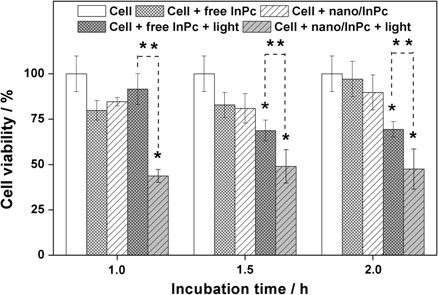
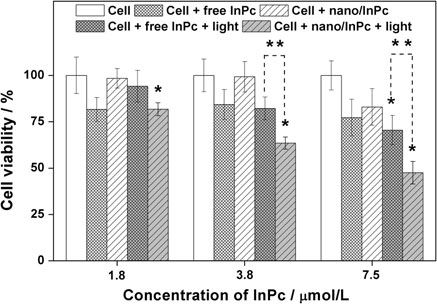
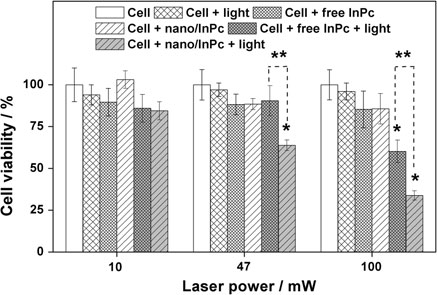
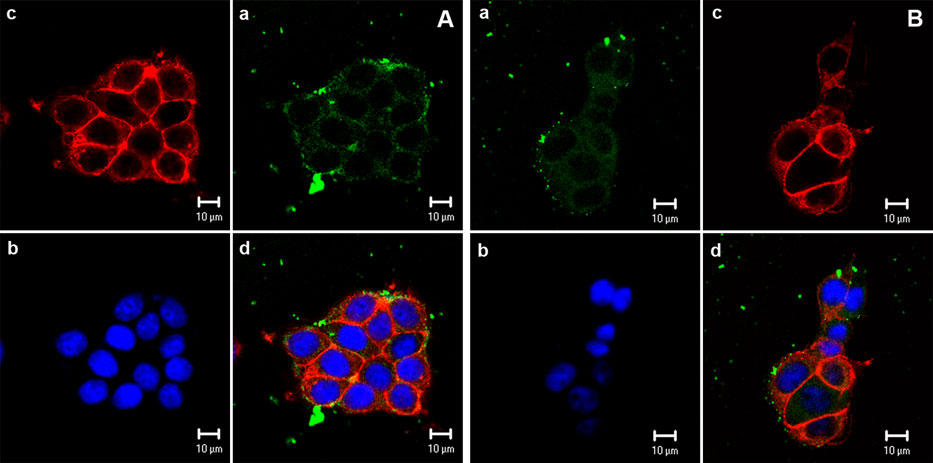
The economics of irreparable harm in pharmaceutical patent litigation
CORNERSTONE RESEARCH FINANCIAL AND ECONOMIC CONSULTING AND EXPERT TESTIMONY The Economics of Irreparable Harm in Pharmaceutical Patent Litigation Rahul Guha, Cornerstone ResearchMaria Salgado, Cornerstone Research TABLE OF CONTENTS The Hatch-Waxman Act greatly simplifies the process of obtaining FDA approval for a generic drug. Under the Act, a generic company need only file an Abbreviated New Drug