Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Synergistic effects of noradrenergic modulation with atomoxetine and 10�hz repetitive transcranial magnetic stimulation on motor learning in healthy humans
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46http://www.biomedcentral.com/1471-2202/15/46
Synergistic effects of noradrenergic modulationwith atomoxetine and 10 Hz repetitivetranscranial magnetic stimulation on motorlearning in healthy humans
Matthias Sczesny-Kaiser1*†, Alica Bauknecht1†, Oliver Höffken1, Martin Tegenthoff1, Hubert R Dinse2, Dirk Jancke2,Klaus Funke3 and Peter Schwenkreis1
Background: Repetitive transcranial magnetic stimulation (rTMS) is able to induce changes in neuronal activity thatoutlast stimulation. The underlying mechanisms are not completely understood. They might be analogous tolong-term potentiation or depression, as the duration of the effects seems to implicate changes in synapticplasticity. Norepinephrine (NE) has been shown to play a crucial role in neuronal plasticity in the healthy and injuredhuman brain. Atomoxetine (ATX) and other NE reuptake inhibitors have been shown to increase excitability indifferent systems and to influence learning processes. Thus, the combination of two facilitative interventions maylead to further increase in excitability and motor learning. But in some cases homeostatic metaplasticity mightprotect the brain from harmful hyperexcitability. In this study, the combination of 60 mg ATX and 10 Hz rTMS overthe primary motor cortex was used to examine changes in cortical excitability and motor learning and toinvestigate their influence on synaptic plasticity mechanisms.
Results: The results of this double-blind placebo-controlled study showed that ATX facilitated corticospinal andintracortical excitability in motor cortex. 10 Hertz rTMS applied during a motor task was able to further increaseintracortical excitability only in combination with ATX. In addition, only the combination of 10 Hz rTMS and ATXwas capable of enhancing the total number of correct responses and reaction time significantly, indicating aninteraction effect between rTMS and ATX without signs of homeostatic metaplasticity.
Conclusion: These results suggest that pharmacologically enhanced NE transmission and 10 Hz rTMS exert asynergistic effect on motor cortex excitability and motor learning in healthy humans.
Keywords: rTMS, Neuromodulation, Norepinephrine, Atomoxetine, Plasticity, Motor cortex
different TMS-protocols. Furthermore, rTMS is capable
Repetitive transcranial magnetic stimulation (rTMS) is a
to influence task performance and learning processes. For
non-invasive tool for brain stimulation and is able to
example, it can improve motor learning The induced
modulate brain activity beyond stimulation The
effects depends on different parameters like coil orientation,
mechanisms underlying these long-term rTMS-effects
total number of pulses and frequency.
could be analogous to long-term potentiation (LTP) or
For the measurement of rTMS-induced changes of
depression (LTD). These rTMS-induced changes in cor-
cortical excitability, a single-pulse TMS (spTMS) protocol
tical excitability and brain activity can be measured by
called "stimulus response curve" (SRC) is used. It testsstimulus intensity-dependent recruitment of corticospinal
* Correspondence:
projections by means of spTMS [. The steepness of the
†Equal contributors1
linear regression line through the data points of the SRC
Department of Neurology, BG-Universitaetsklinikum Bergmannsheil Bochum,
Buerkle-de-la-Camp-Platz 1, 44789 Bochum, Germany
is a measure of corticospinal excitability [Special
Full list of author information is available at the end of the article
2014 Sczesny-Kaiser et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of theCreative Commons Attribution License which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons PublicDomain Dedication waiver applies to the data made available in thisarticle, unless otherwise stated.
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
paired-pulse TMS (ppTMS) protocols can determine
on cortical excitability and motor learning. Moreover, it
intracortical facilitation (ICF) and short-latency intracorti-
has not been investigated if a subsequent high frequency
cal inhibition (SICI) . The normalized ICF and SICI
rTMS can further increase excitability and motor learning.
ratios give information about the activity of excitatory and
Here, we used a sequential 4-finger tapping/10 Hz rTMS
inhibitory intracortical interneuronal circuits [].
combination paradigm previously introduced by Kim and
Despite clear effects of rTMS on cortical excitability,
coworkers [] combined with ATX or placebo intake in
identifying consistent effects of rTMS on sensorimotor
order to evaluate the effect of atomoxetine and rTMS on
learning has proven more difficult. Many experiments
motor cortex plasticity and motor learning in humans.
have found no changes in motor learning after high fre-
Previous studies generally used neuropharmacological
quency rTMS in healthy humans, while others showed only
modulation only. Here, we were particularly interested
mild effects Short lasting improvements, however,
in possible interactional effects of both treatment regimens.
could be elicited using a combination of finger tapping task
We therefore administered placebo or ATX and real rTMS
or sham rTMS in a randomized double-blind study. We hy-
Under these difficult circumstances and to get insight
pothesized that the combination of ATX and 10 Hz rTMS
of the physiology of learning processes, numerous studies
over M1 is capable of increasing excitability and motor
have used pharmacological interventions There are
learning, and that they might have synergistic effects.
several studies on the effect of the positive allostericmodulators of the GABAA receptors, i. e. benzodiazepines
]. For example, Di Lazzaro and coworkers investi-
gated the influence of lorazepam on the excitability on
There were no significant differences in sex (p = 0.26)
human motor cortex. They could demonstrate by means
or age (mean ATX + real rTMS group: 24.1 ± 3.95 years,
of single and paired-pulse TMS that lorazepam depress
placebo + real rTMS group: 24.6 ± 1.74 years, ATX + sham
high-amplitude motor-evoked potentials (MEP) and in-
rTMS group: 24.2 ± 1.98 years, placebo + sham rTMS group:
creases the excitability of inhibitory circuits Moreover,
23.9 ± 2.47 years; F(3,32) = 0.1, p = 0.96). Blood samples were
it could be demonstrated that this interference with the
taken from all participants approximately 1 hour and 2 hours
GABAA-system can reduce learning and use-dependent
after ATX or placebo (PLC) intake. The average ATX
plastic changes []. Similar changes in excitability could
blood serum levels in both ATX-groups were 366.1 ng/ml
be demonstrated by Schwenkreis and coworkers with the
after 1 hour and 296.7 ng/ml after 2 hours after drug intake.
glutamate antagonist riluzole and the NMDA antagonist
Four subjects reported about temporary headache begin-
memantine [. Both agents reduce intracortical facili-
ning 12 hours after the experiment and persisted about 2
tation and increase intracortical inhibition. Similar to the
to 4 hours. We could not distinguish if these symptoms
observations of Butefisch for lorazepam, it was demon-
derived from ATX or rTMS-intervention.
strated that riluzole and memantine were capable to blockuse-dependent plasticity in motor cortex.
Looking for pharmacological interventions that lead to
Considering the slopes of the SRCs, repeated measurement
facilitative effects and might boost learning processes,
analysis of variance (rmANOVA) showed a significant
the influence of norepinephrine (NE) agonists like am-
effect of the within-subject factor "time" (F(1,2) = 19.26,
phetamine (AMP), methyphenidate (MPH), reboxetine
p < 0.000). There was no effect of the between-subject
(RBX) and atomoxetine (ATX) were investigated. MPH,
factor "group" (F(3,32) = 1.99, p = 0.14) or the interaction
RBX and ATX increase ICF and decrease SICI measured by
"time x group" (F(3,6) = 0.95, p = 0.47). Post-hoc paired
paired-pulse TMS after a single dosage Moreover,
t-tests revealed significantly increased slopes from T1
Plewnia and Tegenthoff investigated the modulation of
(baseline measurements) to T2 (measurements 1 hour
use-dependent plasticity in primary motor cortex (M1).
after ATX/placebo intake), from T2 to T3 (measurements
RBX and AMP were able to enhance training-induced
after motor task/rTMS combination) and from T1 to
motor cortex plasticity ]. All these studies clearly show
T3 (mean slope differenceΔT2T1 = 0.006 ± 0.01, t(35) = 2.96,
that NE plays a crucial role in promoting plasticity. Espe-
p = 0.005; mean slope differenceΔT3T2 = 0.01 ± 0.016,
cially, ATX affects the regulation of NE as a highly selective
t(35) = 3.7, p = 0.001; mean slope difference ΔT3T1 = 0.016 ±
inhibitor of the presynaptic NE transporter with low affinity
0.02, t(35) = 5.4, p < 0.001).
for other transmitters whereas AMP and MPH act
ATX-induced excitability changes between T1 and T2,
as indirect NE and dopamine agonists.
being before rTMS intervention, were further assessed
Considering these facts, a combination of rTMS and
by pooling the results of the 2 ATX-groups and the 2
ATX would be a promising intervention that might lead
placebo (PLC)-groups. Looking at the slope, rmANOVA
to clear learning effects. So far, there are no placebo-
with within-subject factor "time" and between-subject
controlled studies on the influence of ATX and rTMS
factor "group" revealed a significant effect of the factor
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
"time" (F(1,34) = 10.66, p < 0.00), of the interaction between
No effect of either ATX or rTMS could be shown for
"time" and "group" (F(1,34) = 8.57, p = 0.01) and of the factor
RMT (within-subject factor "time": F(1,2) = 1.22, p = 0.3;
"group" (F(1,34) = 5.09, p = 0.03), indicating a greater increase
between-subject factor "group": F(3,32) = 1.78, p = 0.17;
in slope in the ATX-groups as compared to the PLC-
interaction "time x group": F(3,6) = 0.66, p = 0.68).
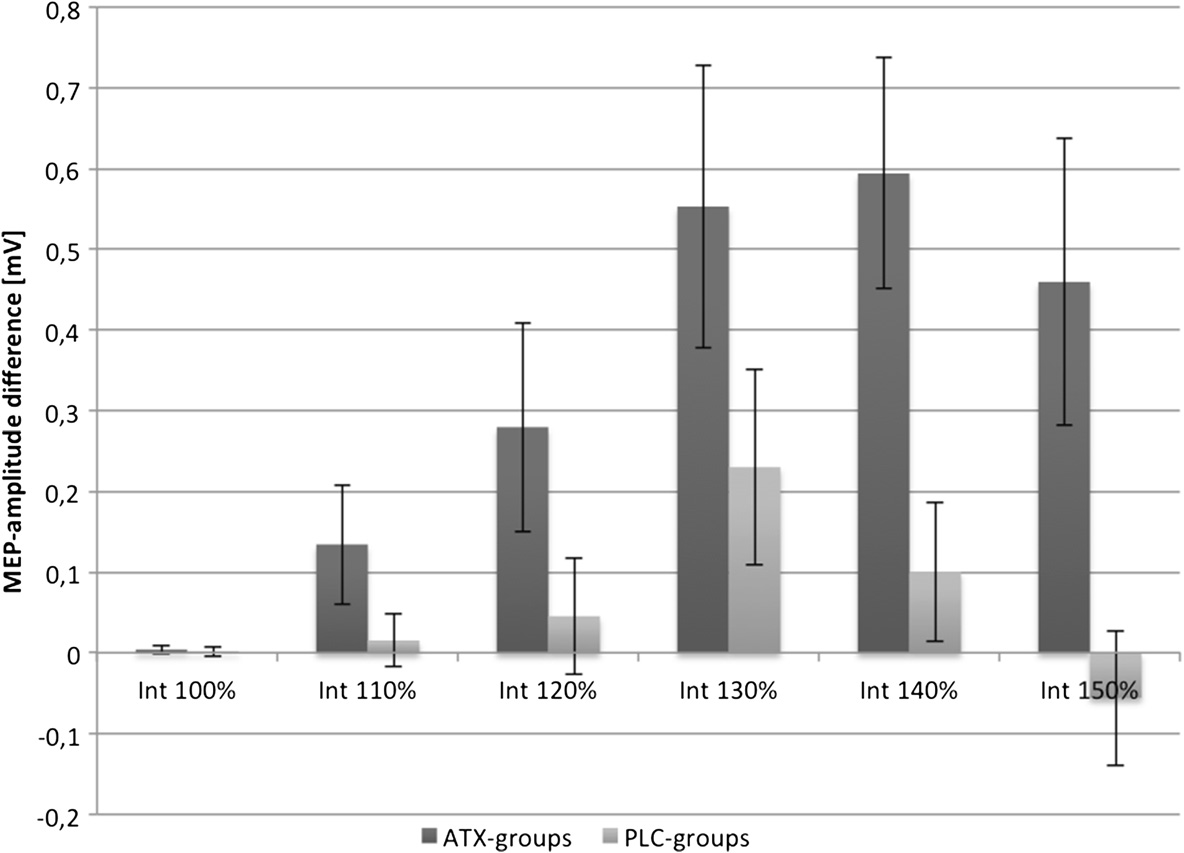
groups. For a better overview, Figure shows the meanMEP-differences of ATX- and PLC-groups between T2
and T1 (= ΔT2T1) for all 6 stimulation intensities.
Analyzing target-score (TS) data, rmANOVA demonstrated
Analyzing the SICI-ratio data, rmANOVA revealed
a significant effect of the within-subject factor "time" and
a significant effect of the within-subject factor "time"
the between-subject factor "group" (F(1,7) = 21.02, p < 0.001;
(F(1,2) = 6.69, p < 0.00), no significant effect of the
F(3,32) = 3.79, p = 0.02) but no significant interaction "time x
between-subject factor "group" (F(3,32) = 1.14, p = 0.35)
group" (F(3,21) = 0.93, p = 0.56; see Figure . Post-hoc inde-
and no interaction between "time x group" (F(3,6) = 0.49,
pendent two-sided t-tests revealed a significant difference
p = 0.81). Post-hoc t-test showed a significant difference
between PLC + sham-rTMS and ATX + real-rTMS group
between T1 and T3 (meanSICI-ratio differenceΔT3T1 =
(t(16) = 3.1, p = 0.007), but not for PLC + sham-rTMS
0.138 ± 0.238, t(35) = 3.5, p = 0.001). The other comparisons
and ATX + sham-rTMS (t(16) = 0.6, p = 0.563) and PLC +
were not significant (T1 vs. T2: t(35) = 2.1, p = 0.047; T2 vs.
sham-rTMS and PLC + real-rTMS (t(16) = 0.3, p = 0.754).
T3: t(35) = 1.9, p = 0.061).
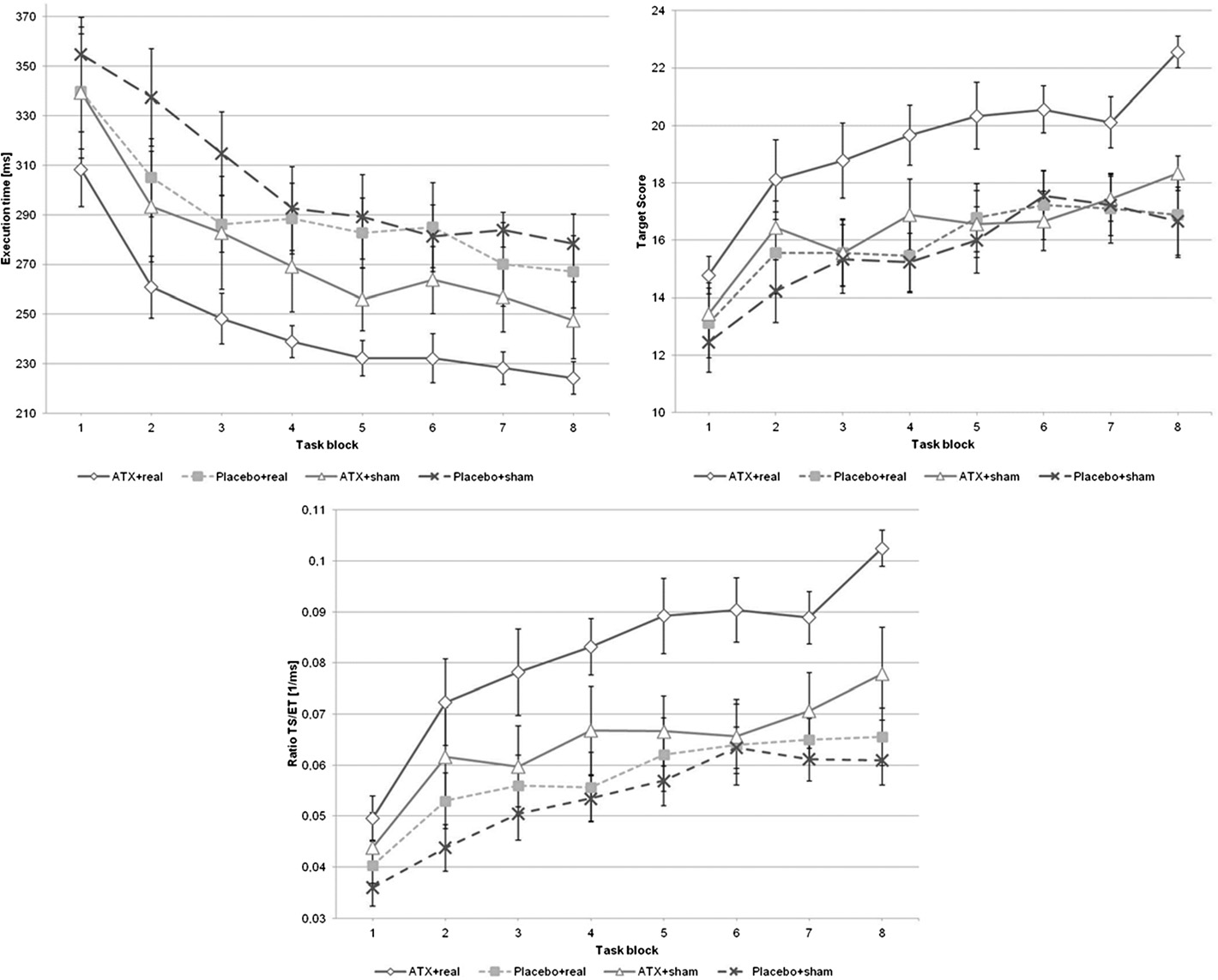
Similar results could be shown for the execution-time
RmANOVA of the ICF-ratio data showed a significant
(ET)- and TSET ratio (= ratio of target score and execution
effect of the within-subject factor "time" (F(1,2) = 13.9,
time)-analyses (see Figure Considering ET, rmANOVA
p < 0.001). Furthermore, a significant interaction be-
showed significant effects for the within-subject factor
tween the factor "time" and the between-subject factor
"time" (F(1,7) = 47.33, p < 0.001) and the between-subject
"group" could be demonstrated (F(3,6) = 2.57, p = 0.03).
factor "group" (F(3,32) = 3.3, p = 0.03) without a significant
The between-subject factor "group" revealed no significant
interaction between both factors (F(3,21) = 0.65, p = 0.87).
effect (F(3,32) = 2.35, p = 0.09). Post-hoc t-test showed a sig-
Post-hoc independent two-sided t-tests demonstrated
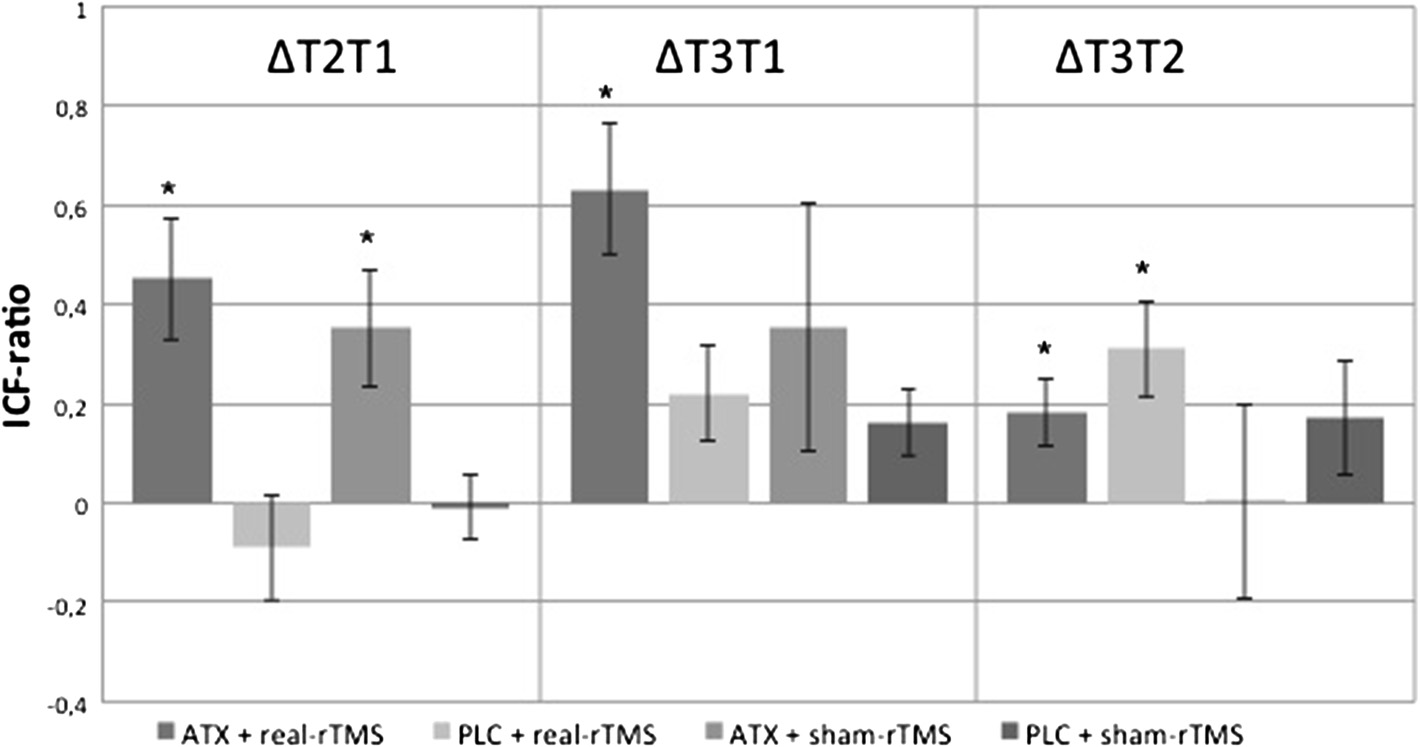
nificant increase of the ICF-ratio 1 hour after drug intake
significant differences only between the PLC + sham-rTMS
in both ATX-groups (ΔT2T1). ICF-ratio increased further
and ATX + real-rTMS group (t(16) = -3.9, p = 0.001).
in the ATX + real-rTMS-group after motor training
RmANOVA of the TSET ratio demonstrated a significant in-
and 10 Hz rTMS (ΔT3T2). This could not be demon-
fluence of the between-subject factor "group" (F(3,32) = 5.13,
strated for the ATX + sham-rTMS-group. Mean ICF-ratio
p = 0.01) and the within-subject factor "time" (F(1,7) = 39.14,
differencesΔT2T1, ΔT3T1 and ΔT3T2 and corresponding
p < 0.001). There was no significant interaction "time x
p-values are shown in Table and Figure
group" (F(3,21) = 1.53, p = 0.07; see Figure . Again, post
Figure 1 ATX effects after 1 hour. Mean-MEP differences of the stimulus-response curves between the measurements T2 and T1. Pooled dataof ATX and PLC groups are compared. Error bars depict standard error of the mean.
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
Table 1 ICF-ratio data
difference ± SEM
difference ± SEM
difference ± SEM
Multiple paired two-sided post-hoc t-test of ICF-ratio data. Bonferroni-corrected p-value threshold (p = 0.025). *significant.
hoc t-test showed significant higher TSET ratio for the
(see Figure Third, only the combination of ATX and
ATX + real-rTMS group compared to the PLC + sham-
10 Hz rTMS significantly improved motor learning with
rTMS group (t(16) = 4.4, p < 0.001). There were no sig-
regard to target score and execution time (see Figure ).
nificant differences between PLC + sham-rTMS groupand PLC + real-rTMS or ATX + sham-rTMS group. No
ATX led to a significant increase of corticospinal and
significant differences could be shown for the ER
intracortical excitability in M1
(rmANOVA: within-subject factor "time": F(1,7) = 0.01,
We could reproduce the facilitative effects of ATX on
p = 0.24; between-subject factor "group": F(3,32) = 1.11,
cortical excitability in M1 as had been previously shown by
p = 0.36; interaction "time x group": F(3,21) = 0.71, p = 0.82).
Gilbert and coworkers . In contrast, we did not see asignificant ATX-induced M1 disinhibition. The reduction
of intracortical inhibition between the beginning and end of
Our study yielded three major findings. First, ATX led to
the study (ΔT3T1) did not depend on the group, i.e. on the
a significant increase of corticospinal and intracortical
type of intervention. Similar effects of NE on M1 excit-
excitability in M1 one hour after intake of 60 mg ATX
ability could be demonstrated for the NE reuptake in-
(see Figures and ). Second, high frequency 10 Hz rTMS
hibitor reboxetine. Plewnia and coworkers
applied over M1 during a finger tapping motor task
showed enhanced corticospinal and intracortical excitabil-
was capable of further increasing intracortical excitability
ity and improved motor skills in healthy subjects suggest-
significantly, but only in combination with 60 mg ATX
ing that this is an effect of NE reuptake inhibitors. This
Figure 2 Intracortical facilitation. Treatment with 60 mg ATX significantly increased ICF after 1 hour (ΔT2T1). Further increase in ICF after rTMS/motor task sequence (ΔT3T1). p-values are shown in Table (p – threshold = 0.025). Error bars depict standard error of the mean. The absolutemean ICF-ratio values T2 from T1, T3 from T1 and T3 from T2 were subtracted (mean ICF-ratio difference ΔT2T1, mean ICF-ratio difference ΔT3T1,mean ICF-ratio difference ΔT3T1) to calculate the absolute difference. * = significant differences.
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
Figure 3 Motor task data. Time course of target score (TS), execution time (ET) and TS/ET-ratio (TSET) is shown for each group. The motor taskconsisted of 8 blocks. Error bars indicate standard error of the mean.
assumption could not be verified by Foster and coworkers
excitability induced by ATX favors the induction of synaptic
[]. They found no improvement of motor learning after
depression by the subsequent 10 Hz rTMS-stimulation and
intake of the NE reuptake inhibitor venlafaxine compared
motor task that themselves would induce LTP-like plas-
to ATX [They concluded that the affinity to other
ticity. Cortical LTP and LTD are typically mediated by
transmitters like serotonin and the lower dosage and
NMDA-receptor activation One reason, why we could
the higher rate of adverse effects of venlafaxine might
not see homeoplastic effects is that our 1st intervention
have led to contradictory results.
(ATX intake) had no effect on NMDA-receptors but onNE-receptors. All classic homeostatic plasticity protocols
High frequency 10 Hz rTMS applied over M1 during a
combine rTMS, transcranial direct current stimulation
finger tapping motor task was capable of further
(tDCS) or paired-associative stimulation protocols that
increasing intracortical excitability significantly, but only
typically involves NMDA-receptors [, for example
in combination with ATX
1 Hz rTMS with cathodal tDCS
In our study, we wanted to extend the approach of neuro-
Instead of homeostatic effects, a synergistic effect of ATX
pharmacological modulation of cortical activity and its use-
and rTMS was observed. The sum of gain in excitability in
dependent plasticity by additionally applying 10 Hz rTMS.
the ATX + 10 Hz rTMS group could not be explained by
Our rTMS paradigm itself had no significant facilitative
the single effects of ATX and 10 Hz rTMS. Because higher
effects on excitability parameters. This might be due to the
cortical excitability is a precondition for neuronal plasticity
low number of total TMS-pulses (i. e. 160 pulses). It is well
and improved learning process, this finding might be closely
known that rTMS effects depend on the number of total
related to the fact that we observed improved motor learn-
pulses, frequency and stimulation intensity [Interest-
ing only after combining both interventions. The significant
ingly, we could see a further increase in excitability only in
increase in ICF-ratio in the PLC + sham-rTMS condition
combination with 60 mg ATX (ATX + real-rTMS group).
could be explained by the motor task itself
This suggests that a premedication with ATX is capableof facilitating the effects of a low pulse number rTMS
Only the combination of ATX and 10 Hz rTMS improved
protocol. Homeostatic plasticity did not play a role in this
motor learning and execution time significantly
study. Following the concept of homeostatic plasticity, we
Looking at the motor task data, significant higher TS
would have expected that the enhancement of motor cortex
and TSET ratio and shorter ET could only be seen in
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
the verum-verum-condition, i. e. ATX + real-rTMS group.
leads to significant increase of activity in error signaling
There was no significant interaction between time and
forebrain areas like bilateral inferior frontal cortex and
group. Graphically, the TS, ET and TSET curves are shifted
pre-supplementary motor cortex, increasing neural sen-
in a parallel fashion. This indicates that the superiority
sitivity for errors in healthy humans [
of the ATX + real-rTMS group was not based upon a
Therefore, the combination of more effective synaptic
different effect on motor learning itself but upon a better
transmission within the motor system along with higher
performance at the beginning of the motor task due to
cognitive/behavioral sensitivity may have led to the
higher cortical excitability as previously mentioned.
synergistic effect of 10 Hz rTMS and ATX seen in our
Another reason might be the well known ATX effects
study. It could also explain the failure of ATX or 10 Hz
in promoting wakening and arousal ]. In contrast to
rTMS alone to be effective.
the results of Kim and coworkers , we did not observehigher TS for the PLC + real-rTMS group compared to
Pharmacological interventions and rTMS
the PLC + sham-rTMS. This may be due to our modified
Studies combining rTMS and neuropharmacological
and easier motor task, which could have prevented smaller
intervention were usually undertaken to investigate the
differences in learning to become visible.
role of transmitters in the induction of rTMS after-effects
Considering detail of our motor task, we decided to
and not to boost performance like we did in our study.
choose the non-dominant hand and according non-
Huang et al. combined a specialized rTMS-protocol called
dominant hemisphere (left hand, right hemisphere)
theta-burst-stimulation (TBS) with the NMDA receptor
for motor task, excitability measurements and rTMS-
antagonist memantine They found that, on one hand,
intervention because we wanted to avoid a ceiling effect
memantine inhibited the facilitatory effect of intermittent
We did not test the excitability parameters of the
TBS (iTBS) on MEP amplitudes. On the other hand, it
contralateral hemisphere and motor learning of the
blocked the suppressive effect of continuous (cTBS). Teo
contralateral hand. Thus, previous studies could dem-
et al. used the NMDA receptor coagonist D-cycloserine
onstrate that there is a hemispheric asymmetry of cor-
that acts at the glycine site of the NMDA receptor. They
ticospinal activation with a higher MEP facilitation for
found that it reversed the aftereffect of iTBS from facilita-
the non-dominant (left) hemisphere [Brouwer
tion to inhibition Lang et al. performed a 1 Hz rTMS
et al. showed that this different level of excitation is
study using the dopamine receptor agonist pergolide and
not related to speed or dexterity of finger movements.
found that the suppression of corticospinal excitability by
Relating to our study, we would expect similar facilitative
rTMS was more pronounced after drug intake compared
effects of ATX and rTMS on dominant hemisphere.
to placebo [These results show in general that NMDA
We also can purpose that there were differences in
and dopaminergic receptors play a role in the induction of
levels of cortical excitability between hemispheres not
rTMS effects.
only in baseline but also later in postinterventional
So far, no study has been undertaken to investigate
measurements. But according to Brouwer's results, these
adrenergic influences on rTMS effects. Furthermore, we
differences had no relation to differences in motor per-
have not found any study that considers the combination of
formance between both hands.
pharmacological intervention, rTMS and motor learning.
Interestingly, motor learning was not affected by ATX
In this case, we describe synergistic effects between rTMS
or 10 Hz rTMS alone. According to the subtraction
and pharmacological modulation for the first time.
method devised by Donders the entire effects on TSor ET should be explained by the cooperativity of the ATX
and rTMS effects. Applying this method, a factor "X"
Considering some limitations of our study, we surely have
remains which cannot be explained by the separate ATX
to mention the relative low number of subject per group
and rTMS effects (see Figure . This kind of synergistic
(n = 9). Furthermore, we did not choose a cross-over
effect "ATX x rTMS" indicates nonlinear interaction
design, which would have allowed intra-subjects compari-
between both components. The neuronal basis of this
son. A cross-over design would have had advantageous for
interaction, however, remains unclear. High frequency
the interpretation of the results, rendering out biases that
rTMS activates the motor network including premotor
comes from inter-individual variability of cortical excit-
cortex, supplemental motor area and ipsilateral primary
ability, partly determined by brain morphology .
motor cortex, and increases effective connectivity between
Moreover, one might argue that the use of circular coil
these areas [The finger tapping task itself activates
for excitability measurement does not extract reliable mea-
a similar system including contralateral primary sensori-
sures compared to a figure-of-eight coil. Ugawa et al. could
motor cortex, premotor cortex, supplementary motor
demonstrate no different results between figure-of-eight
cortex, bilateral secondary somatosensory areas and basal
coil over left hand motor area and circular coil over ver-
ganglia and ipsilateral cerebellum In addition, ATX
tex for the determination of corticocortical facilitation
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
Figure 4 Factorial design. This graphic shows the idea of a synergistic effect that only occurs in the verum-verum-group D (combination ofboth interventions). Furthermore, it shows how to calculate the synergistic effect. Group A = PLC + sham-rTMS; group B = PLC + real-rTMS; groupC = ATX + sham-rTMS; group D = ATX + real-rTMS. X indicates interaction factor "ATX x real-rTMS" (synergistic effect).
and inhibition Moreover, in our TMS-studies, we pre-
(DRKS-ID: DRKS00004653). All subjects were right-handed
fer the use of the circular coil. We could see stable results
as revealed by the Edinburgh Handedness Inventory ].
and the positioning of this coil is less critical
They all denied the practice of fine motor skills presently orin the past, such as playing a guitar or piano or as having
experience in professional typewriting. All participants were
Previous studies could show that high frequency rTMS
free of medication.
and ATX are capable to modulate cortical plasticity andto improve motor learning Possible interaction effects
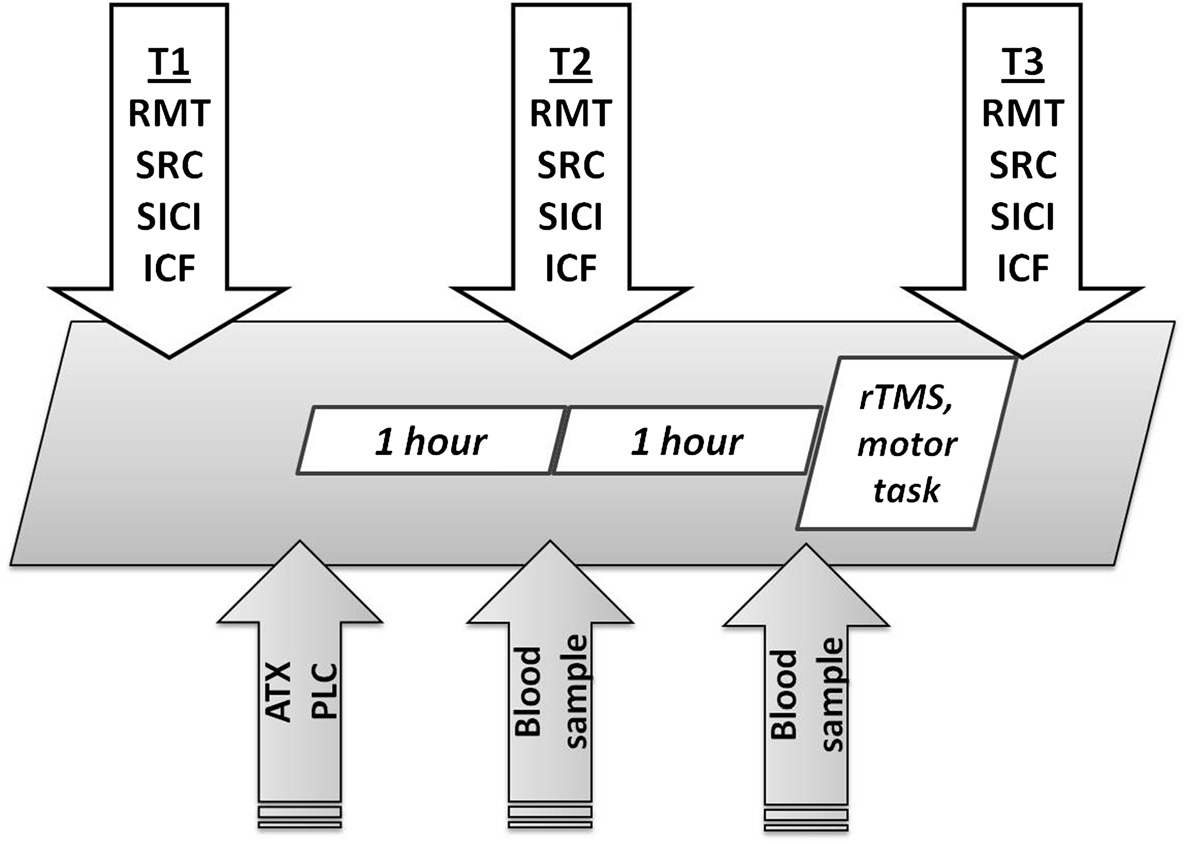
Time course and study design
have never been investigated. In the present study, we could
The study was randomized and placebo-controlled. It
show that the combination of a pharmacologically-induced
was double-blind for the ATX vs. PLC condition and
increase in NE transmission and 10 Hz rTMS exerts syner-
single-blind for the rTMS and sham-rTMS condition.
gistic effects on cortical excitability and motor learning in
A single dose of 60 mg ATX or placebo was given after
healthy humans.
baseline excitability measurements (T1). Blood samples
This could be a promising approach to improve motor
were collected one and two hours after administration
learning in patients with neurological disorders like
of ATX or PLC to determine ATX plasma concentration.
stroke, traumatic brain injury and neuromuscular diseases
One hour after drug intake, excitability measurements were
(e. g. amyotrophic lateral sclerosis). Especially, it would
repeated (T2). Then, all participants performed a sequential
be interesting to investigate the development and consoli-
motor task with their non-dominant left hand while inter-
dation of neuronal plasticity effects in primary motor cor-
mittently receiving single-blinded real 10 Hz rTMS or
tex when rTMS and ATX were administered over several
sham-rTMS in the manner described below. Finally, excit-
days. Furthermore, it remains unclear if such effects would
ability parameters were measured again (T3). Each meas-
be seen with other facilitative drugs like modafinil or
urement session took approximately 30 to 40 minutes, the
amantadine or in combination with other brain stimu-
rTMS + motor practice 10 minutes, and the entire study
lation protocols like tDCS and TBS.
three hours (see Figure
Participants left TMS-laboratory after baseline measure-
Data from 36 healthy subjects (19 women, 17 men) were
ments and went to a special room where the non-blinded
collected and analyzed. Subjects were randomly assigned
examiner (P.S.) who was participated in randomization and
to four equally-sized groups (n = 9). All subjects gave their
administration of the drugs only delivered the capsules.
written informed consent. The protocol was approved
Subjects received either a yellow-blue 60 mg ATX capsule
by the local ethical committee of the Ruhr-University
(Eli Lilly™) or a yellow-red capsule with mannitol (placebo).
of Bochum (registry no. 4317-12) and was performed
They swallowed it with a drink of water in this room and
in accordance with the Declaration of Helsinki. The
stayed for one hour before returning in TMS-laboratory.
study is registered in German Clinical Trials Register
The TMS-examiners have seen neither the capsules nor the
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
Figure 5 Time course of the study and study design.
drug intake itself. The subjects and TMS-examiner did not
Stimulus-response curve
know color code.
For SRC, spTMS was applied at 100%, 110%, 120%, 130%,140% and 150% of individual RMT. For each stimulus
Excitability measurements by TMS
intensity, 12 trials were performed. We used the same
The following parameters of corticospinal and intracortical
TMS-setting for SRC as for RMT-determination. For
excitability in the primary motor cortex were investigated:
analysis of the SRC data, the slope between data points
resting motor threshold (RMT), stimulus-response curve
Ti, i =1, 2, 3 of the SRC as the steepness of the linear re-
and both short intracortical inhibition and intracortical
gression line through the given data points (between
facilitation, assessed using paired-pulse TMS [MEPs
100% and 150% stimulation intensity of the individual
were recorded with Ag-AgCl-surface electrodes using a
RMT) was calculated ].
standard electromyography device (Neuropack M1; NihonKohden, Tokyo, Japan). While stimulating the contralateral
hemisphere, recordings were taken from the left first dorsal
To apply ppTMS, the circular coil was connected to the
interosseus muscle (FDI). The signals were recorded with a
Bistim® device which triggered two Magstim 200® stimu-
sampling rate of 5 kHz, and amplified with a bandpass of
lators. Earlier studies had shown that focal and circular
20 Hz - 3 kHz, a sweep duration of 10 - 50 ms/div and
coils elicited comparable results in pp-TMS studies
a gain of 0.1 mV/div. During the entire measurements,
In this ppTMS assembly, a separate RMT had to be de-
muscle relaxation was monitored by EMG. Subjects were
termined because of the lowered stimulator output in
seated in a comfortable chair in a silent and bright room.
case the Magstim 200 is connected to the Bistim® device.
We tested the interstimulus intervals (ISI) 2, 4, 10 and
Resting motor threshold
15 ms. The second stimulus (test stimulus) was adjusted
RMT was determined with single-pulse TMS to the
to evoke an MEP of approximately 1.0 mV. The condi-
nearest 1% of the stimulator output, and was defined
tioning stimulus was set at 80% of the individual ppTMS
as the minimum intensity which produced four motor
assembly RMT. For each ISI, 12 trials were performed.
evoked potentials > 50 μV out of eight trials Single-
Before and after ppTMS, 12 single control stimuli using
pulse TMS was applied using a Magstim 200® stimulator
the same stimulation intensity as for the second (test)
(Magstim, Whitland, Dyfed, U.K.) connected to a circular
stimulus were applied. The amplitude ratio of the mean
coil (outer diameter 14 cm). The coil was placed with its
conditioned MEP to the mean control MEP was calculated
center near the vertex with the current flowing clockwise
for each ISI. For further statistical analysis, parameters
in the coil in order to activate predominantly the right
of SICI and ICF were defined as the averages of MEP
hemisphere and to produce MEP in the left FDI. This
ratios obtained at inhibitory ISIs of 2 and 4 ms (SICI),
position was marked with a red wax pencil to improve
and at facilitative ISIs of 10 and 15 ms (ICF)
reproducibility of placement.
ICF-ratios between the three time points were compared

Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
by subtracting the means (mean ICF-ratio differenceΔT2T1,
spTMS elicited the highest MEP amplitude (hotspot of
mean ICF-ratio differenceΔT3T1 and mean ICF-ratio
the FDI). At the FDI hotspot, we had to determine a new
RMT because we used a figure-of-eight coil for focalrTMS. Twenty pulses of 10 Hz rTMS were applied for
2 seconds just prior to the beginning of each task block
A modified combined session of motor task and 10 Hz
with an intensity of 80% of individual RMT. A total of
rTMS as previously described by Kim and coworkers
160 pulses were given during each experiment consisting of
was used. The task was designed using Presentation®
eight task blocks. For sham-rTMS, the same stimulation
software (Neurobehavioral Systems Inc., Albany, California,
parameters and the same figure-of-eight-coil were used,
USA). Subjects were seated in a comfortable chair and
except for the stimulation intensity, which was set at the
placed 35 cm in front of a 19 inch LED-monitor. A seven-
lowest possible stimulation intensity (10% of maximal
digit sequence of numbers, namely the combination of "1 2
stimulator output). Previous work of ours confirmed
3 4 1 2 3" was presented on the monitor for 40 seconds.
this intensity to have only local effects at the scalp with no
Subjects were instructed to repeatedly push the four num-
effects on neuronal excitability in the motor cortex ].
bered buttons of the response box (Lumitouch™, PhotonControl, Burnaby, Canada) as accurately, as quickly and as
ATX serum concentration
often as possible using their left hand (one task block).
Blood samples were taken from all participants approxi-
In consideration of this quite easy tapping task, we de-
mately 1 hour and 2 hours after drug or PLC intake. After
cided to choose the non-dominant and non-specialized
finishing the study, ATX serum concentration was deter-
left hand to ensure motor improvement and learning
mined by liquid-chromatography tandem mass spectrom-
and to avoid an early ceiling effect. Our decision was
etry method (Laboratoriumsmedizin Dr. Eberhard & Co.,
not based on electrophysiological aspects like asymmetry
Dortmund, Germany).
in corticospinal activation but on motor task aspects.
Each button relates to one finger (no. 1 to the index finger,no. 2 to the middle finger, no. 3 to the ring finger, and no.
Statistical analysis
4 to the little finger). The motor task required the repetition
RmANOVA was performed with the within-subject factor
of eight identical task blocks with pauses of 28 seconds
"time" and between-subject factor "group" to assess
interleaved (see Figure Within each task block, motor
differences between different points of measurement
practice was preceded by rTMS application. Before starting
for the excitability parameters. Univariate ANOVA was
the entire motor task, subjects were allowed to perform
performed using age as dependent variables and group
two blocks of practice to familiarize themselves with the
as the between-subject factor. A chi-squared test was
equipment and experimental procedure. In this case, we
used to analyze sex differences between the different
used a different combination of numbers.
groups. For analysis of the behavioral data, we consideredthe total number of correct responses of an entire 7-digit
sequence (target score = TS), the execution time (ET)
For rTMS application, a Magstim Rapid® stimulator
defined by the time required to the subsequent correct
(Magstim, Whitland, Dyfed, UK) and a figure-of-eight
response, the ratio of TS and ET (TSET) and the error
shaped coil (outside diameter 8.7 cm, peak magnetic
rate (ER) defined by the equation
field strength 2.2 T, peak electric field strength 660 V/m)were used. The coil predominantly stimulates neuralstructures below the junction of the two coils. During
the entire stimulation procedure the coil was held tangen-
tially to the head in posterior-anterior direction with thehandle pointing backwards.
Real- and sham-rTMS were delivered over the right
RmANOVA was performed with within-subject factor
motor cortex at the scalp position where suprathreshold
"time" and between-subject factor "group". Where it was
Figure 6 Design of rTMS/motor-task sequence.
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
appropriate, post-hoc two-sided t-tests were additionally
Ziemann U, Meintzschel F, Korchounov A, Ilic TV: Pharmacological
applied. The significance level was adjusted by dividing
modulation of plasticity in the human motor cortex. Neurorehabil NeuralRepair 2006, 20:243–251.
it by the number of comparisons (Bonferroni correction).
Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G,
All calculations were performed using IBM SPSS Statistics
Zara F, Kazis A, Mills KR: Lorazepam-induced effects on silent period and
19.0 software package.
corticomotor excitability. Exp Brain Res 2006, 173:603–611.
Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, RothwellJC: Direct demonstration of the effect of lorazepam on the excitability of
the human motor cortex. Clin Neurophysiol 2000, 111:794–799.
ATX: Atomoxetine; ANOVA: Analysis of variance; ET: Execution time; ER: Error
Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG:
rate; NE: Norepinephrine; ppTMS: paired-pulse TMS; rmANOVA: Repeated
Mechanisms of use-dependent plasticity in the human motor cortex.
measures ANOVA; RMT: Resting motor threshold; rTMS: repetitive TMS;
Proc Natl Acad Sci U S A 2000, 97:3661–3665.
SEM: Standard error of the mean; spTMS: single-pulse TMS; SRC:
Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin JP,
Stimulus-response curve; TMS: Transcranial magnetic stimulation; TS: Target
Tegenthoff M: Riluzole suppresses motor cortex facilitation in correlation
score; TSET: Ratio of TS and ET; uANOVA: univariate ANOVA.
to its plasma level. A study using transcranial magnetic stimulation. ExpBrain Res 2000, 135:293–299.
Competing interests
Schwenkreis P, Witscher K, Pleger B, Malin JP, Tegenthoff M: The NMDA
The authors declare that they have no competing interests.
antagonist memantine affects training induced motor cortex plasticity–astudy using transcranial magnetic stimulation. BMC Neurosci 2005, 6:35.
Plewnia C, Hoppe J, Hiemke C, Bartels M, Cohen LG, Gerloff C: Enhancement of
Authors' contributions
human cortico-motoneuronal excitability by the selective norepinephrine
MSK, AB, OH, PS, MT, HRD, KF and DJ participated in the design of the study,
reuptake inhibitor reboxetine. Neurosci Lett 2002, 330:231–234.
the discussion of the results and drafted the manuscript. MSK and AB
Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps TD, Wassermann EM:
conducted the experiments. MSK, AB, OH and PS performed the statistical
Comparison of the inhibitory and excitatory effects of ADHD
analysis. All authors read and approved the final manuscript.
medications methylphenidate and atomoxetine on motor cortex.
Neuropsychopharmacology 2006, 31:442–449.
Plewnia C, Hoppe J, Cohen LG, Gerloff C: Improved motor skill acquisition
This study was supported by a grant from the Deutsche
after selective stimulation of central norepinephrine. Neurology 2004,
Forschungsgemeinschaft (SFB 874) and the Ruhr-University Bochum
(FORUM F729-2011). We thank Lauren Haag for proof reading.
Zerbe RL, Rowe H, Enas GG, Wong D, Farid N, Lemberger L: Clinicalpharmacology of tomoxetine, a potential antidepressant. J Pharmacol Exp
Ther 1985, 232:139–143.
1Department of Neurology, BG-Universitaetsklinikum Bergmannsheil Bochum,
Wong DT, Threlkeld PG, Best KL, Bymaster FP: A new inhibitor of
Buerkle-de-la-Camp-Platz 1, 44789 Bochum, Germany. 2Institute for
norepinephrine uptake devoid of affinity for receptors in rat brain.
Neuroinformatics, Ruhr-University Bochum, 44780 Bochum, Germany.
J Pharmacol Exp Ther 1982, 222:61–65.
3Department of Neurophysiology, Medical Faculty, Institute of Physiology,
Plewnia C, Hoppe J, Gerloff C: No effects of enhanced central
Ruhr-University Bochum, 44780 Bochum, Germany.
norepinephrine on finger-sequence learning and attention.
Psychopharmacology (Berl) 2006, 187:260–265.
Received: 5 January 2014 Accepted: 27 March 2014
Foster DJ, Good DC, Fowlkes A, Sawaki L: Atomoxetine enhances a short-term
Published: 2 April 2014
model of plasticity in humans. Arch Phys Med Rehabil 2006, 87:216–221.
Tsumoto T: Long-term potentiation and long-term depression in the
neocortex. Prog Neurobiol 1992, 39:209–228.
Chen R: Studies of human motor physiology with transcranial magnetic
Huang YZ, Chen RS, Rothwell JC, Wen HY: The after-effect of human theta
stimulation. Muscle Nerve Suppl 2000, 9:S26–S32.
burst stimulation is NMDA receptor dependent. Clin Neurophysiol 2007,
Ridding MC, Rothwell JC: Is there a future for therapeutic use of
transcranial magnetic stimulation? Nat Rev Neurosci 2007, 8:559–567.
Teo JT, Swayne OB, Rothwell JC: Further evidence for NMDA-dependence
Hoogendam JM, Ramakers GM, Di Lazzaro L: Physiology of repetitive transcranial
of the after-effects of human theta burst stimulation. Clin Neurophysiol
magnetic stimulation of the human brain. Brain Stimul 2010, 3:95–118.
Ridding MC, Rothwell JC: Stimulus/response curves as a method of
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC:
measuring motor cortical excitability in man. Electroencephalogr Clin
Preconditioning of low-frequency repetitive transcranial magnetic stimulation
Neurophysiol 1997, 105:340–344.
with transcranial direct current stimulation: evidence for homeostatic
Rosenkranz K, Williamon A, Rothwell JC: Motorcortical excitability and
plasticity in the human motor cortex. J Neurosci 2004, 24:3379–3385.
synaptic plasticity is enhanced in professional musicians. J Neurosci 2007,
Liepert J, Terborg C, Weiller C: Motor plasticity induced by synchronized
thumb and foot movements. Exp Brain Res 1999, 125:435–439.
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe
Berridge CW, Schmeichel BE, Espana RA: Noradrenergic modulation of
S, Asselmann P, Marsden CD: Corticocortical inhibition in human motor
wakefulness/arousal. Sleep Med Rev 2012, 16:187–197.
cortex. J Physiol 1993, 471:501–519.
Brouwer B, Sale MV, Nordstrom MA: Asymmetry of motor cortex
Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone
excitability during a simple motor task: relationships with handedness
P, Tonali P, Rothwell JC: Magnetic transcranial stimulation at intensities
and manual performance. Exp Brain Res 2001, 138:467–476.
below active motor threshold activates intracortical inhibitory circuits.
Daligadu J, Murphy B, Brown J, Rae B, Yielder P: TMS stimulus-response
Exp Brain Res 1998, 119:265–268.
asymmetry in left- and right-handed individuals. Exp Brain Res 2013,
Tegenthoff M, Cornelius B, Pleger B, Malin JP, Schwenkreis P: Amphetamine
enhances training-induced motor cortex plasticity. Acta Neurol Scand
Donders FC: On the speed of mental processes. Acta Psychol (Amst) 1969,
Sczesny-Kaiser M, Tegenthoff M, Schwenkreis P: Influence of 5 Hz
Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ:
repetitive transcranial magnetic stimulation on motor learning.
Acute remapping within the motor system induced by low-frequency
Neurosci Lett 2009, 457:71–74.
repetitive transcranial magnetic stimulation. J Neurosci 2003, 23:5308–5318.
Kim YH, Park JW, Ko MH, Jang SH, Lee PK: Facilitative effect of high
Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RS:
frequency subthreshold repetitive transcranial magnetic stimulation on
Frequency specific changes in regional cerebral blood flow and motor
complex sequential motor learning in humans. Neurosci Lett 2004,
system connectivity following rTMS to the primary motor cortex.
Neuroimage 2005, 26:164–176.
Sczesny-Kaiser et al. BMC Neuroscience 2014, 15:46
Mima T, Sadato N, Yazawa S, Hanakawa T, Fukuyama H, Yonekura Y,Shibasaki H: Brain structures related to active and passive fingermovements in man. Brain 1999, 122(Pt 10):1989–1997.
Graf H, Abler B, Freudenmann R, Beschoner P, Schaeffeler E, Spitzer M,Schwab M, Gron G: Neural correlates of error monitoring modulated byatomoxetine in healthy volunteers. Biol Psychiatry 2011, 69(9):890–897.
Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M:Dopaminergic potentiation of rTMS-induced motor cortex inhibition. BiolPsychiatry 2008, 63:231–233.
Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB,Mattingley JB: Simple metric for scaling motor threshold based onscalp-cortex distance: application to studies using transcranialmagnetic stimulation. J Neurophysiol 2005, 94:4520–4527.
List J, Kubke JC, Lindenberg R, Kulzow N, Kerti L, Witte V, Floel A:Relationship between excitability, plasticity and thickness of the motorcortex in older adults. Neuroimage 2013, 83:809–816.
Ugawa Y, Hanajima R, Kanazawa I: Motor cortex inhibition in patients withataxia. Electroencephalogr Clin Neurophysiol 1994, 93:225–229.
Schwenkreis P, Tegenthoff M, Witscher K, Bornke C, Przuntek H, Malin JP,Schols L: Motor cortex activation by transcranial magnetic stimulation inataxia patients depends on the genetic defect. Brain 2002, 125:301–309.
Schwenkreis P, Janssen F, Rommel O, Pleger B, Volker B, Hosbach I,Dertwinkel R, Maier C, Tegenthoff M: Bilateral motor cortex disinhibition incomplex regional pain syndrome (CRPS) type I of the hand. Neurology2003, 61:515–519.
Schwenkreis P, Scherens A, Ronnau AK, Hoffken O, Tegenthoff M, Maier C:Cortical disinhibition occurs in chronic neuropathic, but not in chronicnociceptive pain. BMC Neurosci 2010, 11:73.
Oldfield RC: The assessment and analysis of handedness: the Edinburghinventory. Neuropsychologia 1971, 9:97–113.
Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W: Magneticstimulation: motor evoked potentials. The International Federation ofClinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 1999,52:97–103.
Ziemann U, Chen R, Cohen LG, Hallett M: Dextromethorphan decreases theexcitability of the human motor cortex. Neurology 1998, 51:1320–1324.
doi:10.1186/1471-2202-15-46Cite this article as: Sczesny-Kaiser et al.: Synergistic effects of noradrenergicmodulation with atomoxetine and 10 Hz repetitive transcranial magneticstimulation on motor learning in healthy humans. BMC Neuroscience 2014 15:46.
Submit your next manuscript to BioMed Central
and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution
Submit your manuscript at www.biomedcentral.com/submit
Source: https://www.ini.rub.de/upload/file/1470692851_ca15bd5efc81ffab05cd/Synergistic_effects_of_noradrenergic_modulation_with_atomoxetine_and_10_Hz_repetitive_transcranial_magnetic_stimulation_on_motor_learning_in_healthy_humans.pdf
DWI/Drug Courts: Defining a National Strategy Judge Jeff Tauber (Ret.) Director C. West Huddleston Deputy Director March 1999 DWI/Drug Courts: Defining a National Strategy Prepared by the National Drug Court Institute, the education, research and scholarship affiliate of the National Association of Drug Court Professionals. Copyright © 1999, National Drug Court Institute; reprinted May 2004 NATIONAL DRUG COURT INSTITUTE This document was prepared in cooperation with the American Council on Alcoholism; the National Commission Against Drunk Driving; the National Sheriffs' Association; the Drug Courts Program Office, Office of Justice Programs, U.S. Department of Justice; and the National Association of Drug Court Professionals. This document was prepared under Cooperative Agreement Number 1999-DC-VX-K001 from the Bureau of Justice Assistance, U.S. Department of Justice, with the support of the Office of National Drug Control Policy, Executive Office of the President. Points of view or opinions in this document are those of the authors and do not necessarily represent the official position of the U.S. Department of Justice or the Executive Office of the President. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the National Drug Court Institute. Printed in the United States of America. Drug courts perform their duties without manifestation, by word or conduct, of bias or prejudice, including, but not limited to, bias or prejudice based upon race, gender, national origin, disability, age, sexual orientation, language or socioeconomic status.
Original Article · Originalarbeit Forsch Komplementmed 2014;21:239–245 Published online: August 5, 2014 Evidence for the Efficacy of a Bioresonance Method in Smoking Cessation: A Pilot Study Aylin Pihtilia Michael Galleb Caglar Cuhadarogluc Zeki Kilicaslana Halim Isseverd Feyza Erkana Tulin Cagataya Ziya Gulbarana a Department of Pulmonary Diseases, Faculty of Medicine, University of Istanbul, Turkeyb Institute for Biophysical Medicine, Idar-Oberstein, Germanyc Department of Pulmonary Diseases, Faculty of Medicine, Acibadem University, Istanbul, Turkeyd Department of Community Health, Faculty of Medicine, University of Istanbul, Turkey








