (), Timo-
), suggesting that genetic composition may contribute to
thy syndrome (CACNA1C), Cowden's syndrome (PTEN), and Angel-man's syndrome (UBE3A) the occurrence of ASD is higher than thegeneral population. Among these potential candidate genes several
⇑ Corresponding author. Address: Department of Microbiology and Immunology,
play important roles in immune function. Proteins within the
The Medical Investigation of Neurodevelopmental Disorders (M.I.N.D) Institute,
phosphoinositide-3-kinase (PI3K) pathway, including those coded
2805, 50th Street, Sacramento, CA 95817, USA. Fax: +1 916 703 0367.
by MET, PTEN, TSC1 and TSC2, have a major role in regulating
E-mail address: (P. Ashwood).
0889-1591/$ - see front matter Ó 2011 Elsevier Inc. All rights reserved.
doi:
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
interleukin (IL)-12 production from myeloid cells and are involved
to some autistic features, including decreased prepulse inhibition
in shifting macrophage phenotypes from inflammatory (M1) to
and latent inhibition, as well as impaired sociability (reviewed in
alternative activated (M2) subsets Additional
These models are becoming more established
candidate genes including the major histocompatibility complex
and can be induced by congenital exposure to bacteria, the bac-
type 2 (MHC-II) haplotypes (),
terial compound LPS, influenza virus or, the viral mimic and toll-
as well as complement 4B (C4B) (), and macro-
phage inhibitory factor (MIF) (are impor-
[poly(I:C)]. In all four versions of the model, IL-6 appears to play
tant in directing and controlling immune responses. Even with
an essential role and exposure to IL-6 alone during gestation is
the recent advancements in identifying candidate genes involved
sufficient to elicit behavioral changes in the offspring
in ASD, all identified genetic risk factors combined account for only
The similarities between
10–20% of the total ASD population (
the behaviors seen in models of MIA and the symptoms of ASD
). A number of these genetic risk factors can also be present
have spurred further investigation into the physiological features
in individuals without ASD, suggesting that many of these muta-
of the offspring. For example, an increase in IL-6 is present up to
tions may increase the risk of developing ASD, but additional risk
24 weeks postnatally in brains of offspring of dams exposed to
factors are also necessary.
poly(I:C) (While elevated numbers of
The absence of a known genetic cause in the majority of
splenic TH17 cells have been observed in offspring after maternal
cases, and the incomplete penetrance of known genetic risk fac-
poly(I:C) exposure ). This evidence suggests
tors, suggests that environmental factors are linked with the
that in the MIA model, there are prolonged inflammatory re-
causation of ASD. Growing research has highlighted maternal
sponses that persist in adult offspring and are likely maintained
immune activation (MIA), especially during the first or second
by alterations in the immune system of the affected offspring.
trimesters of pregnancy, as one potential environmental factor
These data bear more than passing resemblance to features of
that increases the risk for ASD In 1964 a ru-
dysfunctional immune activity frequently observed in children
bella epidemic in the US which affected many pregnant mothers
and adults with ASD.
resulted in a large increase in the number of children who devel-oped ASD (). More-
2. Immune activity in ASD
over, using medical information obtained in a large Danishdatabase, increased risk for ASD is associated with mothers that
2.1. Neuroinflammation
required hospitalization for a viral infection in the first trimesterof pregnancy, or mothers hospitalized for a bacterial infection in
A key finding in ASD research has been the observations of
the second trimester of pregnancy (), sug-
marked ongoing neuroinflammation in postmortem brain speci-
gesting that bacterial and viral infections may confer different
mens from individuals with ASD over a wide range of ages (4–
risks depending on gestational age. Further data suggest that
45 years of age)
season of birth is important, with increased rates of ASD associ-
These findings include prominent microglia activation
ated with experiencing the first trimester of pregnancy during
and, increased inflammatory cytokine and chemokine production,
the winter months, timing which coincided with the influenza
including interferon (IFN)-c, IL-1b, IL-6, IL-12p40, tumor necrosis
season ). Increased psoriasis, asthma and aller-
factor (TNF)-a and chemokine C–C motif ligand (CCL)-2 in the
gies during pregnancy have also been suggested as risk factors
brain tissue and cerebral spinal fluid
for the development of ASD
The potential role of a heightened or activated maternal im-
Microglia are the resident mononuclear phagocytic cells of the
mune response in the risk for ASD is further strengthened by
CNS, and participate in immune surveillance of the CNS as well
epidemiological data from large population based studies that
as synaptic pruning in normal neurodevelopment
show increased rates of autoimmune disorders in the families
Microglia are also activated in the postmortem brain speci-
of individuals with ASD (
mens of individuals with other neurological diseases of unknown
). Separately or coincidentally, the presence of specific
genetic etiology such as Multiple Sclerosis, Alzheimer's and Parkin-
anti-fetal brain antibodies in approximately 12% of mothers of
son's Expression profiling of postmortem
children with ASD, which are absent in mothers of children
brain tissue from ASD individuals revealed increased messenger
who are typically developing or mothers of children with devel-
RNA transcript levels of several immune system associated genes,
opmental delays, suggests a potential inflammatory process that
further implicating neuroinflammatory processes in this disorder
leads to the production of antibodies directed to the developing
). Moreover, a recent study looking at tran-
scriptome organization patterns showed that gene co-expression
). Such fetal brain-specific antibodies could alter
networks reflect abnormalities in cortical patterning in the brain
neurodevelopment as is seen in systemic lupus erythematosis
of ASD individuals (These findings were
(SLE) (). In experiments
associated with changes in microglia and immune activation; sug-
using IgG collected from mothers of children with ASD, admin-
gesting a causative role for immune dysregulation in ongoing neu-
istration of these antibodies to pregnant rhesus macaques, in-
rological dysfunction and synapse plasticity in the brains of
duced stereotypic behavior and hyperactivity in the offspring,
individuals with ASD.
symptoms that share homology to ASD ).
Similarly, anti-brain protein reactive antibodies from motherswho have children with ASD mediate behavioral changes and
2.2. Systemic immune activation
neuro-pathology in the offspring of pregnant dams that are in-jected with these antibodies ). These data
As well as signs of neuroinflammation in ASD, there are mul-
suggest a potential pathogenic/pathological effect of anti-fetal
tiple lines of evidence indicating that immune responses in the
brain antibodies in some mothers who have children that devel-
periphery are also dysfunctional and are associated with in-
creased severity of core and related symptoms of ASD
In rodent models of MIA, several abnormal behavioral fea-
Immune abnormalities were first described in individuals with
tures are exhibited in the offspring that may have face validity
ASD in 1977 Since this report
Table 1Immune dysfunction and behaviors in ASD.
Studies in chronological
Ratio of CD4 to CD8 positive T
Administration of naltrexone led to reduction of ‘‘autistic'' symptomology and increased ratio of CD4 to CD8 cells
No formal testing
immunoglobulin levels
IVIG resulted in improvements in eye contact, calmer behavior, improvement in speech and echolalia
No formal testing
IVIG treatment resulted in transient improvements in attention span and hyperactivity; improvement declined
Improvements in communication and asocial behaviors after vancomycin treatment, but were lost after conclusionof treatment
No formal testing
Treatment with prednisone resulted in marked improvement in social and communication skills
Fewer aberrant behaviors were recorded for subjects with fever (>100.4°F) compared with controls. Improvements
were transient.
ADI-R, ADOS, SCQ,
Plasma levels of active TGFb1
Lower TGFb1 levels were associated with lower adaptive behaviors and worse behavioral symptoms
Plasma levels of P-Selectin
Lower levels of P-Selectin associated with poor social development
IgG levels in plasma
Decreased IgG associated with increased aberrant behaviors
Genotyping of the MIF gene,
Plasma MIF levels were positively correlated with worse scores on ADOS for social impairment and imaginative
and plasma levels of MIF
Induced cytokine response to
Negative correlation between PHA induced IL-23 production and sociability scores of the ADOS
ADI-R, ADOS, SCQ,
Monocyte TLR ligand
More impaired social behaviors and non-verbal communication are associated with increased production of IL-1b
and IL-6 after TLR4 stimulation
ADI-R, ADOS, SCQ,
Induced cytokine response to
Pro-inflammatory or TH1 cytokines were associated with greater impairments in core features of ASD as well as
aberrant behaviors; GM-CSF and TH2 cytokines were associated with better cognitive and adaptive function
ADI-R, ADOS, SCQ,
Antibodies directed against a
Children with antibodies directed against a 45 kDa cerebellum protein had increased, lethargy and stereotypy;
45 or 62 kDa cerebellum
children with antibodies against a 62 kDa cerebellum protein showed increased aberrant behaviors on the VABS
composite standard score
Serum levels of PDGF
Increased serum levels of PDGF-BB homodimers positively associated with increased restricted, repetitive andstereotyped patterns of behavior and interests
ADI-R, ADOS, SCQ,
Plasma chemokines CCL2, CCL5
Plasma chemokine levels associated with higher aberrant behavior scores and more impaired developmental and
adaptive function
ADI-R, ADOS, SCQ,
Plasma levels of cytokines IL-
Elevated cytokine levels in plasma were associated with more impaired communication and aberrant behaviors
1b, IL-6, IL-8 and IL-12p40
BSE = behavior summarized evaluation; ABC = aberrant behavior checklist; CARS = childhood autism rating scale; ADI-R = autism diagnostic interview, revised; ADOS = autism diagnostic observation schedule; SCQ = socialcommunication questionnaire; VABS = vineland adaptive behavior scale; GI = gastrointestinal; MIF = macrophage migration inhibitory factor; PDGF = platelet derived growth factor; n.s. = not significant.
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
several research groups, from around the world, have identified
interaction and communication, as well as associated features
a variety of immune functions that are atypical in ASD; yet,
such as aberrant behaviors (
not surprisingly, these findings are often as heterogeneous as
The occurrence of a differential antibody repertoire has been
the behavioral phenotypes which make up ASD.
studied extensively in ASD. For example, decreased total levels of
Proteomic analysis indicates that the levels of many immune
IgM and IgG classes of immunoglobulin have been reported, with
proteins in plasma/sera, such as cytokines, chemokines, comple-
lower levels found to correlate with more aberrant behaviors
ment proteins, adhesion molecules and growth factors are
However, within this profile, atypical antibody iso-
altered in ASD. Notably, increased plasma levels of pro-inflam-
type levels are frequently reported in the plasma of individuals
matory cytokines such as IL-1b, IL-6, IL-8 and IL-12p40 as well
with ASD including increases in levels of the neutralizing IgG4
as MIF and platelet derived growth factor (PDGF), have been re-
ported in ASD (;
Antibodies reportedly reactive to human and non-human primate
Moreover, elevated levels of these cyto-
brain and CNS proteins have also been described in children and
kines in the plasma were found to be associated with poor com-
adults with ASD. Using Western blotting and ELISA techniques an
munication and impaired social interaction behaviors ().
increased presence of antibodies can be detected in ASD that exhi-
Plasma levels of chemokines CCL2 and CCL5 are also higher in
bit reactivity against a diverse set of targets or specificities. These
ASD and again are associated with worsening behavioral scores
targets vary between studies, many of which have not be repli-
(). In parallel, decreased circulating levels
cated but have at one time included; antibodies against serotonin
of the anti-inflammatory cytokine, transforming growth factor
receptors ), myelin basic protein
(TGF)-b, has been documented in ASD, with lower levels associ-
), heat shock proteins
ated with worsening behavioral scores (
), glial filament proteins ), as
Collectively, these data reveal a trend to-
well as various brain tissue proteins that have yet to be identified
wards pro-inflammatory immune activity and away from regula-
tory measures in ASD. As many of the cytokines have profound
). It is tempting to speculate that the increased diversity
effects on neuronal development, migration, differentiation and
and lack of a single specific target is due to antibody generation as
synapse formation (see below), a disrupted balance in the cyto-
a result of cellular damage and the emergence/revealing of seques-
kine milieu may directly influence neurodevelopment, early
tered or new epitopes. A similar finding is seen in many autoim-
brain development and alter behavior. To this end it is of note
mune diseases where diverse clonal antibody generation occurs
that the shift in cytokine balance in ASD is linked with greater
throughout the course of the disease, such as in SLE and multiple
impairments in key autism behavioral domains including social
sclerosis (MS). Whether a single antibody or cell response is
Central Nervous System
Synaptic Pruning
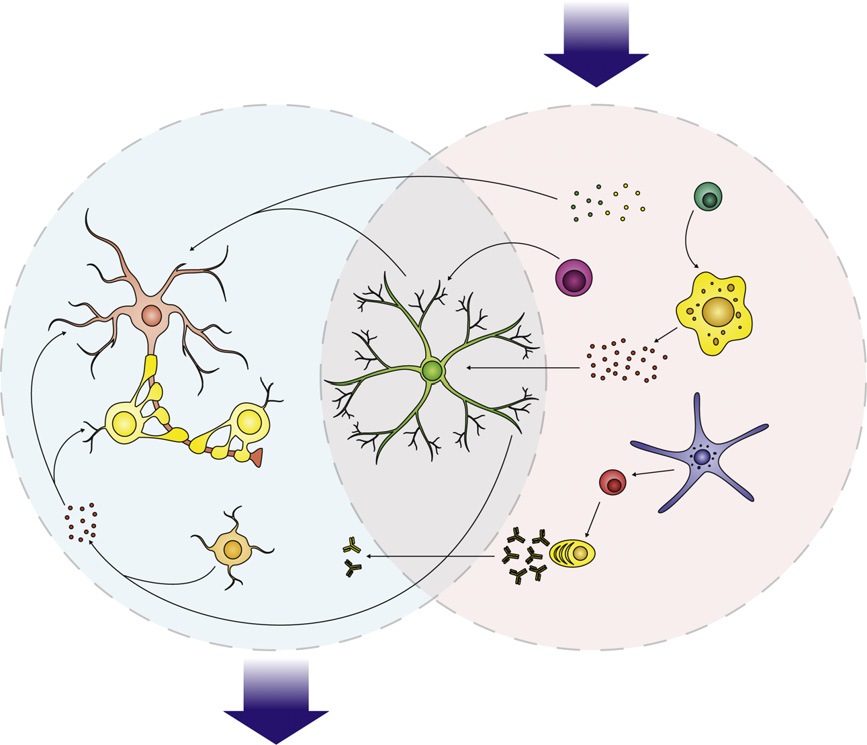
Fig. 1. Immune dysfunction in ASD involves a network of interactions between several cell types, from the innate and adaptive arms of the immune system. The CNS isselective but several immune factors mediate profound effects of CNS function. Increased cytokine production, such TNF-a and IL-1b inhibit neurogenesis and promoteneuron death, while IL-6 may promote the growth and proliferation of neurons and oligodendrocytes. Increased levels of complement proteins can participate in synapticscaling, opsonizing synapses and targeting them for removal by phagocytic microglia. Activated microglia may additionally mediate synaptic pruning via MHCI interactions.
Collectively this immune dysfunction in ASD can exert several negative effects on behavior, including impaired cognitive function, and social withdrawal as well as aberrantbehavior observed in ASD.
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
responsible for initiating this cascade or whether all the antibodies
atypical adaptive T cell responses are repeatedly observed in indi-
reactive to brain tissue hitherto observed in ASD are as a result of
viduals with ASD (A predominance of IL-4+
cell/tissue damage, requires further research. Indeed, in contrast to
IFN-c T cells was observed in the circulating CD4+ T cell popula-
that observed for the maternal antibodies (discussed above)
tion in individuals with ASD ). This bias towards
), what role auto-antibodies
a TH2 phenotype and reduced TH1 responses has been observed in a
play in children with ASD is unknown and it has yet to be demon-
number of other studies. In corroboration with this finding, we
strated whether any of these antibodies block receptor function,
observed increased mononuclear cell production of IL-13 and
activate neuronal/glial cells, induce cellular damage or have any
GM-CSF in response to PHA stimulus, while IFN-c was decreased.
Production of the pro-inflammatory cytokine TNF-a was also in-
In SLE, N-methyl-D-aspartate receptor (NMDAR) reactive anti-
creased in response to in vitro stimulation, and is consistent with
bodies are capable of mediating negative effects on cognitive func-
an activated TH2 immune response in humans. Moreover increased
tion (). Although antibody passage through
TNF-a production was associated with increased stereotypical
the BBB is restricted, these antibodies can be found in the CNS
behaviors a hallmark symptom of ASD ().
). These brain protein reactive antibod-
Analysis of intracellular cytokine production showed an increase
ies are implicitly involved in cognitive impairments in individuals
in frequency of TNF-a+ T cells but reduced frequency of IL-10+ T
who carry them, and are also suspected to exert pathological ef-
cells in both peripheral and intestinal mucosal tissue in children
fects on fetal neurodevelopment during gestation (
). It is possible that the brain protein-reactive antibodies
These studies show, once again, a shift towards a pro-inflammatory
found in the plasma of children with ASD may mediate pathology
cytokine milieu and mirror the plasma cytokine data.
in a similarly direct fashion. Some experimental evidence has
The activation profile of circulating T cell phenotypes was also
shown, that irrespective of the target epitope, antibodies from
different in ASD and CD3+ T cells display higher levels of HLA-DR,
ASD subjects bind specifically to cerebellar interneurons and golgi
a marker of late cellular activation (In addi-
type II cells in tissue obtained from rhesus macaque monkeys
tion, CD26 (dipeptidyl peptidase IV), a marker associated with an
). The binding of these antibodies to specific
effector cell phenotype in human CNS disorders such as multiple
cellular targets could lead to decreased or increased cellular activ-
sclerosis, was increased on CD8+ T cells ().
ity. Moreover, the presence of these antibodies in children with
Following in vitro stimulation an altered pattern of co-stimulatory
ASD correlated strongly with antibody reactivity observed to a
and activation markers was observed, with increased expression of
45 kD cerebellar protein of unknown identity and was positively
CD137 (4-1BB) but decreased CD134 (OX40) and CD25 (IL-2 a
associated with worsening of aberrant behavior (
receptor) on T lymphocytes of children with ASD (
). Elucidating the exact target of these cells and their function
Increased T cell activation may also be linked with de-
on neuronal cell cultures in vitro would help to establish their role
creased apoptosis leading to the survival of activated cells that
in the pathogenesis of ASD.
would otherwise be eliminated (a feature
Cell damage/death can be induced following the binding of
that has been described in chronic inflammatory conditions such
complement proteins to antibodies and may be another mecha-
as Crohn's disease (Collectively, evidence
nism in which auto-reactive antibodies contribute to ASD pathol-
of atypical cytokine production, altered T cell activation and poten-
ogy. Moreover, the complement proteins C1q and C3, which
tial impaired apoptotic activity suggest there is a predisposition to
classically make up a portion of the immune complement cas-
chronic inflammation which could negatively affect healthy cogni-
cade, are also involved in synaptic scaling. Complement mediates
tive development in ASD. Exciting findings from animal models
synaptic pruning by opsonizing synapses, effectively targeting
suggest that neurogenesis is modulated by the interaction between
them for removal by phagocytic microglia
T cells and CNS cells (). Al-
). An increase in complement proteins, including the lytic
tered T cell activation in ASD may therefore directly affect the
component C1q, has been shown in sera from children with
course of neurodevelopment. However, whether the T cells that
ASD compared to age, ethnicity and gender matched typically
are active in the periphery in ASD also interact with CNS tissue is
developing children Co-localization of IgG
and C1q has also been reported in the mucosa of children with
Adhesion molecules known to control the passage of T cells
ASD (). Whether the antibodies spe-
across endothelial barriers play an important role in mediating
cific for CNS proteins are the same as those in the mucosa and
T cell passage and T cell/CNS interactions. Circulating levels of
whether they are capable of fixing complement and eliciting cel-
soluble adhesion molecules P-Selectin, L-Selectin and PECAM-1
lular damage in ASD is not known. In particular, the potential
accurately represent levels on endothelial cells. In high function-
interaction between auto-antibodies specific for GABAergic inter-
ing individuals with ASD levels of sPECAM-1, sP-Selectin and sL-
neurons and complement in children with ASD requires further
selectin were decreased compared with controls
investigation. Increased complement production in ASD may
Furthermore, lower levels of P-
therefore modulate neuronal function in several ways either by
Selectin were associated with more impaired social skills
synapse pruning or through an interaction with specific auto-
). These data suggest that modulating immune cell
reactive antibodies leading to cell death.
access to the brain in ASD may influence abnormal social inter-actions. In line with these findings, during fever episodes, some
2.3. Adaptive cellular response
children with ASD show a transient improvement in behaviorsthat diminishes back to baseline after the child's fever improves
The examination of immune cell function in ASD has been hin-
(During fever, upregulation of adhesion mol-
dered by problems arising from study design including the use of
ecules and changes in endothelial barriers occurs as a result of
small sample sizes, variable diagnostic criteria, non-matching of
pyrogenic cytokine release. In ASD it is possible that fever may
cases and controls for gender or age and, the use of unevaluated
evoke a transient increase in T cell–brain interactions and hence
siblings as controls. Such issues have plagued the field and led to
an improvement in behavior. These data are provocative and
confusion in the interpretation of the various study findings. De-
suggest that immune activation, including activation of T-lym-
spite these drawbacks many studies have observed reproducible
phocyte subsets, could be important in improving behaviors in
findings of altered cellular function in ASD. Among these findings
some individuals with ASD.
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
2.4. Innate cellular response
a profile representative of myeloid cell activation, i.e. the produc-tion of IL-12p40, TNFa, IL-1b and IL-6
As well as changes in adaptive immune responses the activity of
a number of other cell subsets has been described, including atyp-ical natural killer (NK) cell activity. Reduced ability of NK cells to
3. Potential impact of immune dysfunction in ASD on CNS
kill K562 target cells in ASD was first described by
activity and behavior
and has now been confirmed in more contemporary reports(In addition to reduced
Although a singular pathology of ASD remains elusive, a wealth
lytic activity, changes in several factors that contribute to NK cell
of evidence suggests that ASD symptoms may be related to im-
activity such as perforin, granzyme B and IFN-c have been identi-
mune dysfunction (
fied. NK cells appear to produce higher levels of perforin, granzyme
). Further detailed investigations are needed
B, and IFN-c while under resting conditions in children with ASD
to concretely identify whether the immunological findings in ASD
(These data suggest that in vivo, there is in-
converge to a single immunopathology. However, in the following
creased NK cell activation; however, following a strong in vitro sig-
section we will try and identify potential mechanisms of action in
nal, such as with target cells, NK cells from children with ASD are
which the observed immune dysfunction in ASD could impact neu-
unable to produce more of their effector molecules thus leading to
ronal function and behavior in ASD.
the reduced capability to lyse the targets ).
Activity of the immune system can elicit profound effects on
As early responders of the innate immune system, NK cells help
behavior (). Several immune proteins function within the
shape the initial immune response during an inflammatory event
nervous system as mediators of normal neurodevelopment (
and aberrant activity of these cells are likely an important contrib-
). Cytokines, such as TNF-a, IL-1b, the
utor to the atypical immune activity observed in individuals with
TGF-b family of molecules, and the gp130 ligand family mediate di-
rect effects on neuronal activity. For example, TNF-a is produced
Monocytes are among the first responders during inflammation,
by wide variety of cells during an inflammatory event (
and are robust cytokine producers, creating a cytokine milieu that
and, in addition to its role in inflammation, can mod-
profoundly influences the activity of neighboring immune cells.
ulate neuronal cell proliferation or cell death, and play an impor-
Monocytes also serve as precursors for a number of tissue specific
tant role in synaptic pruning
myeloid lineage cells including macrophages, dendritic cells, and
Other neuropoeitic cytokines,
microglia (). An increased
such as IL-1b and IL-6, also exert varied affects on neuronal sur-
number of circulating monocytes has been reported in ASD (
vival, proliferation, synapse formation, migration, and differentia-
). Furthermore, upregulation of activation markers on
tion. IL-6 is member of the gp130 ligand family that includes
circulating CD14+ monocytes suggest that these cells are activated
IL-11, ciliary neurotrophic factor (CNTF), oncostatin M, and leuko-
in vivo In research conducted in our labo-
cyte inhibitory factor (LIF), that play important roles in promoting
ratory, an atypical pattern of cytokine responses were observed
and maintaining oligodendrocyte (ODC) survival in the CNS
following TLR agonist stimulation of isolated CD14+ monocytes
). Generation of IL-6 knock-out mouse
from young children with ASD (Specifically,
models results in viable offspring; however, they display impaired
increased inflammatory cytokine production, IL-1b, TNF-a and IL-
recognition memory, suggesting an essential role for IL-6 in learn-
6, was observed in response to the TLR2 ligand, lipoteichoic acid
ing Both IL-6 and IL-1b
(LTA) and to a lesser extent the TLR4 ligand, LPS. Conversely, re-
are involved in mediating ‘‘sickness behavior,'' an adaptive change
duced cytokine production was observed in response to a TLR9
in behaviors accompanying inflammation that are characterized by
agonist, an unmethylated CpG repeat synthetic oligonucleotide,
lethargy, depression, loss of appetite, anxiety, impaired ability to
resembling bacterial DNA. Furthermore, increased monocyte IL-6
focus and social withdrawal (Notably, the so-
and IL-1b production in response to LPS stimulation was associated
cial withdrawal of sickness behavior can be alleviated when ani-
with more impaired social behavior in individuals with ASD
mals are given minocycline, a broad spectrum antibiotic with
These atypical monocyte responses are intriguing, and indi-
anti-inflammatory qualities (
cate abnormal myeloid involvement in ASD. Hyperactivation of
In addition, the cytokines IL-2 and IL-4 have been shown to
myeloid cells in ASD is implicated in both the periphery and CNS,
influence repetitive and cognitive behaviors. Treatment of mice
as increased infiltration of monocytes and perivascular macro-
with IL-2 results in increased ‘‘climbing behavior'' that are thought
phages are observed in brain specimens from individuals with
to denote repetitive behaviors, a pattern of behaviors that are
ASD (). Moreover, there is a striking similarity
characteristic of ASD (IL-4 knock-out mice show
in cytokine levels in the plasma of children with ASD that exhibit
impaired cognition, possibly as a result of loss of T cell function
Table 2Influence of cytokines on behaviors with relevance to ASD.
CNS effects relevant to the symptoms of ASD
� Sustained expression of IL-1b in hippocampus impairs spatial memory (� IL-1b promotes the adaptive stress responses )� Blockage of IL-1b results in a reversible impairment of long-term potentiation and can alter synaptic plasticity � IL-1 alters sleep patterns � Sickness behavior – social withdrawal )
� IL-2 treatment results in increased climbing (repetitive) behaviors, which are mediated by dopaminergic processes ()
� IL-4 KO mice show cognitive impairments (
� IL-6 KO mice display impaired recognition memory )� Increased plasma levels of IL-6 in subjects with depression (Musselman et al., 2001)
� TNF-a induces cell death on neurons, and is thought to play an important role in synaptic pruning ()� Sickness behavior - Social withdrawal )
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
in the CNS (). There is some evidence in individ-
Peripheral and or neuronal production of cytokines
uals with ASD that cognition is impaired; however, largely due to
could also lead to an activated profile in these cells. For example,
the deficits in verbal and non-verbal communication as well as
increased GM-CSF production has been detected in ASD and will
poor social interaction, the specific nature of these cognitive issues
drive the release of myeloid progenitor cells that can become tissue
has been hard to define and remain somewhat controversial.
macrophages (). In
Collectively these findings suggest that cytokines are both nec-
turn the production of cytokines from microglia and macrophages
essary for normal neurodevelopment and behavior and that any
will influence neuronal survival, proliferation, function and synap-
perturbation in the cytokine network can impact neurodevelop-
tic plasticity .
ment . Observed increases in TNF-a, IL-1b and IL-6 in the
Furthermore, perhaps due to their activities as immunological
blood, CSF and brain tissues from children with ASD represents
sentinels of the CNS, or their role in synaptic pruning, genetic
the first piece of the puzzle of a disrupted neuro-immune network.
abnormalities in microglia can result in profound effects on behav-
Associations with greater impairment in core autistic behaviors
ior. Recently, a landmark paper described the source of repetitive
and increased cytokine levels highlight a potential new avenue of
grooming behavior in Hoxb8 / mice ().
research in which cytokine patterns could be manipulated to ben-
The Hoxb8 protein is expressed exclusively in bone marrow-de-
efit behavioral outcome in ASD. For example, minocycline has been
rived microglia in the CNS, and deficiency in this protein results
shown to successfully alleviate aberrant behavior symptoms in
in reduced numbers of bone marrow-derived microglia cells within
children with Fragile X, a neurodevelopmental disorder with high
the CNS. Transplantation of bone marrow containing functional
rates of ASD ).
Hoxb8 was sufficient to restore normal grooming behavior in these
In ASD, linkage with specific MHC molecules has been fre-
mice, providing solid evidence of the role of microglia cells in the
quently reported (
atypical grooming behavior of Hoxb8 / mice ).
). In addition, two large GWAS studies in schizophrenia high-
These data also suggests that atypical microglia function plays a
light changes within the MHC region on chromosome 6 (
role in repetitive behaviors, a characteristic of several disorders
These data suggest that abnormali-
including ASD. In addition, DAP12 and the triggering receptor ex-
ties in the expression of MHC genes and their effects on brain
pressed on myeloid cells 2 (TREM2) protein form a complex in
development and synaptic function may be involved in the patho-
myeloid cells which is reportedly exclusively expressed in microg-
genesis of complex neurodevelopmental disorders such as ASD and
lia cells within the CNS (This complex is essen-
schizophrenia. Until relatively recently neurons were considered to
tial for the phagocytosis of apoptotic cells, and for limiting
be negative for the MHC I molecules; however, emerging data sup-
inflammatory cytokine production in the CNS
port the role of these molecules in CNS development
A loss of function mutation of genes encoding either TREM2
MHCI is expressed on virtually every cell
or DAP12 results in Nasu–Hakola disease, also known as polycystic
type within the body, and can display cytosolic proteins to CD8+ T
lipomembranous osteodysplasia with sclerosing leukoencephalop-
cells, alerting them to the presence of non-self proteins, for exam-
athy (PLOSL). PLOSL results in cysts within the bone, and early on-
ple, during a viral infection. In the CNS, MHCI plays a dual role in
set dementia (). The disorder is characterized
regulating synaptic scaling, likely by engaging with a CD3f or PIRB
by severe microglia activation in brain tissue. The example of
molecules MHCI is expressed in the CNS under non-
PLOSL suggests that unchecked microglia driven inflammation
inflammatory conditions, and correlates with times and regions of
can contribute to cognitive degeneration, and bears some resem-
synaptic plasticity ). Double knock-out of the
blance to the microglia activation observed in individuals with
b-2-microglobulin (b2m) subunit of the MHCI molecule, and the
ASD. A number of genetic risk factors for ASD such as MET and
transporter with antigen processing (TAP) protein, virtually elimi-
MIF, are known to directly impact the function of myeloid cells
nates all allotypes of MHCI from the cell surface
and their activation status and if these cells are skewed towards
The elimination of functional MHCI results in
a tolerance or pro-inflammatory inducing phenotype. Further
atypical synaptic plasticity in animal models, further illustrating
investigation of genes that control microglia function in ASD could
the essential role for MHCI in synapse remodeling (
provide clues to the pathogenesis and potential treatment of this
Thus, the role of MHC in ASD may be twofold, firstly,
by their ability to modulate synapse formation during develop-
Inflammatory activity of microglia may be influence by polari-
ment and, secondly, in their role in shaping the T cell repertoire
zation towards an inflammatory (M1) or alternatively activated
and the specificity, diversity and conformation of antigens that
(M2) phenotype. Recently, in a model of T cell deficiency and cog-
are presented to T cells.
nitive impairment, administration of M2 microglia was beneficial
Microglial cells are uniquely positioned to robustly respond to
to cognitive performance (The recent finding
immune signals, and influence the CNS environment, through the
of altered transciptome profiles in microglia in brain specimens
production of inflammatory cytokines and the generation of reac-
from individuals with ASD also suggests
tive oxygen species (ROS) within the CNS (
that environmental factors may play a role in the activation of
The presence of activated
these cells. TH2 cells generate IL-4, and IL-10, skewing the microen-
perivascular macrophages and microglia have been described in
vironment and potentially polarize neighboring microglia into an
brain specimens in ASD
alternative M2 phenotype (). In
); however, what the cause of this hyperactive
ASD the data suggest that a TH2 cytokine profile may predominate
state is still unknown. The phagocytosis of dead or dying neurons
(and may thereby skew
by microglia is believed to be a normal and relatively non-inflam-
the microglia/macrophage response. This data highlights the
matory function ). However, upon phagocytosis
importance of all arms of the immune system in immune regula-
of antibody-bound targets, microglia produce increased inflamma-
tion within the CNS, and the bi-directional regulation between
tory cytokines and ROS The potentially pro-
the adaptive and innate immune system required to maintain a
inflammatory activity of microglia in ASD could be associated with
healthy neuro-immune environment, that may be dysfunctional
the presence of brain-reactive antibodies in the CNS. Similarly, dys-
regulated complement production in ASD may have a similar effect
as complement opsoniza-
(especially affect, social functioning and cognition) evidence of
tion can mediate the phagocytosis of neurons by microglia (
extensive immune dysfunction suggest other systems are also
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
disrupted. The findings so far point towards a disruption of many
evidence of immune dysfunction and are associated with impaired behavioral
facets of the immune responses including polymorphisms in im-
outcome. Brain Behav. Immun. 25, 40–45.
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I.N., Van de Water,
mune genes that control and regulate function of the immune cells,
J., 2011c. Altered T cell responses in children with autism. Brain Behav. Immun.
microglia and astroglia activation, the production of pro-inflamma-
25 (5), 840–849.
tory cytokines, increased presence of CNS reactive antibodies, T cell
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I.N., Van de Water,
J., 2011d. Associations of impaired behaviors with elevated plasma chemokines
activation and innate immune activation. Research into the im-
in autism spectrum disorders. J. Neuroimmunol. 232, 196–199.
mune connections in ASD is still in its infancy and, at this stage,
Atladottir, H.O., Pedersen, M.G., Thorsen, P., Mortensen, P.B., Deleuran, B., Eaton,
centralizing the findings so far into one unifying theory is difficult
W.W., Parner, E.T., 2009. Association of family history of autoimmune diseasesand autism spectrum disorders. Pediatrics 124, 687–694.
and will only be achieved through further analysis of immune dys-
Atladottir, H.O., Thorsen, P., Ostergaard, L., Schendel, D.E., Lemcke, S., Abdallah, M.,
function in individuals with ASD.
Parner, E.T., 2010. Maternal infection requiring hospitalization duringpregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40, 1423–1430.
Baier, P.C., May, U., Scheller, J., Rose-John, S., Schiffelholz, T., 2009. Impaired
hippocampus-dependent and -independent learning in IL-6 deficient mice.
Behav. Brain Res. 200, 192–196.
The collective findings of immune aberration in ASD, and the
Bailey, A., Le Couteur, A., Gottesman, I., Bolton, P., Simonoff, E., Yuzda, E., Rutter, M.,
effects of immune dysfunction in normal neurodevelopment, are
1995. Autism as a strongly genetic disorder: evidence from a British twin study.
difficult to ignore. Despite several early challenges the evidence
Psychol. Med. 25, 63–77.
Belmonte, M.K., Bourgeron, T., 2006. Fragile X syndrome and autism at the
of immune cell dysfunction in ASD has continued to grow. In con-
intersection of genetic and neural networks. Nat. Neurosci. 9, 1221–1225.
junction, recent basic research has provided further evidence of
Bessis, A., Bechade, C., Bernard, D., Roumier, A., 2007. Microglial control of neuronal
how the immune system can profoundly impact neurodevelop-
death and synaptic properties. Glia 55, 233–238.
ment, cognitive function, and behavior. The dysfunctional immune
Braunschweig, D., Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Croen,
L.A., Pessah, I.N., Van de Water, J., 2008. Autism: maternally derived antibodies
activity observed in ASD spans both innate and adaptive arms of
specific for fetal brain proteins. Neurotoxicology 29, 226–231.
the immune system, and suggest perturbations in either area
Buxbaum, J.D., Silverman, J.M., Smith, C.J., Greenberg, D.A., Kilifarski, M., Reichert, J.,
may have profound effects on neurodevelopment. Cytokines that
Cook Jr., E.H., Fang, Y., Song, C.Y., Vitale, R., 2002. Association between a GABRB3polymorphism and autism. Mol. Psychiatry 7, 311–316.
have been observed at atypical levels in ASD, including the brain
Cabanlit, M., Wills, S., Goines, P., Ashwood, P., Van de Water, J., 2007. Brain-specific
tissue, CSF, circulating blood, and GI tissues, can alter neuronal sur-
autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann.
vival and proliferation. Similarly, cellular dysfunction observed in
N. Y. Acad. Sci. 1107, 92–103.
Cacci, E., Claasen, J.H., Kokaia, Z., 2005. Microglia-derived tumor necrosis factor-
ASD may contribute to atypical CNS function in a number of ways
alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J.
including the production of cytokines, abnormal cell lysis and gen-
Neurosci. Res. 80, 789–797.
eration of brain-reactive antibodies. Abnormal levels of comple-
Campbell, D.B., Sutcliffe, J.S., Ebert, P.J., Militerni, R., Bravaccio, C., Trillo, S., Elia, M.,
Schneider, C., Melmed, R., Sacco, R., Persico, A.M., Levitt, P., 2006. A genetic
ment proteins and linkage to specific MHC molecules, have been
variant that disrupts MET transcription is associated with autism. Proc. Natl.
repeatedly observed as in ASD, and may suggest a role for in syn-
Acad. Sci. USA 103, 16834–16839.
aptic pruning/plasticity in ASD.
Careaga, M., Van de Water, J., Ashwood, P., 2010. Immune dysfunction in autism: a
pathway to treatment. Neurotherapeutics 7, 283–292.
The underlying cause of immune abnormalities in ASD may ex-
Chen, S.K., Tvrdik, P., Peden, E., Cho, S., Wu, S., Spangrude, G., Capecchi, M.R., 2010.
tend from genetic to maternal immune activation, or any number
Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141,
of unknown causes. Data collected thus far, suggests a wide rang-
ing effect of immune dysfunction on behavioral outcome in ASD.
Chess, S., Fernandez, P., Korn, S., 1978. Behavioral consequences of congenital
rubella. J. Pediatr. 93, 699–703.
Due to the heterogeneous nature of ASD, it is unlikely that all im-
Constantino, J.N., Todd, R.D., 2000. Genetic structure of reciprocal social behavior.
mune perturbations stem from a single origin; however, this can-
Am. J. Psychiatry 157, 2043–2045.
not discount that they share some key commonalities that have a
Corbett, B.A., Kantor, A.B., Schulman, H., Walker, W.L., Lit, L., Ashwood, P., Rocke,
D.M., Sharp, F.R., 2007. A proteomic study of serum from children with autism
pathological effect on behavior and which is not yet fully under-
showing differential expression of apolipoproteins and complement proteins.
stood. While a panacea for such a heterogeneous disorder may
Mol. Psychiatry 12, 292–306.
never exist, evidence suggests that manipulation of the immune
Corriveau, R.A., Huh, G.S., Shatz, C.J., 1998. Regulation of class I MHC gene
expression in the developing and mature CNS by neural activity. Neuron 21,
response could improve core features of ASD as well as associated
aberrant behaviors. A firm understanding of immune dysfunction
Croen, L.A., Grether, J.K., Yoshida, C.K., Odouli, R., Van de Water, J., 2005. Maternal
in ASD will be essential to develop such therapies. Identifying a
autoimmune diseases, asthma and allergies, and childhood autism spectrumdisorders: a case-control study. Arch. Pediatr. Adolesc. Med. 159, 151–157.
convergent effect of immune function on neurodevelopment and
Croen, L.A., Braunschweig, D., Haapanen, L., Yoshida, C.K., Fireman, B., Grether, J.K.,
behavioral symptoms should be a focus future research.
Kharrazi, M., Hansen, R.L., Ashwood, P., Van de Water, J., 2008. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autismstudy. Biol. Psychiatry 64, 583–588.
Croonenberghs, J., Wauters, A., Devreese, K., Verkerk, R., Scharpe, S., Bosmans, E.,
Egyed, B., Deboutte, D., Maes, M., 2002. Increased serum albumin, gamma
Abrahams, B.S., Geschwind, D.H., 2008. Advances in autism genetics: on the
globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol. Med. 32,
threshold of a new neurobiology. Nat. Rev. Genet. 9, 341–355.
APA, 2000. Diagnostic and Statistical Manual of Mental Disorders. American
Curran, L.K., Newschaffer, C.J., Lee, L.C., Crawford, S.O., Johnston, M.V., Zimmerman,
Psychiatric Publishing, Inc., Arlington, VA.
A.W., 2007. Behaviors associated with fever in children with autism spectrum
Ashwood, P., Wakefield, A.J., 2006. Immune activation of peripheral blood and
disorders. Pediatrics 120, e1386–e1392.
mucosal CD3+ lymphocyte cytokine profiles in children with autism and
Daws, M.R., Lanier, L.L., Seaman, W.E., Ryan, J.C., 2001. Cloning and characterization
gastrointestinal symptoms. J. Neuroimmunol. 173, 126–134.
of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol.
Ashwood, P., Anthony, A., Torrente, F., Wakefield, A.J., 2004. Spontaneous mucosal
31, 783–791.
lymphocyte cytokine profiles in children with autism and gastrointestinal
Dantzer, R., O'Connor, J.C., Freund, G.G., Johnson, R.W., Kelley, K.W., 2008. From
symptoms: mucosal immune activation and reduced counter regulatory
inflammation to sickness and depression: when the immune system subjugates
interleukin-10. J. Clin. Immunol. 24, 664–673.
the brain. Nat Rev Neurosci 9, 46–56.
Ashwood, P., Enstrom, A., Krakowiak, P., Hertz-Picciotto, I., Hansen, R.L., Croen, L.A.,
DeGiorgio, L.A., Konstantinov, K.N., Lee, S.C., Hardin, J.A., Volpe, B.T., Diamond, B.,
Ozonoff, S., Pessah, I.N., Van de Water, J., 2008. Decreased transforming growth
2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2
factor beta1 in autism: a potential link between immune dysregulation and
glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193.
impairment in clinical behavioral outcomes. J. Neuroimmunol. 204, 149–153.
Derecki, N.C., Cardani, A.N., Yang, C.H., Quinnies, K.M., Crihfield, A., Lynch, K.R.,
Ashwood, P., Corbett, B.A., Kantor, A., Schulman, H., Van de Water, J., Amaral, D.G.,
Kipnis, J., 2010. Regulation of learning and memory by meningeal immunity: a
2011a. In search of cellular immunophenotypes in the blood of children with
key role for IL-4. J. Exp. Med. 207, 1067–1080.
autism. PLoS One 6, e19299.
Derecki, N.C., Quinnies, K.M., Kipnis, J., 2011. Alternatively activated myeloid (M2)
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I., Van de Water, J.,
cells enhance cognitive function in immune compromised mice. Brain Behav.
2011b. Elevated plasma cytokines in autism spectrum disorders provide
Immun. 25, 379–385.
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
Deverman, B.E., Patterson, P.H., 2009. Cytokines and CNS development. Neuron 64,
Takei, Mori, N., 2010. Serum levels of platelet-derived growth factor BB
Djukic, M., Mildner, A., Schmidt, H., Czesnik, D., Bruck, W., Priller, J., Nau, R., Prinz,
Neuropsychopharmacol. Biol. Psychiatry 34, 154–158.
M., 2006. Circulating monocytes engraft in the brain, differentiate into
Kates, W.R., Burnette, C.P., Eliez, S., Strunge, L.A., Kaplan, D., Landa, R., Reiss, A.L.,
microglia and contribute to the pathology following meningitis in mice. Brain
Pearlson, G.D., 2004. Neuroanatomic variation in monozygotic twin pairs
129, 2394–2403.
discordant for the narrow phenotype for autism. Am. J. Psychiatry 161, 539–
Enstrom, A., Krakowiak, P., Onore, C., Pessah, I.N., Hertz-Picciotto, I., Hansen, R.L.,
Van de Water, J.A., Ashwood, P., 2009a. Increased IgG4 levels in children with
Kim, S.U., de Vellis, J., 2005. Microglia in health and disease. J. Neurosci. Res. 81,
autism disorder. Brain Behav. Immun. 23, 389–395.
Enstrom, A.M., Lit, L., Onore, C.E., Gregg, J.P., Hansen, R.L., Pessah, I.N., Hertz-
Korade, Z., Mirnics, K., 2011. Gene expression: the autism disconnect. Nature 474,
Picciotto, I., Van de Water, J.A., Sharp, F.R., Ashwood, P., 2009b. Altered gene
expression and function of peripheral blood natural killer cells in children with
Lee, J.Y., Huerta, P.T., Zhang, J., Kowal, C., Bertini, E., Volpe, B.T., Diamond, B., 2009.
autism. Brain Behav. Immun. 23, 124–133.
Neurotoxic autoantibodies mediate congenital cortical impairment of offspring
Enstrom, A.M., Van de Water, J.A., Ashwood, P., 2009c. Autoimmunity in autism.
in maternal lupus. Nat. Med. 15, 91–96.
Curr. Opin. Invest. Drugs 10, 463–473.
Lee, L.C., Zachary, A.A., Leffell, M.S., Newschaffer, C.J., Matteson, K.J., Tyler, J.D.,
Enstrom, A.M., Onore, C.E., Van de Water, J.A., Ashwood, P., 2010. Differential
Zimmerman, A.W., 2006. HLA-DR4 in families with autism. Pediatr. Neurol. 35,
monocyte responses to TLR ligands in children with autism spectrum disorders.
Brain Behav. Immun. 24, 64–71.
Li, X., Chauhan, A., Sheikh, A.M., Patil, S., Chauhan, V., Li, X.M., Ji, L., Brown, T., Malik,
Evers, M., Cunningham-Rundles, C., Hollander, E., 2002. Heat shock protein 90
M., 2009. Elevated immune response in the brain of autistic patients. J.
antibodies in autism. Mol. Psychiatry 7 (Suppl. 2), S26–S28.
Neuroimmunol. 207, 111–116.
Fairweather, D., Cihakova, D., 2009. Alternatively activated macrophages in
Mandal, M., Marzouk, A.C., Donnelly, R., Ponzio, N.M., 2011. Maternal immune
infection and autoimmunity. J. Autoimmun. 33, 222–230.
stimulation during pregnancy affects adaptive immunity in offspring to
Fukao, T., Tanabe, M., Terauchi, Y., Ota, T., Matsuda, S., Asano, T., Kadowaki, T.,
promote development of TH17 cells. Brain Behav. Immun. 25, 863–871.
Takeuchi, T., Koyasu, S., 2002. PI3K-mediated negative feedback regulation of IL-
Martin, L.A., Ashwood, P., Braunschweig, D., Cabanlit, M., Van de Water, J., Amaral,
12 production in DCs. Nat. Immunol. 3, 875–881.
D.G., 2008. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG
Garbett, K., Ebert, P.J., Mitchell, A., Lintas, C., Manzi, B., Mirnics, K., Persico, A.M.,
from mothers of children with autism. Brain Behav. Immun. 22, 806–816.
2008. Immune transcriptome alterations in the temporal cortex of subjects with
McAllister, A.K., van de Water, J., 2009. Breaking boundaries in neural-immune
autism. Neurobiol. Dis. 30, 303–311.
interactions. Neuron 64, 9–12.
Garden, G.A., Moller, T., 2006. Microglia biology in health and disease. J.
MMWR, 2009. Prevalence of autism spectrum disorders – autism, developmental
Neuroimmune Pharmacol. 1, 127–137.
disabilities monitoring network, United States. MMWR Surveill. Summ. 58, 1–
Gasque, P., Neal, J.W., Singhrao, S.K., McGreal, E.P., Dean, Y.D., Van, B.J., Morgan, B.P.,
2002. Roles of the complement system in human neurodegenerative disorders:
Moessner, R., Marshall, C.R., Sutcliffe, J.S., Skaug, J., Pinto, D., Vincent, J.,
pro-inflammatory and tissue remodeling activities. Mol. Neurobiol. 25, 1–17.
Zwaigenbaum, L., Fernandez, B., Roberts, W., Szatmari, P., Scherer, S.W., 2007.
Geissmann, F., Manz, M.G., Jung, S., Sieweke, M.H., Merad, M., Ley, K., 2010.
Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum.
Development of monocytes, macrophages, and dendritic cells. Science 327,
Genet. 81, 1289–1297.
Monteleone, I., Monteleone, G., Fina, D., Caruso, R., Petruzziello, C., Calabrese, E.,
Goddard, C.A., Butts, D.A., Shatz, C.J., 2007. Regulation of CNS synapses by neuronal
Biancone, L., Pallone, F., 2006. A functional role of flip in conferring resistance of
MHC class I. Proc. Natl. Acad. Sci. USA 104, 6828–6833.
Crohn's disease lamina propria lymphocytes to FAS-mediated apoptosis.
Goines, P., Haapanen, L., Boyce, R., Duncanson, P., Braunschweig, D., Delwiche, L.,
Gastroenterology 130, 389–397.
Hansen, R., Hertz-Picciotto, I., Ashwood, P., Van de Water, J., 2011.
Moore, A.H., Wu, M., Shaftel, S.S., Graham, K.A., O'Banion, M.K., 2009. Sustained
Autoantibodies to cerebellum in children with autism associate with
expression of interleukin-1beta in mouse hippocampus impairs spatial
behavior. Brain Behav. Immun. 25, 514–523.
memory. Neuroscience 164, 1484–1495.
Goshen, I., Yirmiya, R., 2009. Interleukin-1 (IL-1): a central regulator of stress
Morgan, J.T., Chana, G., Pardo, C.A., Achim, C., Semendeferi, K., Buckwalter, J.,
responses. Front Neuroendocrinol 30, 30–45.
Courchesne, E., Everall, I.P., 2010. Microglial activation and increased microglial
Greer, J.M., Capecchi, M.R., 2002. Hoxb8 is required for normal grooming behavior
density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry
in mice. Neuron 33, 23–34.
68, 368–376.
Grigorenko, E.L., Han, S.S., Yrigollen, C.M., Leng, L., Mizue, Y., Anderson, G.M.,
Nagarajan, R.P., Patzel, K.A., Martin, M., Yasui, D.H., Swanberg, S.E., Hertz-Picciotto,
Mulder, E.J., de Bildt, A., Minderaa, R.B., Volkmar, F.R., Chang, J.T., Bucala, R.,
I., Hansen, R.L., Van de Water, J., Pessah, I.N., Jiang, R., Robinson, W.P., LaSalle,
2008. Macrophage migration inhibitory factor and autism spectrum disorders.
J.M., 2008. MECP2 promoter methylation and X chromosome inactivation in
Pediatrics 122, e438–e445.
autism. Autism Res. 1, 169–178.
Gruys, E., Toussaint, M.J., Niewold, T.A., Koopmans, S.J., 2005. Acute phase reaction
Neefjes, J.J., Momburg, F., 1993. Cell biology of antigen presentation. Curr. Opin.
and acute phase proteins. J. Zhejiang Univ. Sci. B 6, 1045–1056.
Immunol. 5, 27–34.
Gupta, S., Aggarwal, S., Rashanravan, B., Lee, T., 1998. Th1- and Th2-like cytokines in
Odell, D., Maciulis, A., Cutler, A., Warren, L., McMahon, W.M., Coon, H., Stubbs, G.,
CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 85, 106–109.
Henley, K., Torres, A., 2005. Confirmation of the association of the C4B null
Hanisch, U.K., Kettenmann, H., 2007. Microglia: active sensor and versatile effector
allelle in autism. Hum. Immunol. 66, 140–145.
cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394.
Okada, K., Hashimoto, K., Iwata, Y., Nakamura, K., Tsujii, M., Tsuchiya, K.J., Sekine, Y.,
Harden, L.M., du Plessis, I., Poole, S., Laburn, H.P., 2008. Interleukin (IL)-6 and IL-1
Suda, S., Suzuki, K., Sugihara, G., Matsuzaki, H., Sugiyama, T., Kawai, M., Minabe,
beta act synergistically within the brain to induce sickness behavior and fever
Y., Takei, N., Mori, N., 2007. Decreased serum levels of transforming growth
in rats. Brain Behav. Immun. 22, 838–849.
factor-beta1 in patients with autism. Prog. Neuropsychopharmacol. Biol.
Henry, C.J., Huang, Y., Wynne, A., Hanke, M., Himler, J., Bailey, M.T., Sheridan, J.F.,
Psychiatry 31, 187–190.
Godbout, J.P., 2008. Minocycline attenuates lipopolysaccharide (LPS)-induced
Onore, C., Enstrom, A., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Van de Water, J.,
neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation 5,
Ashwood, P., 2009. Decreased cellular IL-23 but not IL-17 production in children
with autism spectrum disorders. J. Neuroimmunol. 216, 126–134.
Heuer, L., Ashwood, P., Schauer, J., Goines, P., Krakowiak, P., Hertz-Picciotto, I.,
Opp, M.R., Krueger, J.M., 1991. Interleukin 1-receptor antagonist blocks interleukin
Hansen, R., Croen, L.A., Pessah, I.N., Van de Water, J., 2008. Reduced levels of
1-induced sleep and fever. Am J Physiol 260, R453–457.
immunoglobulin in children with autism correlates with behavioral symptoms.
Paloneva, J., Autti, T., Raininko, R., Partanen, J., Salonen, O., Puranen, M., Hakola, P.,
Autism Res. 1, 275–283.
Haltia, M., 2001. CNS manifestations of Nasu-Hakola disease: a frontal dementia
Hryniewicz, A., Bialuk, I., Kaminski, K.A., Winnicka, M.M., 2007. Impairment of
with bone cysts. Neurology 56, 1552–1558.
recognition memory in interleukin-6 knock-out mice. Eur. J. Pharmacol. 577,
Paribello, C., Tao, L., Folino, A., Berry-Kravis, E., Tranfaglia, M., Ethell, I.M., Ethell,
D.W., 2010. Open-label add-on treatment trial of minocycline in fragile X
Hsiao, E.Y., Patterson, P.H., 2011. Activation of the Maternal Immune System
syndrome. BMC Neurol. 10, 91.
Induces Endocrine Changes in the Placenta via IL-6. Brain Behav. Immun 25,
Patterson, P.H., 2009. Immune involvement in schizophrenia and autism: etiology,
pathology and animal models. Behav. Brain Res. 204, 313–321.
Iwata, Y., Tsuchiya, K.J., Mikawa, S., Nakamura, K., Takai, Y., Suda, S., Sekine, Y.,
Plioplys, A.V., 1998. Intravenous immunoglobulin treatment of children with
Suzuki, K., Kawai, M., Sugihara, G., Matsuzaki, H., Hashimoto, K., Tsujii, M.,
autism. J Child Neurol 13, 79–82.
Sugiyama, T., Takei, N., Mori, N., 2008. Serum levels of P-selectin in men with
Samuelsson, A.M., Jennische, E., Hansson, H.A., Holmang, A., 2006. Prenatal exposure
high-functioning autism. Br. J. Psychiatry 193, 338–339.
to interleukin-6 results in inflammatory neurodegeneration in hippocampus
Jamain, S., Quach, H., Betancur, C., Rastam, M., Colineaux, C., Gillberg, I.C.,
with NMDA/GABA(A) dysregulation and impaired spatial learning. Am. J.
Soderstrom, H., Giros, B., Leboyer, M., Gillberg, C., Bourgeron, T., 2003.
Physiol. Regul. Integr. Comp. Physiol. 290, R1345–1356.
Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are
Sandler, R.H., Finegold, S.M., Bolte, E.R., Buchanan, C.P., Maxwell, A.P., Vaisanen,
associated with autism. Nat. Genet. 34, 27–29.
Jorde, L.B., Hasstedt, S.J., Ritvo, E.R., Mason-Brothers, A., Freeman, B.J., Pingree, C.,
oral vancomycin treatment of regressive-onset autism. J Child Neurol 15,
McMahon, W.M., Petersen, B., Jenson, W.R., Mo, A., 1991. Complex segregation
analysis of autism. Am. J. Hum. Genet. 49, 932–938.
Schneider, H., Pitossi, F., Balschun, D., Wagner, A., del Rey, A., Besedovsky, H.O.,
Kajizuka, M., Miyachi, T., Matsuzaki, H., Iwata, K., Shinmura, C., Suzuki, K., Suda, S.,
1998. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc
Tsuchiya, K.J., Matsumoto, K., Iwata, Y., Nakamura, K., Tsujii, M., Sugiyama, T.,
Natl Acad Sci USA 95, 7778–7783.
C. Onore et al. / Brain, Behavior, and Immunity 26 (2012) 383–392
Scifo, R., Cioni, M., Nicolosi, A., Batticane, N., Tirolo, C., Testa, N., Quattropani, M.C.,
Swisher, C.N., Swisher, L., 1975. Letter: congenital rubella and autistic behavior. N.
Morale, M.C., Gallo, F., Marchetti, B., 1996. Opioid-immune interactions in
Engl. J. Med. 293, 198.
autism: behavioural and immunological assessment during a double-blind
Takahashi, K., Rochford, C.D., Neumann, H., 2005. Clearance of apoptotic neurons
treatment with naltrexone. Ann Ist Super Sanita 32, 351–359.
without inflammation by microglial triggering receptor expressed on myeloid
Shatz, C.J., 2009. MHC class I: an unexpected role in neuronal plasticity. Neuron 64,
cells-2. J. Exp. Med. 201, 647–657.
Todd, R.D., Hickok, J.M., Anderson, G.M., Cohen, D.J., 1988. Antibrain antibodies in
Shenoy, S., Arnold, S., Chatila, T., 2000. Response to steroid therapy in autism secondary
infantile autism. Biol. Psychiatry 23, 644–647.
to autoimmune lymphoproliferative syndrome. J Pediatr 136, 682–687.
Torrente, F., Ashwood, P., Day, R., Machado, N., Furlano, R.I., Anthony, A., Davies, S.E.,
Shi, J., Levinson, D.F., Duan, J., Sanders, A.R., Zheng, Y., Pe'er, I., Dudbridge, F.,
Wakefield, A.J., Thomson, M.A., Walker-Smith, J.A., Murch, S.H., 2002. Small
Holmans, P.A., Whittemore, A.S., Mowry, B.J., Olincy, A., Amin, F., Cloninger, C.R.,
intestinal enteropathy with epithelial IgG and complement deposition in
Silverman, J.M., Buccola, N.G., Byerley, W.F., Black, D.W., Crowe, R.R., Oksenberg,
children with regressive autism. Mol. Psychiatry 7, 375–382, 334.
J.R., Mirel, D.B., Kendler, K.S., Freedman, R., Gejman, P.V., 2009. Common
Torrente, F., Anthony, A., Heuschkel, R.B., Thomson, M.A., Ashwood, P., Murch, S.H.,
variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460,
2004. Focal-enhanced gastritis in regressive autism with features distinct from
Crohn's and Helicobacter pylori gastritis. Am. J. Gastroenterol. 99, 598–605.
Silva, S.C., Correia, C., Fesel, C., Barreto, M., Coutinho, A.M., Marques, C., Miguel, T.S.,
Torres, A.R., Maciulis, A., Odell, D., 2001. The association of MHC genes with autism.
Ataide, A., Bento, C., Borges, L., Oliveira, G., Vicente, A.M., 2004. Autoantibody
Front Biosci. 6, D936–943.
repertoires to brain tissue in autism nuclear families. J. Neuroimmunol. 152,
Torres, A.R., Maciulis, A., Stubbs, E.G., Cutler, A., Odell, D., 2002. The transmission
disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism
Singer, H.S., Morris, C.M., Williams, P.N., Yoon, D.Y., Hong, J.J., Zimmerman, A.W.,
spectrum disorder. Hum. Immunol. 63, 311–316.
2006. Antibrain antibodies in children with autism and their unaffected
Torres, A.R., Sweeten, T.L., Cutler, A., Bedke, B.J., Fillmore, M., Stubbs, E.G., Odell, D.,
siblings. J. Neuroimmunol. 178, 149–155.
2006. The association and linkage of the HLA-A2 class I allele with autism. Hum.
Singer, H.S., Morris, C., Gause, C., Pollard, M., Zimmerman, A.W., Pletnikov, M., 2009.
Immunol. 67, 346–351.
Prenatal exposure to antibodies from mothers of children with autism produces
Tsuchiya, K.J., Hashimoto, K., Iwata, Y., Tsujii, M., Sekine, Y., Sugihara, G., Matsuzaki,
neurobehavioral alterations: a pregnant dam mouse model. J. Neuroimmunol.
H., Suda, S., Kawai, M., Nakamura, K., Minabe, Y., Yagi, A., Iyo, M., Takei, N., Mori,
211, 39–48.
N., 2007. Decreased serum levels of platelet-endothelial adhesion molecule
Singh, V.K., Warren, R.P., Odell, J.D., Warren, W.L., Cole, P., 1993. Antibodies to
(PECAM-1) in subjects with high-functioning autism: a negative correlation
myelin basic protein in children with autistic behavior. Brain Behav. Immun. 7,
with head circumference at birth. Biol. Psychiatry 62, 1056–1058.
Ulvestad, E., Williams, K., Matre, R., Nyland, H., Olivier, A., Antel, J., 1994. Fc
Singh, V.K., Singh, E.A., Warren, R.P., 1997a. Hyperserotoninemia and serotonin
receptors for IgG on cultured human microglia mediate cytotoxicity and
receptor antibodies in children with autism but not mental retardation. Biol.
phagocytosis of antibody-coated targets. J. Neuropathol. Exp. Neurol. 53, 27–36.
Psychiatry 41, 753–755.
Vargas, D.L., Nascimbene, C., Krishnan, C., Zimmerman, A.W., Pardo, C.A., 2005.
Neuroglial activation and neuroinflammation in the brain of patients with
autoantibodies to neuronal and glial filament proteins in autism. Pediatr.
autism. Ann. Neurol. 57, 67–81.
Neurol. 17, 88–90.
Voineagu, I., Wang, X., Johnston, P., Lowe, J.K., Tian, Y., Horvath, S., Mill, J., Cantor,
Skaar, D.A., Shao, Y., Haines, J.L., Stenger, J.E., Jaworski, J., Martin, E.R., DeLong, G.R.,
R.M., Blencowe, B.J., Geschwind, D.H., 2011. Transcriptomic analysis of autistic
Moore, J.H., McCauley, J.L., Sutcliffe, J.S., Ashley-Koch, A.E., Cuccaro, M.L.,
brain reveals convergent molecular pathology. Nature 474, 380–384.
Folstein, S.E., Gilbert, J.R., Pericak-Vance, M.A., 2005. Analysis of the RELN gene
Vojdani, A., Campbell, A.W., Anyanwu, E., Kashanian, A., Bock, K., Vojdani, E., 2002.
as a genetic risk factor for autism. Mol. Psychiatry 10, 563–571.
Antibodies to neuron-specific antigens in children with autism: possible cross-
Smith, S.E., Li, J., Garbett, K., Mirnics, K., Patterson, P.H., 2007. Maternal immune
reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and
activation alters fetal brain development through interleukin-6. J. Neurosci. 27,
Streptococcus group A. J. Neuroimmunol. 129, 168–177.
Vojdani, A., Mumper, E., Granpeesheh, D., Mielke, L., Traver, D., Bock, K., Hirani, K.,
Stefansson, H., Ophoff, R.A., Steinberg, S., Andreassen, O.A., Cichon, S., Rujescu, D.,
Neubrander, J., Woeller, K.N., O'Hara, N., Usman, A., Schneider, C., Hebroni, F.,
Werge, T., Pietilainen, O.P., Mors, O., Mortensen, P.B., Sigurdsson, E., Gustafsson,
Berookhim, J., McCandless, J., 2008. Low natural killer cell cytotoxic activity in
O., Nyegaard, M., Tuulio-Henriksson, A., Ingason, A., Hansen, T., Suvisaari, J.,
autism: the role of glutathione, IL-2 and IL-15. J. Neuroimmunol. 205, 148–154.
Lonnqvist, J., Paunio, T., Borglum, A.D., Hartmann, A., Fink-Jensen, A.,
Warren, R.P., Foster, A., Margaretten, N.C., 1987. Reduced natural killer cell activity
Nordentoft, M., Hougaard, D., Norgaard-Pedersen, B., Bottcher, Y., Olesen, J.,
in autism. J. Am. Acad. Child Adolesc. Psychiatry 26, 333–335.
Breuer, R., Moller, H.J., Giegling, I., Rasmussen, H.B., Timm, S., Mattheisen, M.,
Widera, D., Mikenberg, I., Elvers, M., Kaltschmidt, C., Kaltschmidt, B., 2006. Tumor
Bitter, I., Rethelyi, J.M., Magnusdottir, B.B., Sigmundsson, T., Olason, P., Masson,
necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-
G., Gulcher, J.R., Haraldsson, M., Fossdal, R., Thorgeirsson, T.E., Thorsteinsdottir,
kappaB signaling. BMC Neurosci. 7, 64.
U., Ruggeri, M., Tosato, S., Franke, B., Strengman, E., Kiemeney, L.A., Melle, I.,
Wills, S., Cabanlit, M., Bennett, J., Ashwood, P., Amaral, D.G., Van de Water, J., 2009.
Djurovic, S., Abramova, L., Kaleda, V., Sanjuan, J., de Frutos, R., Bramon, E.,
Detection of autoantibodies to neural cells of the cerebellum in the plasma of
Vassos, E., Fraser, G., Ettinger, U., Picchioni, M., Walker, N., Toulopoulou, T.,
subjects with autism spectrum disorders. Brain Behav. Immun. 23, 64–74.
Need, A.C., Ge, D., Yoon, J.L., Shianna, K.V., Freimer, N.B., Cantor, R.M., Murray, R.,
Wills, S., Rossi, C.C., Bennett, J., Martinez-Cerdeno, V., Ashwood, P., Amaral, D.G., Van
Kong, A., Golimbet, V., Carracedo, A., Arango, C., Costas, J., Jonsson, E.G.,
de Water, J., 2011. Further characterization of autoantibodies to GABAergic
Terenius, L., Agartz, I., Petursson, H., Nothen, M.M., Rietschel, M., Matthews,
neurons in the central nervous system produced by a subset of children with
P.M., Muglia, P., Peltonen, L., St Clair, D., Goldstein, D.B., Stefansson, K., Collier,
autism. Mol. Autism 2, 5.
D.A., 2009. Common variants conferring risk of schizophrenia. Nature 460, 744–
Wiznitzer, M., 2004. Autism and tuberous sclerosis. J. Child Neurol. 19, 675–679.
Wu, S., Jia, M., Ruan, Y., Liu, J., Guo, Y., Shuang, M., Gong, X., Zhang, Y., Yang, X.,
Steffenburg, S., Gillberg, C., Hellgren, L., Andersson, L., Gillberg, I.C., Jakobsson, G.,
Zhang, D., 2005. Positive association of the oxytocin receptor gene (OXTR) with
Bohman, M., 1989. A twin study of autism in Denmark, Finland, Iceland, Norway
autism in the Chinese Han population. Biol. Psychiatry 58, 74–77.
and Sweden. J. Child Psychol. Psychiatry 30, 405–416.
Zalcman, S.S., 2002. Interleukin-2-induced increases in climbing behavior:
Stellwagen, D., Malenka, R.C., 2006. Synaptic scaling mediated by glial TNF-alpha.
inhibition by dopamine D-1 and D-2 receptor antagonists. Brain Res. 944,
Nature 440, 1054–1059.
Steup-Beekman, G., Steens, S., van Buchem, M., Huizinga, T., 2007. Anti-NMDA
Zerbo, O., Iosif, A.M., Delwiche, L., Walker, C., Hertz-Picciotto, I., 2011. Month of
receptor autoantibodies in patients with systemic lupus erythematosus and
conception and risk of autism. Epidemiology. 22, 469–475.
their first-degree relatives. Lupus 16, 329–334.
Ziv, Y., Schwartz, M., 2008. Immune-based regulation of adult neurogenesis:
Stubbs, E.G., Crawford, M.L., 1977. Depressed lymphocyte responsiveness in autistic
implications for learning and memory. Brain Behav. Immun. 22, 167–176.
children. J. Autism Child Schizophr. 7, 49–55.
Ziv, Y., Ron, N., Butovsky, O., Landa, G., Sudai, E., Greenberg, N., Cohen, H., Kipnis, J.,
Sweeten, T.L., Posey, D.J., McDougle, C.J., 2003. High blood monocyte counts and
Schwartz, M., 2006. Immune cells contribute to the maintenance of
neopterin levels in children with autistic disorder. Am. J. Psychiatry 160, 1691–
neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9,
OnoreC_2012_Immune dysfunction in the pathophysiology of autism.pdf
Source: http://www.jocdp.jp/php/info2/data/20121024100034.pdf
en Languedoc-Roussillon LE MOT DES PREFETS En 2010, les services de l'Etat se sont recomposés pour mieux rendre service aux habitants du Languedoc-Roussillon et contribuer à la reprise de l'économie. Nous les remercions, eux qui se sont fortement mobilisés, ainsi que tous ceux qui nous accompagnent dans nos missions de service public : collectivités, entreprises,
Doro PhoneEasy® 624 15. Charging socket 16. Headset socket 17. Assistance button Left selection button 20. External display Input method/Silent 22. Green light = New message 10. Message shortcut 23. Red light = Battery level low 11. Volume control 24. Charging stand 12. End call/Power on/off 14. Right selection button The items supplied with your phone might vary depending on the soft-ware and accessories available in your region or offered by your serviceprovider. You can obtain additional accessories from your local Doro deal-er. The supplied accessories provide the best performance with yourphone.






