Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Doi:10.1016/j.jalz.2009.05.027
Alzheimer's Imaging Consortium IC-P: Poster Presentations
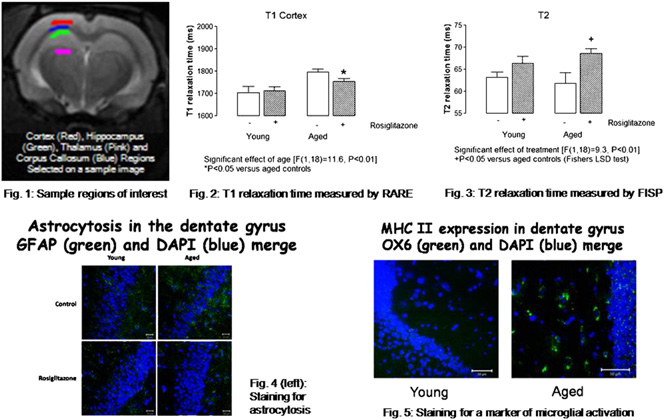
Background: Rosiglitazone, a peroxisome proliferator-activated receptor
copy. Because of their high iron content, plaques typically appear as hypo-
[gamma] (PPAR[gamma]) agonist, has an anti-inflammatory effect in the
intense spots on T2-weighted scans. One of the challenges in imaging
brain, decreasing interleukin-1[beta] concentrations in hippocampus and re-
plaques is to achieve high-enough resolution and contrast to detect these
storing the age-related deficit in long-term potentiation. It also attenuates
50-mm large lesions. While most high-field systems can reach high resolu-
learning and memory deficits in a mouse model of Alzheimer's disease. Ev-
tion, the lack of contrast between the plaques and the parenchyma often
idence suggests that activation of microglia and astrocytes contribute to age-
impedes their detection. Methods: Transgenic mice over-expressing muta-
related neuroinflammatory changes. In this study, relaxometry measure-
tions of the human genes responsible for familial forms of AD were stud-
ments were assessed in hippocampus of young and aged rats because there
ied. A total of eight APP/PS1 mice and seven wild-type mice were used for
have been recent reports in the literature linking spin-lattice (T1) and spin-
the in vivo study. The mice were injected in the lateral ventricles with
spin (T2) relaxation times to astrocytic activation and microglial activation
a non-specific gadolinium(Gd)-based contrast agent (CA). Within 6 hours
respectively. Methods: Male Wistar rats aged 3 and 18 months (n ¼ 5-6
after injections, the animals were imaged in a 7 T Siemens system (Syngo
per group) were treated either with 3 mg/day rosiglitazone maleate or
MR VB15) using a 3D turbo spin-echo sequence (resolution ¼
with vehicle only for 56 days and MR images obtained under anesthesia
503503200 mm, scan time ¼ 2.5 hours). A total of six APP mice were
using a 7-Tesla MRI scanner (Bruker) with slices selected to give optimal
used for the ex vivo study where the extracted brains were fixed and stained
regions of interest in dorsal hippocampus (Fig. 1). Images were acquired
with a Gd CA for at least 24 hours, then imaged (3D FLASH gradient-echo
using rapid acquisition with relaxation enhancement (RARE) and echo-
sequence, resolution ¼ 653653200 mm and scan time ¼ 1 hour, or reso-
train multi-slice-multi-echo (MSME) sequences, from which T1 and T2
lution ¼ 23323390 mm and scan time ¼ 12 hours). Results: There was
maps were generated, respectively; and a fast imaging with steady-state
a continuous diffusion of the CA into the parenchyma for up to 40 min
precession (FISP) protocol, from which both T1 and T2 maps were gener-
after injection, until it reached a plateau and remained in the brain for at
ated. Data from manually-selected regions of interest were analyzed using
least 5 hours. The images showed that prior to injection of CA, no plaques
software scripts in IDL language (ITTVIS). One- or two-way analyses of
were detectable in the cortex or hippocampus, whereas some were clearly
variance (ANOVA) with Fischer's post-test were used to statistically assess
visible after injection of CA. The ex vivo results showed that 50-mm large
the data. Tissue from the animals was examined for markers of astrogliosis
plaques could be detected after staining with the CA. However, individual
and microglial activation. Results: T1 in hippocampus and cortex (p <
plaques were only well-defined at high resolution, enabling quantification
0.01, Fig. 2) were significantly increased with age whilst T2 in hippocam-
of the plaque load which was about 10% in the cortex. Conclusions: This
pus (p < 0.05) and cortex (p < 0.01, Fig. 3) were significantly decreased
study shows that amyloid plaques can be detected both in vivo and ex vivo
with age. Treatment with rosiglitazone significantly attenuated the age-re-
using a non-specific Gd-contrast agent. The level of detail achieved should
lated T1 increase in both regions (p < 0.05, Fig. 2) and it increased T2
be sufficient for pharmacology studies.
relaxation time in hippocampus of aged, but not young rats (p < 0.05,Fig. 3). Conclusions: The data demonstrate an age-related increase in
MEMORY LOSS IN YOUNG APPSWE/PS1DE9 MICE
T1 in hippocampus and cortex, which is attenuated by rosiglitazone; this
AND ASSOCIATED CHANGES IN BRAIN
correlates with astrocytosis (Fig. 4). We also show a decrease in T2 relax-
METABOLISM ANALYZED USING A 3-D VOXEL-
ation time in hippocampus and cortex, which correlates with an increase in
microglial activation (Fig. 5), and report that rosiglitazone-treatment ofaged rats increases hippocampal T2.
Anne-Sophie He´rard, Thierry Sylvie Cornet,Jessica LebenbergPierre-Etienne Chabrier, Philippe Marc 1CEA-MIRCen-CNRS URA 2210, Fontenay-Aux-Roses,France; 2SCRAS-IHB, Les Ulis, France. Contact e-mail: anne-sophie.
[email protected]
Background: APPswe/PS1dE9 is a mouse model of Alzheimer's diseasethat starts to develop amyloid plaques by the age of 4 months. This studyevaluated early alterations of spatial memory and brain glucose metabolismin this mouse strain. The latter biomarker is of great interest in translationalstudies, as it can be evaluated both in animals and humans. Methods:APPswe/PS1dE9-transgenic (Tg) and C57BL/6 J amyloid free control(WT) mice were studied at 4.5 months, i.e. at the very beginning of amy-loid deposition. Spatial memory was evaluated using the Morris watermaze (nTg ¼ 14; nWT ¼ 14). During the training (4 trials/day for 4days) the latency and distance to reach platform (PF) were evaluated.
For the memory retention test, the PF was removed and mice were allowedto navigate for 60 seconds. The time spent in the target quadrant, the dis-tance moved, the number of crossing into PF zone, and the swim speedwere evaluated (Ethovision videotracking system, Noldus). Glucose uptakewas quantified in awake mice (nTg ¼ 6; nWT ¼ 7) by [14C]-2-deoxyglu-cose autoradiography of the whole brain (20-mm serial sections). 3D auto-radiographic brain volumes were reconstructed using BrainRAT (freely
IN VIVO AMYLOID PLAQUE DETECTION USING
available software, http://www.brainvisa.info) and statistical analyses
CONTRAST-ENHANCED MAGNETIC RESONANCE
were performed without any a priory hypothesis using SPM5. Cerebral am-
MICROSCOPY IN TRANSGENIC MICE
yloid deposits were examined in the same animals (BAM10 immunostain-ing and Congo red). Results: APPswe/PS1dE9 mice displayed significant
Alexandra PetietAnne Christopher
spatial memory alterations that could be detected in training (increased dis-
Diane Thomas Debeir, Thomas , Marc ,
tance and latencies to reach the PF) and retention sessions (reduced time
1Sanofi-Aventis R&D, Vitry-sur-Seine, France; 2CNRS URA 2210, Mircen,
spent in the quadrant). Despite these alterations, SPM analysis of glucose
Orsay, France; 3CEA, DSV, I2BM, Neurospin, Gif-sur-Yvette, France.
metabolism revealed no statistically significant hypometabolic voxels in
Contact e-mail: [email protected]
Tg as compared to WT animals. On the contrary, hypermetabolic voxels
Background: Amyloid plaques have previously been identified in mouse
were identified bilaterally. They were located in lateral olfactory tracts
models of Alzheimer's disease (AD) using magnetic resonance micros-
and in the somatosensory and motor parietal cortex (increases of 12.0%/
Alzheimer's Imaging Consortium IC-P: Poster Presentations
13.7% and 13.9%/13.0% for the left and right sides of these structures).
Background: Intracellular inclusions of pathological tau fibrils are hall-
Conclusions: Although young Tg mice displayed spatial memory impair-
mark lesions in Alzheimer's disease (AD) and associated tauopathies,
ment, no obvious hypometabolic brain regions was detected. Strikingly, Tg
and there has been a growing interest in the mechanistic links between fi-
mice even present with discrete areas of hypermetabolism in the somato-
brillar tau accumulation and neuronal deterioration. In-vivo visualization
sensory and motor parietal cortex and in olfactory areas. The fine relation-
of tau lesions would thus serve the preclinical and clinical needs for pur-
ship between these behavioral and metabolic changes remains to be
suing the molecular etiology of neurodegenerative disorders and evaluat-
ing candidate disease-modifying therapies. This notion has led us todevelop imaging agents capable of capturing tau aggregates in a living
ALTERATIONS OF BRAIN GLUCOSE
mouse model of tauopathies. Methods: An array of fluorescent chemicals
METABOLISM IN AGED APP/PS1 MICE: AN
were screened by assaying their in-vitro binding to recombinant tau fibrils
ORIGINAL VOXEL-BASED STATISTICAL
and neuronal tau inclusions on brain sections from patients with diverse
ANALYSIS USING BRAINRAT AND SPM
tauopathies and mice transgenic for the pathogenic P301S mutant tau.
Anne-Sophie He´Albertine , Nicolas
The selected compounds were intravenously injected into the tau trans-
Benoıˆt Delatour, Philippe Hantraye, Marc Dhenain, Thierry Delzescaux,
genics, and ex-vivo labelings of tau lesions with these agents in excised
1CEA-MIRCen-CNRS URA 2210, Fontenay-Aux-Roses, France;
brain samples were microscopically assessed. Pulse-laser optical and pos-
2CEA-SHFJ-INSERM U803, Orsay, France; 3Universite´ Paris Sud-
itron emission tomographic (PET) systems were then applied to in-vivo
NAMC-CNRS UMR 8620, Orsay, France. Contact e-mail: anne-sophie.
detections of tau pathologies in the transgenic mice with near-infrared
fluorescent and 11C-labeled compounds, respectively. The PET datawere also supplemented by in-vitro and ex-vivo autoradiographic analyses.
Background: Glucose brain metabolism is a widely used clinical bio-
Results: Chemical properties of the compounds such as structural dimen-
marker in Alzheimer's disease (AD), usually examined on 2D post mortem
sions and hydrophilicities were associated with their affinities for tau in-
autoradiographic data in AD animal models. We propose an innovative
clusions in a wide range of tauopathies as well as transgenics. A class
method to analyze these data in 3D without a priori anatomical informa-
of chemicals sharing the common core structure enabled ex-vivo visualiza-
tion, to derive brain metabolic activity maps using voxel-based approach
tion of aggregates in the transgenic mice. One of these putative imaging
in APP/PS1 mice. Results will be discussed relative to previous findings
agents was suitable for the near-infrared optics, and was proven to bind
in this field. Methods: Uptake of [14C]-2-deoxyglucose was measured in
to tau aggregated in living mouse brains based on the intensity and life-
adult awake APP/PS1 (64 6 1 weeks, n ¼ 4) and PS1 (65 6 2 weeks,
time of its fluorescent signals. Two other compounds of the same group
n ¼ 3) transgenic mice. Glucose uptake was evaluated by autoradiography
were radiolabeled with 11C, and were demonstrated to allow both autora-
on the right hemisphere while the left hemisphere was processed for Congo
diographic and PET detections of tau lesions in the transgenics. Conclu-
red staining. Data acquisition was performed with a digital camera (block-
sions: The present neuroimaging approaches using mouse models of the
face photographs) and a high resolution flatbed scanner (autoradiography,
diseases provide insights into the structural basis of molecular interactions
histology). Blockface, autoradiographic and histological post mortem vol-
between tau fibrils and exogenous compounds, which would facilitate the
umes were 3D-reconstructed using BrainRAT in-house software (freely
development of diagnostic and therapeutic agents for AD and non-AD
available, http://brainvisa.info) while statistical analysis was achieved us-
ing SPM5. Results: We successfully combined BrainRAT (computerizedprocedures for acquisition and 3D reconstruction of anatomic and func-
DIAGNOSTIC UTILITY OF PLATELET AMYLOID
tional volumes) and SPM5 (voxel-wise statistical analysis) methodologies
PRECURSOR PROTEIN RATIO, RED BLOOD CELLS
to data sets mapping brain metabolic activity in a transgenic mouse model
AND PLASMA BETA AMYLOID (Ab 1-42) LEVELS
of AD. We were able to extract accurate parametric mapping of both hypo-
IN LATE-ONSET ALZHEIMER'S DISEASE
and hypermetabolic regions in the APP/PS1 animals relative to PS1 con-
Ben B'joe Vasudevan DevanathaSenthil Kumar,
trols. Decreased glucose uptake was observed within cortex (cingulate
Jeyakumar 1Sri Ramachandra Medical College, Chennai, India;
-36%, retrosplenial -26%, somatosensory -23%), striatum (-22%), thalamus
2Kamakshi Memorial Hospital, Chennai, India; 3Central Leather Research
(-29%) and hippocampus (-25%). Increased glucose uptake was also de-
Institute, Chennai, India; 4Stanford University, Stanford, CA, USA.
tected within other cortical areas (piriform þ22%, perirhinal þ19%),
Contact e-mail: [email protected]
amygdala (þ23%), dorsal endopiriform (þ34%) and accumbens (þ20%)nuclei, dentate gyrus (þ25%) and dorsal hippocampus (þ26%). Conclu-
Background: Alzheimer's disease (AD) is a severe neurodegenerative dis-
sions: We have demonstrated the ability to robustly and automatically re-
ease with a characteristic progressive decline in cognitive functions and de-
construct post mortem functional volumes. We also provided accurate
mentia. It is believed that the majority of all AD patients are affected by the
cerebral glucose uptake mapping in a murine AD model, in 3D, without
sporadic form, caused by the combined effects of several risk factors. In-
a priori hypotheses. We offer an efficient and standardized method to com-
creasing evidence suggests that abnormal processing of amyloid precursor
pare local changes in mice cerebral glucose utilization across ages, lines or
protein (APP) may play an important role in the pathogenesis of AD. The
drugs. Furthermore, our work demonstrates the possibility of using func-
present study focused on finding reliable biochemical markers in peripheral
tional brain imaging and dedicated software to help bridge the gap, in
venous blood and their relation to AD. Methods: The biochemical markers,
translational studies, between features of neurodegenerative diseases in hu-
platelets APP ratio, red blood cells (RBC), and plasma beta amyloid (Ab
man beings and animal models.Acknowledgements: Sanofi-Aventis for
1-42) were quantitated in 40 probable/possible sporadic AD patients and
sharing the mouse strains.
60 non demented age matched healthy controls. Results: The presentstudy found a reduction in platelet APP ratio. The magnetitude of the
IN VIVO OPTICAL AND PET DETECTIONS OF
APP ratio reduction is proportional to the severity of the cognitive loss
FIBRILLAR TAU LESIONS IN A MOUSE MODEL OF
in AD. The mean plasma Ab1-42 levels were higher in AD compared to
age matched healthy subjects. In erythrocytes the mean RBC Ab1-42
Masahiro Maruyama, Jun Bin Ji, Ming-Rong
levels were found to be decreased in AD but there was substantial individ-
Takashi Maiko Ono, Satoko John Q. Trojanows
ual variability and overlap in plasma and RBC Ab1-42 levels between
Virginia M.-Y. Lee., Toshimitsu Fukumura, Makoto ,
these groups. Conclusions: Since platelet APP ratio is decreased at differ-
Tetsuya Suhara, 1National Institute of Radiological Sciences, Chiba, Japan;
ent stages of AD and abnormal APP processing is related to the neuropath-
2University of Pennsylvania, Philadelphia, PA, USA.
ological changes in AD brain, the present study suggests that the APPr
Contact e-mail: [email protected]
may assist in the early diagnosis of AD in individuals at risk for the disease
Source: http://jessica.lebenberg.free.fr/conferences/Herard-Lebenberg_ICAD2009.pdf
PRODUCT INFORMATION NAME OF THE MEDICINE Granisetron Kabi Concentrated Injection Granisetron hydrochloride has the following chemical structure: Empirical formula: Molecular weight: The systematic chemical name is endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride. DESCRIPTION Granisetron hydrochloride is a white to off-white crystalline powder which is freely soluble in water and sodium chloride 0.9% at 20°C. Granisetron Kabi Concentrated Injection contains granisetron hydrochloride equivalent to granisetron free base 1 mg/mL. It also contains sodium chloride, citric acid monohydrate, hydrochloric acid, sodium hydroxide and water for injections. PHARMACOLOGY Granisetron is a potent anti-emetic and highly selective antagonist of 5-hydroxytryptamine (5-HT3) receptors. Radioligand binding studies have demonstrated that granisetron has negligible affinity for other receptor types, including 5-HT, alpha1 and alpha2, beta-adrenoreceptors, histamine H1, picrotoxin, benzodiazepine, opioid and dopamine D2 binding sites. Antagonism of 5-HT receptors located peripherally on vagal nerve terminals
Medication Policy (example document for e-learning course) CONTENTS Roles and Responsibilities 2.1 Registered Manager 2.2 Care Staff (Nurses & Senior Care Workers) Training and Competency 3.1 Basic training in safe handling of medicines 3.2 Specialised training to give medicines Ordering medicines from a Pharmacy 4.1 Repeat prescription requests for the GP 4.2 How to check the prescription on return from the GP 4.3 How to check medicines delivered from the pharmacy 4.4 Obtaining acute medicines 4.5 Verbal Orders




