Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Euco-net.eu
The Open Infectious Diseases Journal, 2011, 5, (Suppl 1-M5) 51-59
Open Access
The Situation of HIV/M. tuberculosis Co-Infection in India
Seth Research Foundation, 1, Aradhana Colony, Sector 13, R.K. Puram, Ring Road, New Delhi-110066, India
Abstract: In November 2008, European Commission initiated a collaborative research and information dissemination
project entitled "European Network for global cooperation in the field of AIDS and TB (EUCO-Net)" involving
Institutions in Europe (Germany, Belgium, Italy), Latin America (Brazil, Argentina, Colombia), Russia, South Africa, and
India with the following objectives: a) to provide an overview of the state of art in HIV and TB research and disease
management in different partner countries; b) to identify global research priorities; and c) to boost International
cooperation between leading HIV and TB experts from Europe and those countries mainly affected by these two diseases.
Therefore, in this report from India these objectives have been addressed under the following topics: i) Basic demographic
data; ii) Basic epidemiological Data of HIV and TB; iii) Medical treatment standards; iv) Diagnostic Standards.
Keywords: India, HIV/
M. tuberculosis co-infection, revised national TB control programme (RNTCP).
causes of morbidity and one of the leading causes of mortality in people living with HIV/AIDS.
The first HIV estimation in India was done in 1994 based
on data from 52 sites. Since then, the process of estimation
In recent years, India has witnessed rapid expansion of the
of HIV infected persons in the country has evolved to a very
DOTS strategy for TB control and scaling up of interventions to
great extent. Since, the samples from which data were
combat HIV/AIDS, including access for treatment with
collected through sentinel surveillance were not exactly
antiretroviral drugs. With the establishment of new mechanisms
representative of the general population certain assumptions
of funding like the Global Funds for AIDS, TB and Malaria,
were used to generate estimates for the general population.
financing of interventions against MTB/HIV is no longer an
Over the years, these assumptions were gradually refined
issue. Additionally, over the last few years, considerable
with the help of other available data sources. The year 2006
experience has been gained on TB and HIV programme
provided a unique opportunity when multiple data sources
coordination, as well as individual patient care. Progress in
such as a community based HIV prevalence study of
improving HIV/MTB coordination and linkages in service
National Family Health Survey-III, Integrated Bio-
delivery will contribute greatly to achieving these goals.
behavioural Assessment Survey, Endline Behavioural
BASIC DEMOGRAPHIC DATA
Surveillance Survey could be utilized along with the data from the expanded sentinel surveillance system to arrive at
The demographical information of India was obtained from
more robust HIV estimates that are closer to reality.
a website "Census of India" [1]. The information collected
Moreover, in 2006, the Workbook Model of WHO-UNAIDS
during a countrywide census conducted in 2001. Census in
was adopted that allowed international comparability.
India is undertaken every ten years.
Special statistical packages such as Random effects model
India is a large country comprising 28 states and 7 union
and Spectrum Projection Software are utilized to make more
territories. These states and the union territories are divided into
accurate and reliable estimates.
districts. At the time of Census 2001 there were in all 593
The burden of MTB/TB and HIV/AIDS pose districts. Each district is further divided in to sub-districts, which
unprecedented challenges on the public health system in
are known differently in different parts in the country.
India. Globally, India has the highest burden of TB and the
As of 1st March, 2001 the population of India stood at
number of HIV-infected people estimated in India is second
1,027,015,247 comprising 531,277,078 males and 495,738, 169
highest after South Africa. In addition to morbidity and
females. Thus, India becomes the second country in the world
mortality, the two diseases cause substantial economic and
after China to cross the one billion mark. Life expectancy at
social burden on the nation. TB and HIV are overlapping
birth is 64 years. Urban population constitutes only 27.2% of
epidemics. The HIV pandemic presents a massive challenge
the total population. Meaning thereby, almost three-fourth's of
to the control of TB at all levels. People living with HIV
India's population lives in rural areas. One-third of India's
have increased susceptibility to active tuberculosis, and HIV
households are in urban areas, with two-thirds in rural areas.
infection is the greatest risk factor worldwide for tuberculosis disease. TB is also one of the most common
BASIC EPIDEMIOLOGIC DATA
*Address correspondence to this author at the Seth Research Foundation, 1,
In India, countrywide control and prevention of
Aradhana Colony, Sector 13, R.K. Puram, Ring Road, New Delhi-110066,
HIV/AIDS and Tuberculosis is the responsibility of the
India; Tel: +91-9810311407; Fax: +91-11-26876639; E-mail:
[email protected]
Government of India, Ministry of Health and Family
1874-2793/11
52 The Open Infectious Diseases Journal, 2011, Volume 5
Pradeep Seth
Welfare (MOHFW). Two independent departments have
regions. NACO utilizes this information for effective
been created under the MOHFW to achieve the desired
planning and implementation of its programs. Standardized
effect. National AIDS Control Organization (NACO) is
methods supported by WHO/UNAIDS are employed for
responsible for control and prevention of HIV/AIDS; and
estimating the burden of the epidemic overtime. Adult HIV
Central TB Division of the Directorate General of Health
prevalence has been estimated using WHO/UNAIDS
Services, looks after tuberculosis prevention and control.
Workbook restructured in 2006 for Indian epidemic
Epidemiological data released by these sources were chosen
situation. It included five subpopulations: intravenous drug
because they were based on sound epidemiological methods
users (IDU), men having sex with men (MSM), female sex
accepted by the WHO and UNAIDS. The data were
workers (FSW), long distance truckers and the general
comprehensive and permitted comparison with global data
population represented by the antenatal clinic attendees.
released by the WHO and UNAIDS.
Age-wise stratification of data was done by Spectrum
Epidemiological data on HIV/AIDS were obtained from
NACO report on HIV sentinel surveillance and HIV
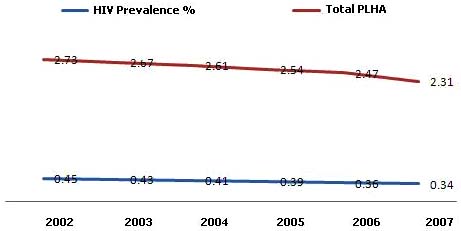
The total number of people living with HIV/AIDS
estimation in India-2007 [2] and Annual Report NACO
(PLHA) in India in 2007 is estimated to be 2.31 million (1.8-
2008-09 [3]. The data on Tuberculosis (TB) and multiple
2.9 million, Fig. 1). Females constitute around 39% of the
drug resistance tuberculosis MDR) were obtained from the
burden (0.9 million). Children below 15 years constitute
reports from Central TB Division [4, 5].
3.5% of the estimated number of PLHA while elderly people
HIV/AIDS epidemiologic data from NACO did not
with age greater than 49 years constitute 7.8%. Adults aged
stratify the estimated prevalence data as per EUCO-Net
15-49 years constitute 88.7% of the estimated number of
questionnaire requirement particularly prevalence of HIV
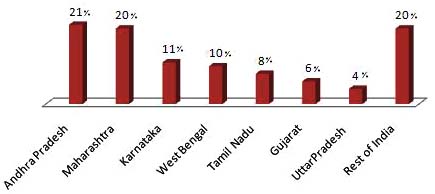
PLHA. The highest number of PLHA is in Andhra Pradesh
infection in children (0 to 12 years). The cut-off age as per
and Maharashtra, with nearly half-a-million PLHA each.
NACO document is <15 years for children. Therefore in the
Besides Tamil Nadu and Karnataka, West Bengal, Gujarat
present report also we used <15 years cut-off age for
and Uttar Pradesh are estimated to have higher burden of the
disaggregation of data between children and adults.
epidemic with greater than 0.1 million PLHA in each of these states. The four South Indian states contribute 60% of
Epidemiological data on TB were obtained from Revised
all PLHA in the country and along with West Bengal,
National Tuberculosis Control Programme (RNTCP) Report
Gujarat and Uttar Pradesh they contribute 80% of PLHA in
-2009 [4]. Here also, Central TB Division did not stratify the
India. Though Manipur and Nagaland have the highest HIV
estimated prevalence data age-wise. Indeed, it is only from
prevalence in the country, due to small population size, the
the year 2006 that the pediatric patients are being covered
estimated number of PLHA in these two states is less than
under RNTCP. In addition, other reports of the Central TB
25,000. The states of Kerala, Bihar and Rajasthan have more
Division like Technical and operational Guidelines of TB
than 50,000 PLHA each though the HIV prevalence in these
control [6] Pediatric Guidelines, RNTCP Guidelines for
states is low. Fig. (2) shows the distribution of PLHA among
diagnosis of TB [7], RNTCP DOTS plus guidelines [8] etc.
the high burden states of India.
Estimated Adult HIV prevalence in India in 2007 is
0.34% (0.25%-0.43%) (Fig. 1). Estimated HIV prevalence
among males (0.40%) continues to be higher than among
HIV-AIDS Epidemiology
females (0.27%). Estimated Adult HIV prevalence remains
The data generated through HIV sentinel surveillance is
>1% in Manipur (1.57%) and Nagaland (1.20%) in 2007.
used to estimate the level of infections in the country at
Andhra Pradesh has an estimated adult HIV prevalence of
regular intervals. The annual surveillance and estimation
0.97% while Karnataka and Maharashtra have estimated
helps to understand the course of epidemic stage in different
adult HIV prevalence <1%. Tamil Nadu, West Bengal,
Fig. (1). Estimated Adult HIV Prevalence and total number of PLHA (million) in India 2002-07 (Adapted from HIV Sentinel Surveillance
Report).
The Situation of HIV/M. tuberculosis Co-Infection in India
The Open Infectious Diseases Journal, 2011, Volume 5 53
Fig. (2). Distribution of PLHA among High Burden States (2007) (Adapted from Annual Report NACO).
Gujarat and Delhi have estimated adult HIV prevalence of
Maharashtra state: Pune, Mumbai and Thane have shown
0.4%. Fig. (2) shows the year-wise estimated adult HIV
>30% HIV prevalence among FSW.
prevalence from 2002 to 2007 derived from Spectrum
However, there is a decline in HIV prevalence among
Package. Fig. (3) shows the state-wise estimated adult HIV
FSW in South Indian States reflecting the impact of
prevalence in selected groups for the years 2006 and 2007
interventions, while rising trends are evident in the North
derived from Workbook model as well as Spectrum Package.
East suggesting a dual nature of the epidemic. In the low
However, there are considerable differences in the
prevalence states, the trends are stable.
prevalence rates across different geographical regions. HIV
Men who have Sex with Men
Prevalence amongst ANC clinic attendees has been around 1% in the states of Andhra Pradesh, Karnataka in South
Expansion of surveillance among MSM has revealed new
India and in Manipur, Nagaland and Mizoram in North-
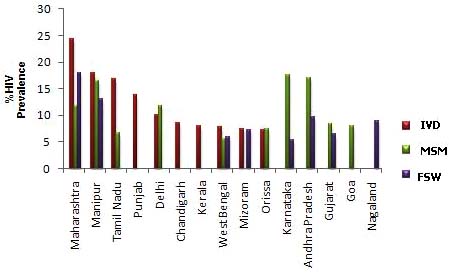
pockets of epidemic. Among MSM, high HIV prevalence is
eastern region of India. Tamil Nadu and Maharashtra have
recorded in the states of Karnataka (17.6%) followed by
recorded less than 1% HIV prevalence in ANC clinic
Andhra Pradesh (17.04%), Manipur (16.4%), Maharashtra
attendees. The epidemic is greater in urban areas than rural
(11.80%) and Delhi (11.73%), Goa (7.93%) and Gujarat
areas, greater among males than females, decreases with
(8.40%) (Fig. 3). In total, 10 states have shown greater than
increasing education level, and is found to be highest among
5% HIV prevalence among MSM. Thirty out of forty
women whose spouses work in transport industry.
districts with MSM sites show HIV prevalence greater than 5%. Among MSM, HIV trends are rising in south Indian
HIV Epidemic Among High Risk Groups
states. Rising trends are also noted Delhi while trends are
Female Sex Workers
stable at the single MSM site in Manipur.
At the state level, HIV prevalence among FSWs is very
STD Clinic Attendees
high in Maharashtra (17.91%), followed by Manipur
Among the STD clinic attendees, Andhra Pradesh
(13.07%), Andhra Pradesh (9.74%), Nagaland (8.91%) and
continues to show the highest prevalence (19.72% (7.60%-
Mizoram (7.2%). Among the other states, Gujarat,
39.20%) followed by Maharashtra (16.18% (7.20%-32.20%),
Karnataka, and West Bengal have HIV prevalence greater
Karnataka (7.15% (1.60%-10.80%)) and Tamil Nadu
than 5% among FSW. Fig. (3) shows state-wise HIV
(12.04% (1.60%-38.40%)). Mizoram (7.13%) and Goa
prevalence among high risk groups. Pockets in three cities of
Fig. (3). States with high prevalence among different groups, 2007; IVD, injecting drug users; MSM, men who have sex with men; FSW,
female sex workers (Adapted from HIV Sentinel Surveillance Report).
54 The Open Infectious Diseases Journal, 2011, Volume 5
Pradeep Seth
(5.60%) have also shown HIV prevalence greater than 5%
estimated at 3.8 million bacillary cases for the year 2000, by
among STD clinic attendees. Trends among STD clinic
the expert group of Govt. of India. In addition, an estimated
attendees are declining at all India level and in high
40% of the Indian population is latently infected with M.
prevalence states, while rising trends are noted in
tuberculosis.
Chhattisgarh, Mizoram and Gujarat. Stable trends are noted
By any measure the burden of TB in India is staggering.
in other low prevalence states.
More than 80% of the burden of tuberculosis is due to
Injecting Drug Users
premature death, as measured in terms of disability-adjusted
High HIV prevalence among IDUs has been noted in
life years (DALYs) lost. Every day, more than 5,000 people
Maharashtra (24.4%), Manipur (17.90%), Tamil Nadu
develop TB disease, and nearly 1,000 people die of TB, i.e. 2
(16.80%), Punjab (13.79%), Delhi (10.10%), Orissa (7.3%)
deaths every 3 minutes. As per WHO estimates in 2006,
and Kerala & West Bengal (7.8%). Trends among IDUs are
nearly 322,000 persons in India died of tuberculosis
on a decline in Manipur, Nagaland and Chennai reflecting
(mortality rate 28 per 100,000 persons), which was estimated
impact of interventions while rising trends are noted in
at over 500,000 annually at the beginning of the RNTCP.
Meghalaya, Mizoram, West Bengal, Mumbai, Kerala and
Data from specific surveys, however, suggest that case
fatality rates prior to RNTCP were generally greater than 25%. In the RNTCP era, case fatality has remained below
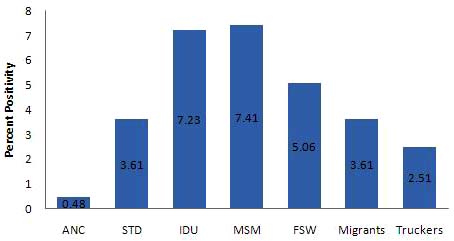
The overall HIV prevalence among different population
5% for new cases registered for treatment under the
groups in 2007 continues to portray the concentrated
programme. Deaths due to TB exceed the combined deaths
epidemic in India, with a very high prevalence among High
from all other communicable diseases and account for 26%
Risk Groups - IDU (7.2%), MSM (7.4%), FSW (5.1%) &
of all avoidable adult deaths. TB is also the leading killer of
STD clinic attendees (3.6%) (Fig. 4) and low prevalence
women, causing more orphans than those produced by all
among ANC clinic attendees (age adjusted - 0.48%).
causes of maternal mortality combined.
Though heterosexual mode of transmission is still the
MDR/XDR Tuberculosis
predominant mode of HIV transmission in India, HIV epidemic in India appears to be dual epidemic driven by
The emergence of resistance to drugs used to treat TB,
sexual and IDU routes of transmission, concentrated in
and particularly MDR-TB, has become a significant public
nature with high HIV prevalence among high risk groups
health problem in a number of countries and an obstacle to
and heterogeneous in spread with pockets of infection found
effective TB control. Several small surveys conducted across
in various districts of the country.
the country have shown the prevalence rates of MDR-TB in the country at around 1%- 3% among new cases, and 12%
Tuberculosis Epidemiology
among retreatment cases. A retrospective analysis of various
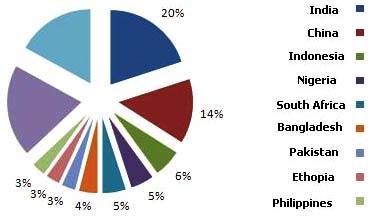
India is the highest TB burden country globally (29.9
randomized clinical trials conducted by the Tuberculosis
million patients), accounting for one fifth of the global
Research Centre (Chennai, India) with various rifampicin
incidence and 2/3rd of the cases in South East Asia (Fig. 5).
containing regimens in the initial intensive phase, and with
Nearly 40% of the Indian population is infected with the
or without rifampicin in the continuation phase, revealed an
MTB bacillus. Each year, 1.9 million new cases of TB occur
overall emergence of resistance to rifampicin in only 2%
in the country, of which about 0.8 million are infectious new
patients despite a high level (18%) of initial resistance to
smear positive pulmonary TB cases. The estimate of TB
isoniazid either alone or in combination with other anti-TB
incidence in India is based on findings of the nationwide
drugs. A large scale population based survey in the states of
Annual Risk of MTB Infection (ARTI) study conducted in
Gujarat and Maharashtra has also indicated similar resistance
2000-03. The national ARTI was estimated at 1.5% i.e. 75
levels (new-3% and retreatment-12-17%). Available
new smear positive pulmonary TB cases are expected per
information suggests that the proportion of MDR-TB is
100,000 population annually. The prevalence of TB has been
relatively low in India. However, this translates into a large absolute number of cases, with an estimated annual
Fig. (4). HIV prevalence among different population groups (Adapted from Annual Report NACO).
The Situation of HIV/M. tuberculosis Co-Infection in India
The Open Infectious Diseases Journal, 2011, Volume 5 55
Fig. (5). Global Incidence of TB Burden (Adapted from RNTCP Status Report).
incidence of 110,000 cases of MDR-TB. XDR-TB has been
However, BCG vaccination has no effect on the transmission
reported in India by isolated studies with non-representative
of the disease. The most effective step in the prevention of
and highly selected clinical samples. The magnitude of the
TB is to cure infectious cases in order to break the chain of
problem remains to be determined due to the absence of
transmission. The BCG Vaccination is given at birth under
laboratories capable of conducting quality assured second
UIP to all children including HIV infected child if the child
line Drug Susceptibility Testing (DST). However, what is
is asymptomatic or mildly symptomatic. The guidelines
frightening is the potential threat of XDR-TB in India, with
issued by the NACO, recommend withholding all live
unregulated availability and injudicious use of the second
vaccines for symptomatic and severely immune-
line drugs along with non-existence of systems to ensure
compromised HIV infected children.
standardized regimens and treatment adherence for MDR-TB outside the national programme. The problem of MDR and
MEDICAL TREATMENT STANDARDS
XDR-TB in India and across the world raises the possibility
HIV Medical Treatment Standards
that the current TB epidemic of mostly drug susceptible TB will be replaced with a form of TB with severely restricted
The free ART programme was launched on 1 April 2004
treatment options. If this happens it would jeopardize the
by the Government of India. The technical guidelines were
progress made in recent years to control TB globally as well
initially modified from "WHO guidelines for ART in
as in India and would also put at risk the plans to progress
resource constraint settings". As the global experience
towards a world where TB ceases to be a public health
increased a need was felt to revise the 2004 guidelines. The
final guidelines were developed which were again modified in view of revised WHO 2006 ART guidelines [10]. As
A major limiting factor in making State wise
recommended in these guidelines any person who has a
representative data on MDR/XDR-TB is the lack of quality
confirmed HIV infection is subjected to further evaluation
assured culture and drug sensitivity testing (DST) laboratory
for determining whether he requires ART or not by
facilities. It is important to note that the diagnosis of
performing CD4 count and other baseline investigations. All
MDR/XDR-TB is laboratory based. RNTCP is in the process
those eligible as per technical guidelines are started on ART.
of establishing in a phased manner a nationwide network of
Initially eight tertiary-level government hospitals in the six
quality assured culture and DST laboratory facilities.
high-prevalence states of Andhra Pradesh, Karnataka,
Maharashtra, Tamil Nadu, Manipur and Nagaland, as well as the NCT of Delhi were included in this programme. In Phase
In India BCG vaccination is a part of Universal
I of the implementation of this programme, the subgroups of
Immunization Programme (UIP) under which vaccines for
the people living with HIV/AIDS (PLHA) targeted on a
six preventable diseases (tuberculosis, diphtheria. Pertusis,
priority basis were: i) sero-positive mothers who have
tetanus, poliomyelitis and measles) are available to all
participated in the prevention of parent-to-child transmission
eligible children free of cost. This programme was launched
(PPTCT) programme; ii) sero-positive children below the
in 1985 with the aim to cover all districts by 1990 and to
age of 15 years; and iii) people with AIDS who seek
immunize 85% of all infants against six vaccine preventable
treatment in government hospitals. The ART centres were
diseases. BCG vaccination has a complementary role in TB
scaled up in a phased manner and to provide treatment to
control, with particular impact in the prevention of severe
100,000 patients by the end of 2007 and 300,000 patients by
forms of TB in children [9]. The vaccination has a non-
2011 in 250 centres across the country.
specific beneficial effect on infant survival and a BCG scar is a marker of better survival among children in areas with
The free ART programme has adopted the public health
high child mortality. Important contributing factors for the
approach to administration and distribution of ART all of
variable efficacy observed for the present BCG vaccine are
which are manufactured in India. This implies a
said to include background immunity induced by non-
comprehensive prevention, care and treatment programme,
tuberculous environmental mycobacteria, diversity of BCG
with a standardized, simplified combination of ART
strains, and over- attenuation of presently used strains.
regimens, a regular secure supply of good-quality ARV
56 The Open Infectious Diseases Journal, 2011, Volume 5
Pradeep Seth
drugs, and a robust monitoring and evaluation system. Since
NACO. Nonetheless, NACO has developed guidelines in
all first line and second line ART drugs are being
2008 on roll out of second line ART [11]. According to these
manufactured in India the Government of India has been able
guidelines failure of first line ART should be suspected
to scale up ART under NACO with the aim to provide care
among the patients who having received first line ART for at
and treatment to as many people as possible, while working
least six months either show clinical deterioration after initial
towards universal access to care and treatment. The selection
improvement or no improvement despite good adherence to
of first-line regimens is determined on the basis of a number
therapy. The guidelines further define ART failure as
of considerations, such as potency, profile of side-effects,
clinical, immunological (fall in CD4 count to pre-therapy
ability to keep future treatment options open, ease of
level or persistent CD4 levels below 100 cells/ul), and
adherence, cost, risk during pregnancy and potential of the
virological (plasma viral load >10,000 copies/mL).
development of resistant viral strains. The current global
After the failure of first line therapy the second-line ART
recommendation in all circumstances is a triple drug
is the next regimen immediately used in sequence. Second
line ART was initiated in January 2008 at only two site
The national ART programme aims at i) providing long-
(Tamil Nadu and Mumbai). During following 12 months it
term ART to eligible patients, ii) monitoring and reporting
was expanded to 10 more centres. Though provision was
treatment outcomes on a quarterly basis; iii) attaining
made to provide second line drugs to 3000 patients during
individual drug adherence rates of 95% or more; iv)
2008-09, only 344 patients were receiving second line drugs.
increaseing life span so that 50% of patients on ART are
The NACO standard second line regimen comprise
alive 3 years after starting the treatment; and v) ensuring that
Tenofovir DF+ Lamivudine+ Zidovudine+
50% of patients on ART are engaged in or can return to their
Lopinavir/Ritonavir (TDF + 3TC + ZDV + LPV/r) aims to
previous employment.
achieve viral suppression for as long as possible, so that
Eligibility for ART
survival can be prolonged. Current NACO treatment
guidelines recommend that the protease inhibitor (PI) class is
The national programme offers ART to the following
reserved for, and therefore characterizes second-line ART.
groups of persons: i) All persons with HIV infection who are clinically eligible to receive ART; and ii) those who are
Transmission of Drug Resistant HIV Strains
already on ART (outside the national programme) and want
There is no study from NACO on this subject. Studies
to enrol with the national programme for the available ART
from academic institutions on small sample size suggest
regimens, after written informed consent.
about 10% primary infections may be due to resistant strains
Antiretroviral Therapy Regimens
(Seth P. unpublished). Nevertheless, there is low prevalence of primary drug resistant infections. This scenario offers an
Currently, the national programme provides the opportunity to maintain low levels of drug resistant HIV
following combinations for first-line regimens
strains by provision of better treatment monitoring.
Stavudine (30 mg) + Lamivudine (150 mg)
However, compliance is an issue in patients undergoing
ARV treatment even though it is free. Therefore, there is
Zidovudine (300 mg) + Lamivudine (150 mg)
likelihood of rise in drug resistant HIV strains and their
(iii) Stavudine (30 mg) + Lamivudine (150 mg) +
subsequent transmission.
Nevirapine (200 mg)
Tuberculosis Medical Treatment Records
(iv) Zidovudine (300 mg) + Lamivudine (150 mg) +
RNTCP launched in 1997 is based on DOTS strategy.
Nevirapine (200 mg)
RNTCP uses intermittent short-course chemotherapy (SCC)
regimens to facilitate direct observation of treatment. This is
consistent with the World Health Organization guidelines. RNTCP ensures that there is no interruption in drugs and
Current NACO treatment guidelines for first-line ART
treatment once a person is diagnosed with TB. Sufficient
recommends two classes of drugs for initial treatment ie 2
anti-TB drugs in patient-wise boxes are made available at all
NRTI + 1 NNRTI. Fixed-dose combinations (FDCs) are
the appropriate levels (Peripheral Health Institution/TB
preferred because they are easy to use, have distribution
unit/District/ State/National) ensuring that the treatment does
advantages (procurement and stock management), improve
not stop mid-way due to lack of drugs.
adherence to treatment and thus reduce the chances of development of drug resistance. The current national
The uninterrupted supply of drugs to each patient is made
experience shows that bid (twice a day) regimens of FDCs
possible through the "patient-wise box." Patient-wise drug
are well tolerated and complied with.
boxes (both adult and pediatric) are an innovation of RNTCP
wherein a box of medications for the entire duration of the
Initiative to provide free ART to pediatric patients was
treatment is earmarked for every patient registered. This
launched on Nov 30, 2006 by NACO. So far 47,784 children
ensures the availability of the full course of medication to the
living with HIV/AIDS (CLHA) have registered at ART
patient the moment s/he is registered for treatment. Patient-
centres and 14,383 CLHA have been receiving free ART as
wise drug boxes have helped to improve patient care,
adherence, drug supply and drug stock management.
Since monitoring of patients on first line ART is done by
Under RNTCP, all sub-centres, primary health centres,
periodic peripheral blood CD4 T cell count at ART centres,
community health centres, and other health facilities provide
data on emerging ART resistant strains are not available with
DOTS services to patients. Since TB patients may also seek
The Situation of HIV/M. tuberculosis Co-Infection in India
The Open Infectious Diseases Journal, 2011, Volume 5 57
treatment from private physicians, the government has taken
initiatives to provide DOTS services through the private sector and through community volunteers.
India has well developed laboratory approaches for ensuring blood safety and donation safety (including tissue
By March 2006, 1114 million populations have been
and organ); surveillance of high risk groups; sentinel
covered in a nationwide programme for tuberculosis
surveillance; diagnosis and research. Most HIV antibody
detection. So far 10 million patients have been treated with a
screening and supplemental testing is performed at various
success rate of 86%, reducing the death rate due to
testing centres under NACO whereas Reference centres
tuberculosis from 29% in 1990 to 4% in 2006.
provide reference diagnostic services for problematic sera
Direct Observed Treatment (DOTS and DOTS-Plus)
and perform assays like Western Blots, PCR and viral load assays etc.
Directly observed treatment (DOT) is one of the key elements of the DOTS strategy. Under DOTS programme
Primary screening is done at Integrated Counselling and
RNTCP uses four oral anti-TB drugs (Isoniazid, Rifampicin,
Testing (ICTC) by rapid tests. Rapid tests approved by the
Ethambutol and Pyrazinamide). In DOTS, an observer
NACO for use as primary screening tests are based on the
(health worker or trained community volunteer who is not a
principle of EIA or particle agglutination. These are provided
family member) watches and supports the patient in taking
free of cost to all ICTCs and other testing centres under
drugs. It is this DOTS provider who ensures that the patient
NACO. Samples found positive in primary screening are
takes the right drugs, in the right doses, at the right intervals,
further tested by two different EIAs/ELISAs or by Western
for the right duration. 30000 DOTS providers have been
Blot assay for validation of results. This service is provided
recruited nationwide to provide treatment to the patients.
free of cost to all individuals reporting at ICTCs.
Under optimal programme conditions, treatment without
Since HIV infection in infants (<18 months) born to
observation achieves a success rate of 30-60%, whereas,
HIV–infected mothers cannot be established on the basis of
direct observation results in a much higher success rate of
serological tests because of transplacentally transmitted
85-95%. DOTS helps to reduce development of drug
maternal antibodies HIV specific DNA PCR is
resistance, because direct observation ensures adherence and
recommended. Facility for such a test is provided at all the
hence reduces the probability of emergence of drug-resistant
Reference centres by the NACO. These tests are also
organisms. Further, following a correct treatment regimen
provided free of cost only in these centres.
reduces the spread of infection in the community and helps
Although NACO recommends CD4+ T-cell enumeration
in controlling development of new cases.
and HIV plasma viral load by real time PCR assay for
To address the MDR-TB problem through appropriate
monitoring patients on ART, it has been providing only
management of patients and strategies to prevent the
CD4+ T-cell enumeration to all patients free of costs.
propagation and dissemination of MDR-TB, the Indian
Tuberculosis Diagnosis
Government introduced DOTS-Plus programe under RNTCP in 2009 and issued DOTS-Plus guidelines promoting full
Sputum microscopy continues to be the primary tool for
integration of DOTS and DOTS-Plus activities so that
detection of infectious TB, as it provides information on the
patients with MDR-TB are both correctly identified and
extent of infection of the patient, helps in categorization of
properly managed under this programme.
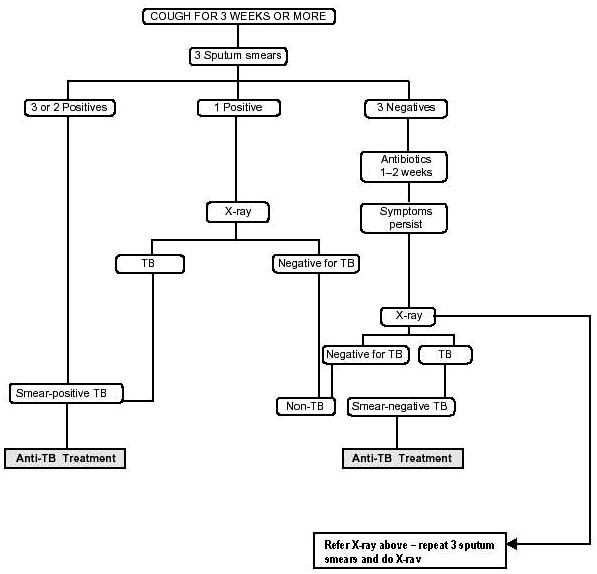
the patient for treatment and is an objective method to monitor the patient's progress. Moreover, the result is
Under DOTS-plus programme RNTCP uses oral second
available within two days and the correct treatment can be
line six anti TB drugs (Kanamycin, Ofloxacin, Ethionamide,
started immediately. Apart from sputum microscopy,
Pyrazinamide, Ethambutol and Cycloserine) during 6-9
RNTCP also uses standardized diagnostic algorithms to
months of the Intensive Phase and 4 drugs (Ofloxacin,
diagnose and treat all forms of TB wherein X-ray plays a
Ethionamide, Ethambutol and Cycloserine) during the 18
supporting role (Fig. 6).
months of the Continuation Phase. p-aminosalicylic acid
(PAS) is included in the regimen as a substitute drug if any
MDR/XDR-TB Diagnosis
bactericidal drug (Kanamycin, Ofloxacin, Z Pyrazinamide
A major limiting factor in making State wise
and Ethionamide) or 2 bacteriostatic (Ethambutol and
representative data on MDR/XDR-TB is the lack of quality
Cycloserine) drugs are not tolerated. All the drugs used in
assured culture and drug susceptibility testing (DST)
DOTS and DOTS-plus strategies of RNTCP are laboratory facilities. It is important to note that the diagnosis
manufactured in India.
of MDR/XDR-TB is laboratory based. The programme is in
DIAGNOSTIC STANDARDS AND AVAILABILITY
the process of establishing a network of 27 accredited
DIAGNOSTIC TESTS
Culture and Drug Susceptibility testing Intermediate
Reference Laboratories (IRLs) across the country in a phased
HIV Diagnosis
manner for diagnosis and follow up of MDR-TB patients.
Six culture and DST Labs including 6 IRLs (Andhra Pradesh, Delhi, Gujarat, Kerala, Maharashtra and Tamil
All laboratories including those in the private sector
Nadu) and 2 private sector labs (BPRC Andhra Pradesh and
undertaking HIV testing activities follow guidelines issued
CMC Vellore) have been accredited in 2008. Another 8 IRLs
by the National AIDS Control Organization [12]. These
(Chattisgarh, Haryana, Jharkhand, Orissa, Rajasthan,
guidelines provide all information regarding the diagnosis of
Uttarakhand, Uttar Pradesh, West Bengal) and 5 medical
HIV infection, monitoring of patients on ART etc.
college laboratories were under accreditation process and
58 The Open Infectious Diseases Journal, 2011, Volume 5
Pradeep Seth
Fig. (6). Tuberculosis Diagnosis Algorithm (Adapted from RNTCP Status Report).
expected to be accredited in 2010. The remaining IRLs will
Prevalence States. Three other states namely Goa, Gujarat
be accredited in 2011.
and Pondicherry, have been classified as Moderate HIV prevalence states. Even within the high prevalence states,
HIV/MTB CO-INFECTION
there are districts which have ante-natal HIV levels below
The emergence and spread of HIV and drug-resistant
tuberculosis further threaten to complicate the tuberculosis
Tuberculosis is one of the earliest opportunistic diseases
situation in the country. India, the third highest HIV
to develop amongst persons infected with HIV. HIV
burdened country, had an estimated 2.47 million people
infection is the most powerful risk factor for the progression
living with HIV/AIDS (PLHAs) in 2006 (that is, 0.36% of
of latent MTB infection to TB disease. An HIV positive
adult population in the country), emphasizing the enormous
person co-infected with MTB has 50-60% life time risk of
challenge ahead. All States and Union Territories of the
developing TB disease, as compared to an HIV negative
country have reported HIV/AIDS patients. However, the
person who has a 10% life-time risk of developing TB
HIV epidemic pattern shows great variance across the
disease. Thus TB mortality could well be influenced by the
country. The worst affected states are Andhra Pradesh,
MTB/HIV co-infection al least in certain districts in the
Karnataka, Maharashtra, Manipur, Nagaland and Tamil
country particularly in districts in India with high prevalence
Nadu. These six states have reported more than 75% of all
of HIV in TB patients.
the AIDS cases in India and are classified as High
The Situation of HIV/M. tuberculosis Co-Infection in India
The Open Infectious Diseases Journal, 2011, Volume 5 59
In India, the TB epidemic is pre-dominantly driven by
Programme of the European Commission. Author of this
the non-HIV positive TB cases. It is estimated that nearly
report was Core group expert on HIV and Tuberculosis from
5% of all TB patients are infected with HIV. The periodic
India in this project. He was very ably assisted by additional
HIV survey in TB patients, which was carried out in 4
experts (Dr. Naveet Wig, Dr. Kamini Walia and Dr. Akash
districts in 2005-06, was scaled up to 15 districts in 2006-07.
Gulalia) in collection of information used for preparation of
The 2007 survey represents the most detailed evaluation to
the Country report and this manuscript. Collation of
date of HIV epidemiology among TB patients in India. The
information and assistance in writing the manuscript by
survey demonstrated that the prevalence of HIV among TB
Kartik Yadav is greatly appreciated.
patients varied substantially across the geographic regions between 1% and 13.8% across the 15 surveyed districts [13].
REFERENCES
A national policy to coordinate common activities for
Census of India, Registrar General & Census Commissio-ner, India, Ministry of Home Affairs, Government of India 2001
HIV/AIDS and TB has been formulated by the National
(available at http://censusindia.gov.in)
AIDS Control Organization and the Central TB Division
HIV Sentinel Surveillance and HIV Estimation in India 2007: A
[14]. TB and TB/HIV interventions are reciprocally included
technical brief. National AIDS Control Organization, Ministry of
in the national policies of both programmes. TB/HIV
Health and Family Welfare, Government of India 2008 (available at http://www.nacoonline.org)
coordination activities are conducted nationwide, and are
Annual Report 2008-2009. National AIDS Control Organization
being intensified in 9 states with districts considered to have
Ministry of Health and Family Welfare, Government of India 2010
the highest HIV burdens in the country. Training and
(available at http://www.nacoonline.org)
sensitization of all health staff is being conducted using
TB India 2009: RNTCP Status Report Central TB Division,
jointly developed TB/HIV training modules. Surveillance for
Directorate General of Health Services, Ministry of Health and Family Welfare, Nirman Bhawan, New Delhi-110001. 2009
HIV infection among TB patients that has been previously
(available at http://www.tbcindia.org)
conducted in special surveys now relies upon routine
TB India 2010: RNTCP Status Report Central TB Division,
reporting of HIV status of TB patients. In 2008 0.16 million
Directorate General of Health Services, Ministry of Health and
HIV positive individuals were referred to RNTCP
Family Welfare, Nirman Bhawan, New Delhi-110001, 2010 (available at http://www.tbcindia.org)
programme for MTB detection and 0.13 million MTB
Technical and Operational Guidelines for Tuberculosis Control.
infected individuals were referred to ICTC (Integrated
Central TB Division, Directorate General of Health Services,
Counselling and treatment Centres) for HIV detection. 35000
Ministry of Health and Family Welfare, Nirman Bhawan, New
and 20000 patients were found to be infected with MTB and
Delhi-110001, 2005 (available at http://www.tbcindia.org)
HIV, respectively.
Guidelines for Quality Assurance of smear microscopy for diagnosing tuberculosis. Central TB Division, Directorate General
In response to the dual epidemics of AIDS and TB, the
of Health Services, Ministry of Health and Family Welfare, Nirman
World Health Organization (WHO) has recommended has
Bhawan, New Delhi-110001. 2005 (available at http:// www.tbcindia.org)
recommended guidelines for AIDS and TB prevention, care
DOTS-Plus Guidelines. Central TB Division, Directorate General
and treatment services. These include interventions that
of Health Services, Ministry of Health and Family Welfare, Nirman
reduce the morbidity and mortality from TB in people living
Bhawan, New Delhi-110001. 2010 (available at http://
with HIV, such as the provision of antiretroviral therapy
www.tbcindia.org)
V.G. Rao, P.G. Gopi, J. Bhat, R. Yadav, D.F. Wares. Role of BCG
(ART) and the Three I's for HIV/TB: intensified case-
vaccination in tuberculosis control. Curr Sci 2009; 96: 1307-8.
finding of TB (ICF), isoniazid preventive therapy (IPT), and
Antiretroviral Therapy Guidelines for HIV-infected Adults and
infection control for TB. These guidelines emphasize that
Adolescents Including Post-exposure Prophylaxis. National AIDS
IPT is a core component of HIV prevention and care, and
Control Organization, Ministry of Health and Family Welfare,
should be the primary responsibility of AIDS programmes
Government of India 2007 (available at http://www.naco online.org)
and HIV service providers. In addition, the provision of IPT
[11] National Guidelines on Second-line ART for adults and
should not be viewed as an isolated intervention for people
adolescents. National AIDS Control Organization, Ministry of
living with HIV. Rather, it should be part of a TB prevention
Health and Family Welfare, Government of India 2008 (available
package along with infection control for TB, ICF and
at http://www. nacoonline.org)
Guidelines on HIV Testing. National AIDS Control Organization,
provision of ART. However, in contrast with many other
Ministry of Health and Family Welfare, Government of India 2007
countries, isoniazid preventative therapy is not
(available at http://www.nacoonline.org)
recommended in clinical care guidelines for human
The HIV-TB Co-infection. National AIDS Control Organization &
immunodeficiency virus (HIV)-infected persons with latent
Central TB Division. Ministry of health and Family Welfare,
tuberculosis (TB) in India.
Government of India 2008 (available at http://www.naco online.org)
National Framework for Joint TB/HIV Collaborative Activities. Central TB Division and National AIDS Control Organization,
The work presented in this manuscript is derived from
Ministry of Health and Family Welfare, Government of India 2008
the EUCO-Net project funded by the 7th Framework
(available at http://www.tbcindia.org)
Received: November 26, 2010
Revised: February 27, 2011
Accepted: March 2, 2011
Pradeep Seth; Licensee Bentham Open.
This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/ by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Source: http://www.euco-net.eu/fileadmin/euco-net/Downloads/OpenAccessJournal/India.pdf
Dispositivos del orden neoliberal en la política educativa chilena reciente. Imperativos para quien osa María Angélica Oliva2 ¿Qué poseen en común el Objetivo Educacional, de 1965; el Objetivo Fundamental, de 1990; el Aprendizaje Esperado, de 2009; y el Objetivo de Aprendizaje, de 2012? Al responder a esta interro-
Mayo Clinic Proceedings Access this article on Remission of Disseminated Cancer After Systemic Oncolytic Published Online: May 13, 2014 Publication stage: In Press Corrected Proof No data is available Need help playing this video? Supplemental Video AbstractMV-NIS is an engineered measles virus that is selectively destructive to myeloma plasma cells and can be monitored bynoninvasive radioiodine imaging of NIS gene expression. Two measles-seronegative patients with relapsing drug-refractory myeloma and multiple glucose-avid plasmacytomas were treated by intravenous infusion of 10 TCID









