Viagra gibt es mittlerweile nicht nur als Original, sondern auch in Form von Generika. Diese enthalten denselben Wirkstoff Sildenafil. Patienten suchen deshalb nach viagra generika schweiz, um ein günstigeres Präparat zu finden. Unterschiede bestehen oft nur in Verpackung und Preis.
Dbkweb.mib.man.ac.uk
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 2010, p. 2075–2085
Copyright 2010, American Society for Microbiology. All Rights Reserved.
Monitoring the Effects of Chiral Pharmaceuticals on Aquatic
Microorganisms by Metabolic Fingerprinting䌤
Emma S. Wharfe, Catherine L. Winder, Roger M. Jarvis, and Royston Goodacre*
School of Chemistry and Manchester Interdisciplinary Biocentre, University of Manchester,
131 Princess Street, Manchester M1 7DN, United Kingdom
Received 2 October 2009/Accepted 22 January 2010
The effects of the chiral pharmaceuticals atenolol and propranolol on Pseudomonas putida, Pseudomonas
aeruginosa, Micrococcus luteus, and Blastomonas natatoria were investigated. The growth dynamics of
exposed cultures were monitored using a Bioscreen instrument. In addition, Fourier-transform infrared
(FT-IR) spectroscopy with appropriate chemometrics and high-performance liquid chromatography
(HPLC) were employed in order to investigate the phenotypic changes and possible degradation of the
drugs in exposed cultures. For the majority of the bacteria studied there was not a statistically significant
difference in the organism's phenotype when it was exposed to the different enantiomers or mixtures of
enantiomers. In contrast, the pseudomonads appeared to respond differently to propranolol, and the two
enantiomers had different effects on the cellular phenotype. This implies that there were different meta-
bolic responses in the organisms when they were exposed to the different enantiomers. We suggest that our
findings may indicate that there are widespread effects on aquatic communities in which active pharma-
ceutical ingredients are present.
Active pharmaceutical ingredients (APIs) and their metab-
Despite the fact that little is known about the effects of APIs
olites are ubiquitous in the environment (12), and the occur-
in the environment, the fact that they are designed to have a
rence of APIs in the aquatic environment is a growing concern
specific mode of action in humans must be taken into account
(13). There are a number of routes by which APIs and their
(12). Adverse side effects may occur in humans at higher doses
metabolites and degradation products may enter these ecosys-
of the APIs, and it can be expected that any beneficial or
tems, and a common avenue is through excretion of the APIs
adverse effect may also be observed in aquatic organisms with
and their metabolites in urine and feces. It is known that APIs
similar biological functions or receptors. It must also be noted
have different rates of metabolism in humans. For example,
that similar targets may control different metabolic processes
the -blocker propranolol is almost completely metabolized in
in different species (43), and therefore APIs and their metab-
the liver, and only 1 to 4% of an oral dose is excreted as the
olites may have additional modes of action in aquatic organ-
unchanged API and its metabolites. In contrast, 40 to 50% of
isms. The effects of the APIs may be subtle due to the very low
an oral dose of atenolol (also a -blocker) is excreted as the
concentrations observed in the aquatic environment, and as a
API or its metabolites (2, 6, 7). Subsequent degradation of the
result these effects may go unnoticed (12). It is also likely that
APIs and their metabolites may also occur at sewage treatment
the effect of an API has an impact on the local population
plants (STPs); this degradation is usually substrate specific and
dynamics in the whole ecosystem, from bacteria to higher or-
varies greatly for different APIs. The rates of adsorption to
ganisms. To explore the effects of APIs on biological systems,
activated sewage sludge during treatment differ for different
a wide range of concentrations should be employed along with
APIs and are dependent on the hydrophobic and electrostatic
appropriate analytical platforms to profile the complement of
interactions of the APIs with the particulates and microorgan-
biochemical components in cells. Indeed, it is known that APIs
isms in the activated sewage sludge (13). Any remaining APIs
can become concentrated in the benthic environment of river
and relevant metabolites are diluted in the surface water when
beds, and as bacteria inhabit this niche, the bacterial commu-
the effluent is released from the STP. Hence, many APIs are
nity may be exposed to higher-than-expected levels of these
present at low concentrations (ng liter⫺1 to g liter⫺1) in
compounds (16, 37, 49).
aquatic environments, such as rivers, streams, and estuaries (2,
While the effect of APIs in the environment is currently a
6, 12). The majority of APIs are neither persistent nor highly
growing area of research, there is very little understanding of
bioaccumulative; however, the continuous release of APIs into
the environmental effects of chiral pharmaceuticals (5, 14). A
the aquatic environment poses a potential risk to aquatic or-
chiral molecule is a molecule that lacks an internal plane of
ganisms even though the concentrations of APIs in the receiv-
symmetry. The nonsuperimposable mirror images are termed
ing waters are quite low (12).
enantiomers and are labeled (
R) or (
S) according to a prioritysystem (Cahn Ingold Prelog priority rules) based on the atomicnumber of the molecule's substituents. Approximately 56% of
* Corresponding author. Mailing address: School of Chemistry and
the APIs currently in use are chiral compounds, and 88% of
Manchester Interdisciplinary Biocentre, University of Manchester, 131
these chiral APIs are administered therapeutically as the race-
Princess Street, Manchester M1 7DN, United Kingdom. Phone: 0161
mate [i.e., an equal mixture of the two enantiomers, indicated
3064480. Fax: 0161 3064519. E-mail:
[email protected].
䌤 Published ahead of print on 29 January 2010.
by (⫾)]. The chirality of environmental contaminants, such as
WHARFE ET AL.
APPL. ENVIRON. MICROBIOL.
TABLE 1. Microorganisms and conditions used for batch growth
Concn of API (g ml⫺1)
P. putida KT2440
10, 20, 30, 40, 50, 60, 70, 80, 90,
(
R)-to-(
S) ratios of 0:100, 25:75,
50:50, 75:25, 100:0
(⫾), (
R), and (
S)
90, 100, 110, 120, 130
P. aeruginosa PA14
(⫾), (
R), and (
S)
40, 50, 60, 70, 80
(
R)-to-(
S) ratios of 0:100, 25:75,
50:50, 75:25, and 100:0
(⫾), (
R), and (
S)
40, 50, 60, 70, 80
M. luteus 2.13
(⫾), (
R), and (
S)
B. natatoria 2.1
(⫾), (
R), and (
S)
APIs, must be taken into consideration in order to fully un-
used for identification of metabolic changes in fermentations
derstand the environmental fate and effects of these com-
(22). FT-IR spectroscopy is an automated high-throughput
pounds. The enantiomers of a chiral API are able to interact
technique (10 to 60 s per sample is typical) that requires min-
differently with other chiral compounds, such as enzymes, and
imal sample preparation, and this makes it relatively inexpen-
therefore potentially have different effects when they are re-
sive. It is therefore an ideal screening method to explore the
leased into the environment (5, 14, 33). It is widely known that
effects of APIs on a number of bacterial systems.
the enantiomers of a chiral API may have different toxicolog-
In this study the chirality-specific metabolism of the  -
ical and biological effects than each other and than the race-
selective adrenergic blocking agent atenolol and the nonselec-
mate (an equal mixture of the two enantiomers) (25, 54). It has
tive -adrenergic blocking agent propranolol by a range of
been shown that the (
S) enantiomers of the -blocking agents
environmental microorganisms was investigated (14, 41, 48).
atenolol and propranolol are more potent in humans than the
FT-IR spectroscopy was employed to monitor biochemical
corresponding antipodes (3, 11, 35, 45) and that a number of
changes in the spectral fingerprints of whole bacterial cells
the biotransformation pathways for -blockers are stereoselec-
during growth of the microorganisms in the presence of the
tive in humans (30). The mode of action of the drugs and their
selected APIs. In addition, we monitored the fate of the APIs
enantiomers in prokaryotic systems is not known. It is there-
with chiral high-performance liquid chromatography (HPLC),
fore necessary to increase our understanding of the fate and
which allowed quantification of the enantiomers.
biological effects of chiral pharmaceuticals on typical micro-flora in the aquatic environment in order to fully appreciate
MATERIALS AND METHODS
the risks (19). Of particular interest is the group of APIstermed -blockers as they all contain at least one chiral center
Cultivation of bacteria. In order to monitor the effects of the APIs used in the
aquatic environment, a variety of microorganisms were selected for this investi-
and are generally administered therapeutically as the racemate
gation. All of the microorganisms employed in this study have been reported to
(30). In addition, they are widely used, and for example, ap-
be common in the aquatic environment and are amenable to growth in the
proximately 29 and 12 tonnes of atenolol and propranolol,
laboratory. The following four bacteria were selected for this study:
Pseudomo-
respectively, are consumed each year in the United Kingdom
nas putida KT2440, which is known to inhabit freshwater streams and activatedsewage sludge (21, 29);
Pseudomonas aeruginosa PA14, which is commonly iso-
lated from freshwater streams (47); and
Micrococcus luteus 2.13 (40) and
Blas-
In order to explore the effects of the APIs on biological
tomonas natatoria 2.1 (40, 44), which have been isolated from freshwater bio-
systems, we employed Fourier-transform infrared (FT-IR)
films. The bacteria were cultured in R2A medium (38) at 15°C for 24 h at 200
spectroscopy; this is a phenotypic typing technique which has
rpm in a Multitron (INFORS HT, Switzerland) orbital shaker unless otherwise
previously been used to generate metabolic fingerprints of
stated. The pure enantiomers [(
R) and (
S) enantiomers] of both atenolol andpropranolol (as a hydrochloride) were purchased from Sigma-Aldrich Company
bacteria (22, 53). Previous studies have successfully discrimi-
Limited (Poole, Dorset, United Kingdom).
nated bacteria to the subspecies level (31, 50, 53) through
Screening of microorganisms for growth in the presence of APIs. The growth
detection of subtle changes in the biochemical phenotypes of
of each bacterium was monitored (using optical density at 600 nm determined
the bacteria. We recently demonstrated use of FT-IR spectros-
with a Bioscreen spectrophotometer [Labsystems, Basingstoke, United King-dom]) at a range of concentrations (10 to 130 g ml⫺1) of each enantiomer of
copy coupled with suitable chemometrics to physiologically
each API and the racemate. The data collected in these investigations were used
assess bioprocesses (unpublished data). In addition, a combi-
to calculate the specific growth rate and death rate (the death rate is the rate
nation of FT-IR spectroscopy and trajectory analysis has been
when the rate of cell death or lysis exceeds the rate of growth so that there is a
EFFECTS OF PHARMACEUTICALS ON AQUATIC MICROORGANISMS
tion in the spectra. Three replicates of each of the samples were randomlyapplied to the ZnSe plates, and triplicate spectra were obtained using differentpositions in each well; a total of nine spectra (called technical replicates) persample were collected. Each plate was loaded onto an HTS-XT motorizedmicroplate module under computer control by the OPUS software (version 4)(53). Spectra were collected with an Equinox 55 FT-IR spectrometer (BrukerOptics Ltd.) in transmission mode using a deuterated triglycine sulfate detectorover a wavelength range from 4,000 to 600 cm⫺1 and with a resolution of 4 cm⫺1.
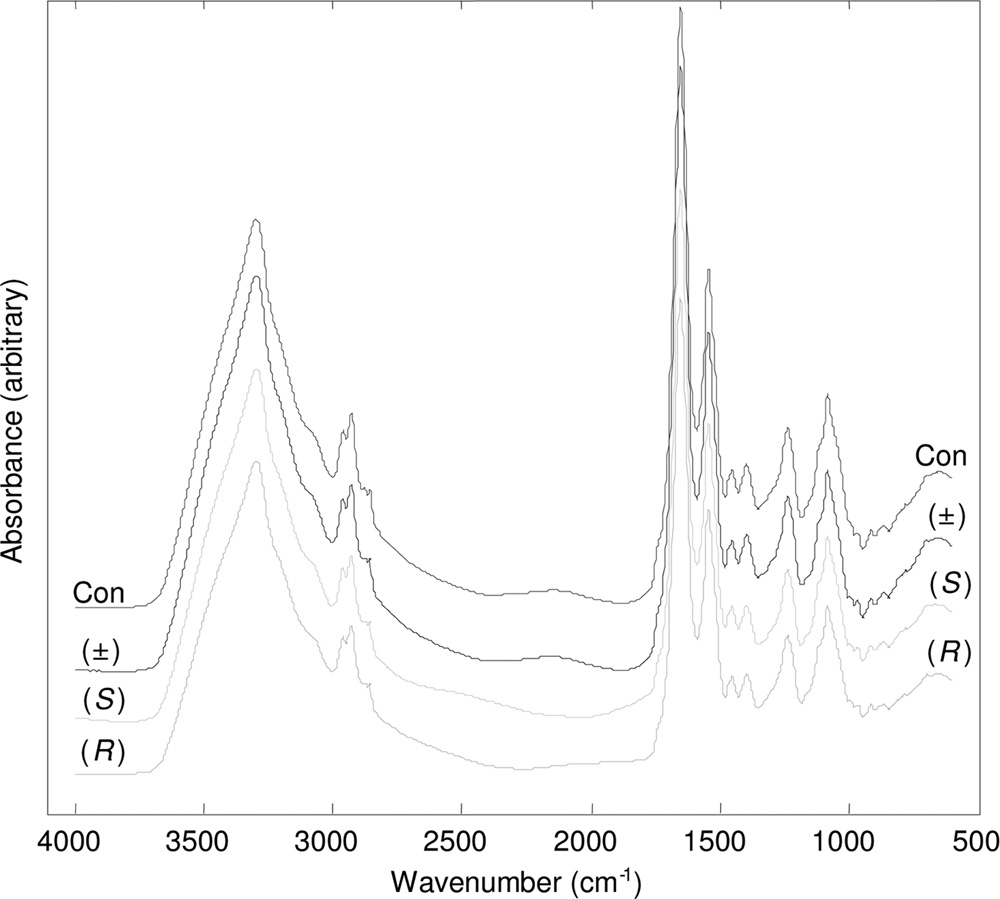
Sixty-four spectra were co-added to improve the signal-to-noise ratio. The spec-tra were displayed in terms of absorbance (Fig. 1 shows typical example spectra).
Analysis of FT-IR spectroscopy data. (i) Spectral preprocessing. The ASCII
data were imported into Matlab version 7.1 (The MathWorks, Inc., Natick, MA),and in the initial step spectral regions which were dominated by CO vibrations
arising from the atmosphere (2,403 to 2,272 cm⫺1 and 683 to 656 cm⫺1) wereremoved and filled with a linear trend. The spectra were corrected using ex-tended multiplicative scatter correction (EMSC), which normalizes andsmoothes spectra by application of a polynomial smoothing function (28). Theresulting preprocessed spectra were used for subsequent multivariate analyses.
(ii) Multivariate analysis. The protocol used for multivariate analysis was a
protocol developed previously (1, 15). Principal component analysis (PCA) is anunsupervised method for reducing the dimensionality of multivariate data whilepreserving the variance. This transformation was performed prior to canonicalvariate analysis (CVA). CVA is a supervised learning method that seeks tominimize within-group variance while it maximizes between-group variance, andit can be used in conjunction with PCA to discriminate between groups on the
FIG. 1. Typical processed FT-IR spectra for P. aeruginosa PA14
basis of retained principal components (PCs), given a priori knowledge of group
exposed to 80 g ml⫺1 (R)-propranolol, (S)-propranolol, and (⫾)-
membership of spectral replicates (26, 52). In this study, PC-CVA models were
propranolol. Control samples (Con) which were not exposed to pro-
constructed with a priori knowledge of the biological replicates. In order to make
pranolol were included. The spectra are offset for clarity.
sure that these models were not over- or undertrained, validation was performedusing the full cross-validation method, where two of the biological replicateswere used for model training and the third replicate was projected into the model
decrease in the turbidity of the culture [39]) for the exponential phases using the
for cluster validation (20). Finally, CVA also allowed statistical significance to be
following equation: ⫽ 2.303(log OD2 ⫺ log OD1)/t ⫺ t ), where is the
displayed on the score plots, and circles were used to indicate the 95% 2
specific growth rate or death rate, log OD1 is the log
optical density at time
confidence region constructed around each group mean based on the 2 distri-
point 1, log OD2 is the log
optical density at time point 2, t is time point 1,
bution with 2 degrees of freedom (24).
and t is time point 2. The growth rate data (see below) were used to select a
Partial least-squares (PLS) (27) regression is a multivariate linear regression
reduced range of concentrations for further investigation; the concentrations
method which allows the quantitative relationship between different variables
selected were based on identifiable differences in the growth rate which did not
(e.g., API concentration and FT-IR spectra) to be modeled, and it can deal
result in cell death.
efficiently with data sets that are highly correlated. In this study, PLS regression
Batch growth. Bacterial cultures were exposed in triplicate to a range of
was employed to predict the API concentration values from the FT-IR spectros-
concentrations of the chosen API; both the pure enantiomers and a range of
copy data. Like the PC-CVA, the regression models were calibrated with two of
enantiomeric mixtures were used (Table 1). An aliquot (1 ml) of sterile water was
the three biological replicates, and the third replicate was used as an independent
added to an additional set of bacterial samples as a control. Samples were
test set to validate the model and establish whether the models could generalize.
maintained at 15°C and 200 rpm in a Multitron orbital shaker for 24 h.
Aliquots (2 ml) were taken in triplicate from each flask and centrifuged (5 min, 0°C,
RESULTS AND DISCUSSION
16,089 ⫻ g) to harvest the biomass. The supernatant and pelleted biomass werestored at ⫺80°C before further analysis.
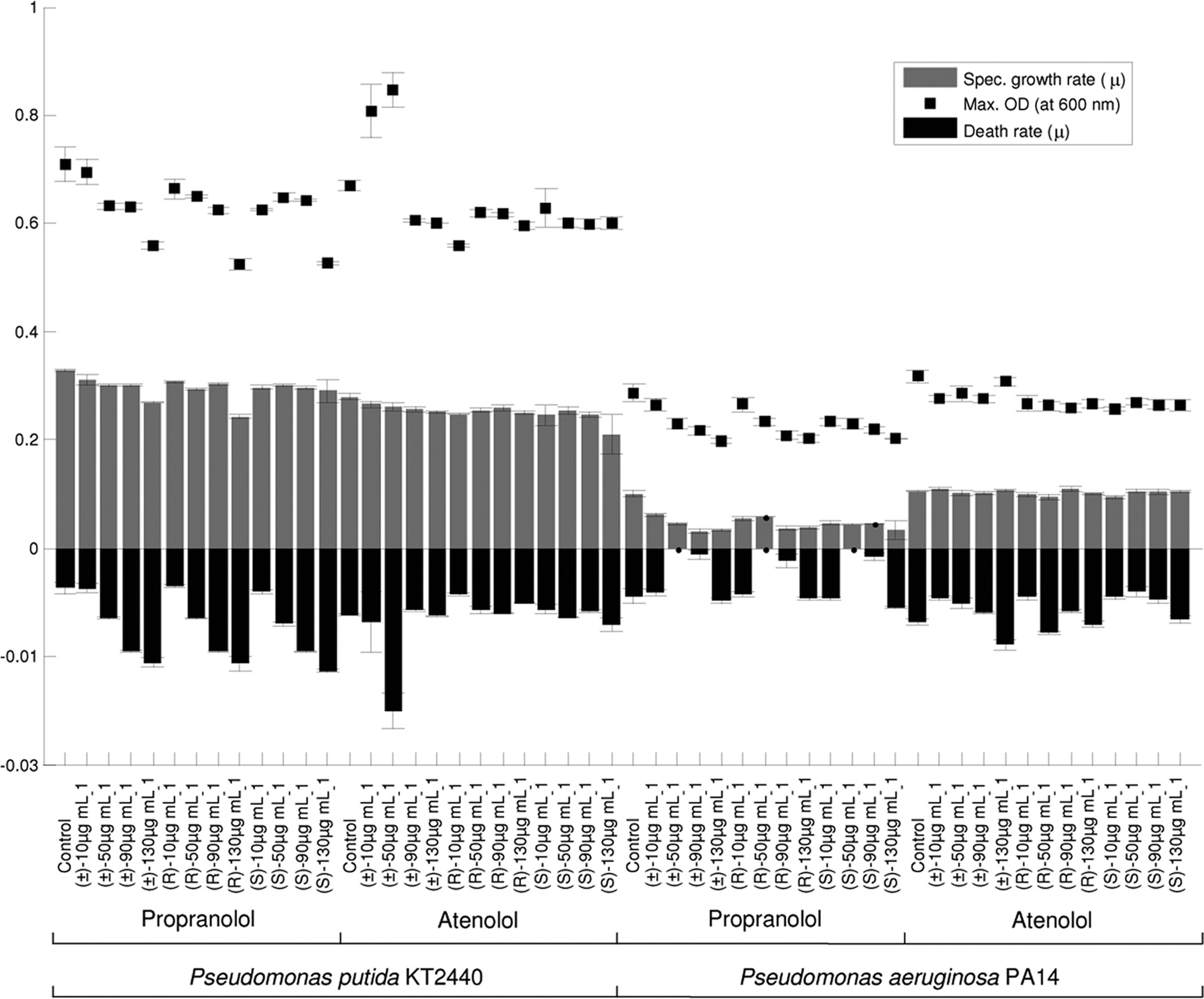
Effects of the chiral APIs on the bacterial growth rates. A
Quantitative analysis of API concentration by HPLC. Concentrations of
number of aquatic microorganisms were exposed to the chiral
atenolol and propranolol were determined by HPLC (Agilent 1100 series). Thesupernatant samples were allowed to thaw at room temperature and were filtered
APIs atenolol and propranolol, and growth rates, death rates,
(0.22 m; Millipore) in order to remove any microbial cells remaining in the
and maximum optical densities were determined to monitor
medium. Aliquots (25 l) were injected onto the HPLC column in a random
the effects of the APIs on culture progress; Fig. 2 shows data
order. Each sample was injected three times during the analysis, resulting in
for 0, 10, 50, 90, and 130 g ml⫺1 of the (R) and (S) enantio-
three analytical replicates for each biological sample. The HPLC system was
mers and the racemic mixtures. Slight variations in the specific
equipped with a Chirobiotic V2 column (250 mm by 4.6 mm [inside diameter];particle size, 5 m; ASTEC, Whippany, NY) and a UV detector operating at a
growth rates were observed for the Pseudomonas species ex-
wavelength of 230 nm. The column was eluted with an isocratic mixture of
posed to different concentrations (10 to 130 g ml⫺1) of (R)-,
methanol and water (90:10, vol/vol) and 1.0% triethylamine acetate (TEAA)
(S)-, and (⫾)-propranolol. There was a considerable difference
buffer (pH 5.0). The pH of the buffer was adjusted with acetic acid prior to the
in the growth rates of species, and a marked effect was ob-
addition of methanol. The measurements were obtained at 25 ⫾ 1°C at a flowrate of 1 ml min⫺1 (4).
served for P. aeruginosa PA14 exposed to propranolol. In con-
Analysis of microbial cells by FT-IR spectroscopy. Ninety-six-well zinc sele-
trast, minimal changes were detected in the growth rates, death
nide plates were cleaned by rinsing them with 2-propanol and deionized water
rates, and maximum amounts of biomass of both Pseudomonas
(three times) and were allowed to dry at room temperature (18, 53). The cell
species exposed to 10 to 130 g ml⫺1 of (R)-, (S)- and (⫾)-
pellets stored at ⫺80°C were allowed to thaw at room temperature and washed
in order to remove any traces of residual API. Ice-cold sterile water (2 ml) wasadded to each sample and gently vortexed. The samples were centrifuged for 10
An interesting effect was observed for P. aeruginosa PA14
min (0°C, 16,089 ⫻ g), and the supernatants were discarded; this cycle was
exposed to both of the propranolol enantiomers and the race-
repeated three times. A final 100-l aliquot of sterile water was added to each
mate. At concentrations of 50 to 70 g ml⫺1 there appeared to
sample, and the solution was vortexed. Aliquots (20 l) of each resuspended
be no death of the microbial cells. In contrast, for cells exposed
sample were applied to ZnSe plates and oven dried at 50°C for 10 min. Dryingwas used to minimize any signal arising from the absorption of water in the
to 10 to 40 g ml⫺1 and to 80 to 130 g ml⫺1 the death rate
mid-IR region, which would mask the biologically important chemical informa-
was equivalent to that of the control cells. This was probably
WHARFE ET AL.
APPL. ENVIRON. MICROBIOL.
FIG. 2. Specific growth rate data for P. putida KT2440 and P. aeruginosa PA14 exposed to 0 to 130 g ml⫺1 of propranolol or atenolol. The
maximum optical densities (OD) at 600 nm and specific death rates are also shown. The data are averages from five biological replicates, and theerror bars indicate standard deviations.
because the lower concentrations (⬍40 g ml⫺1) of propran-
the higher concentrations of propranolol a slight increase in
olol had very little effect on metabolism so cells quickly
the amount of biomass was immediately followed by a notice-
reached the stationary and death phases and because the
able decrease in the optical density of the culture (death
higher concentrations (⬎80 g ml⫺1) had a negative impact on
phase), the maximum amount of biomass was severely inhib-
metabolism and killing cells (as also indicated by the fact that
ited by the presence of the API. Our observations suggest that
the final turbidity measurements were significantly lower than
the API has different effects depending on the concentration
the turbidity measurements for the control cells), while the
applied. At lower concentrations the growth is not affected by
intermediate concentrations (50 to 70 g ml⫺1) slowed growth
the API, and at high concentrations death occurs during the
but the cells did not enter the death phase. Inspection of the
growth period. However, at intermediate concentrations pro-
growth curves indicated that there was a second phase of
duction of biomass occurs throughout the growth period (onset
growth several hours into the stationary phase. While a second
of the death phase may have been observed if the growth had
phase may indicate that there is utilization of a secondary
been monitored for extended periods). The APIs were not
carbon source, this was not observed in the control cultures
metabolized during growth (Table 2), and we hypothesize that
and thus is not the likely explanation for this observation. The
the intermediate concentrations of propranolol affected either
biomass of the culture decreased as the concentration of the
the transport of nutrients into the cell or the rate of metabo-
API increased, and thus the original carbon source was poten-
tially not depleted at the onset of stationary phase. While at
The growth rate data for the cells exposed to propranolol
EFFECTS OF PHARMACEUTICALS ON AQUATIC MICROORGANISMS
TABLE 2. Quantification of propranolol from HPLC data for bacterial cells exposed to different ratios of (R)-propranolol
to (S)-propranolol at a concentration of 50 g ml⫺1
Amt (g ml⫺1) with the following ratio of (R)-propranolol to (S)-propranololb:
P. putida KT2440
P. aeruginosa PA14
a Control experiments (with labeled medium) were performed to determine the experimental effect on the drug concentration (i.e., loss of API during incubation
in growth medium).
b The values are averages for five measurements. The values in parentheses are standard deviations.
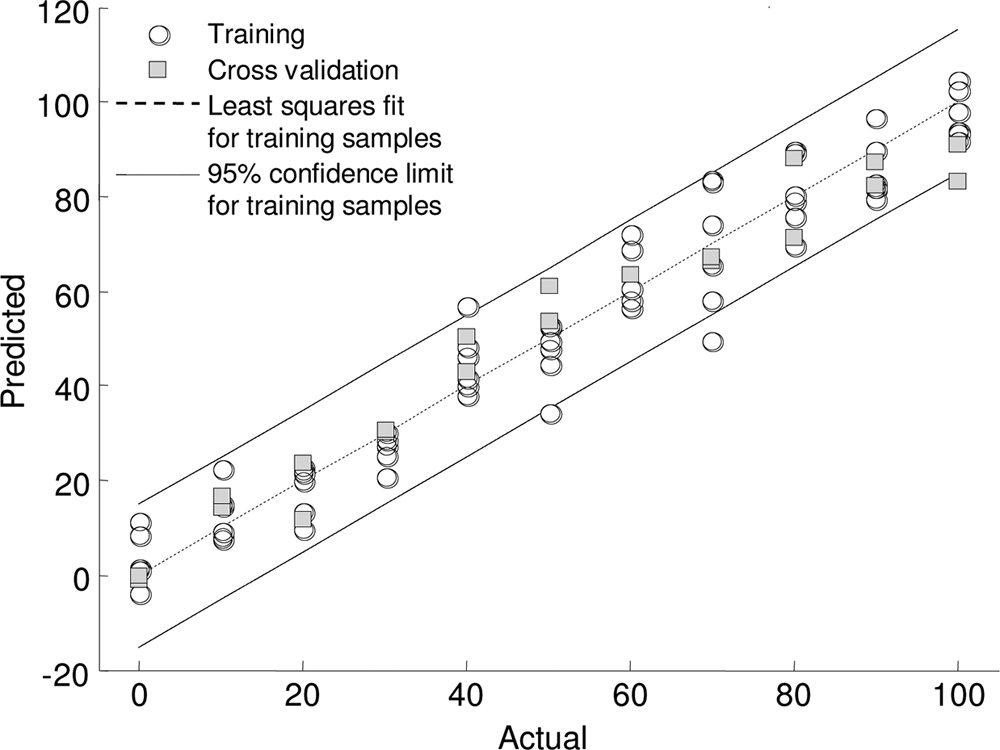
clearly showed that the specific growth rate decreases as the
quantitative drug effect was explored further using FT-IR spec-
concentration of the API increases. This trend was also ob-
troscopy and P. putida KT2440 exposed to (⫾)-propranolol.
served in the maximum optical density data and the death rate
Quantitative effects of APIs on bacteria measured using
data for both pseudomonads. Our findings indicate that pro-
FT-IR spectroscopy. In order to assess possible quantitative
pranolol has considerably different effects on the two Pseudo-
effects of propranolol on the phenotype of P. putida KT2440,
monas species. These findings are rather surprising as these
we employed partial least-squares (PLS) regression analysis to
species are genetically closely related. Estimates have shown
investigate whether the effect on the phenotype as measured
that there is greater similarity (60% of the predicted coding
using FT-IR spectroscopy was directly proportional to the con-
sequences) between these two pseudomonads than between
centration of API applied (Fig. 3). A clear linear relationship
any other complete microbial genomes obtained to date (32).
was observed between the concentration of (⫾)-propranolol to
In addition, comparative genome analysis has shown that 85%
which the P. putida KT2440 cells were exposed and the meta-
of the genes in the P. putida KT2440 genome have homologues
bolic fingerprint. In addition, we were able to predict the con-
in the P. aeruginosa PAO1 genome (46).
centration of propranolol to which the bacterial cells were
The toxic effects of the APIs observed here for aquatic
exposed with an accuracy of 95.45%. This is perhaps not sur-
organisms have been previously reported. Toxicity studies car-
prising as the inhibitory effect of the propranolol on the cells
ried out by Choi and coworkers with the crustacean Thamno-
was proportional to the concentration of API. This was a clear
cephalus platyurus and a fish species (Oryzias latipes) showed
phenotypic effect as we were unable to collect a propranolol
that propranolol caused acute toxicity in T. platyurus at a con-
spectrum at these concentrations when using FT-IR spectros-
centration of 10.61 g ml⫺1 and in O. latipes at a concentration
copy. We also performed PLS regression analysis with the
of 11.40 g ml⫺1. In contrast to the results presented here,
profiles of P. aeruginosa PA14 exposed to the intermediate
these workers found that atenolol did not have toxic effects in
concentrations of propranolol to determine if the secondary
the aquatic organisms at the concentrations that they used(⬍100 g ml⫺1) (9). In addition, toxicity studies have beencarried out with a range of APIs (including propranolol) usingthe Japanese medaka fish (O. latipes), an amphipod (Hyalellaazteca), and two crustaceans (Ceriodaphnia dubia and Daphniamagna). It was found that propranolol had the greatest effecton the organisms studied. The crustacean C. dubia displayedresponses to toxicity at a concentration of 0.25 g ml⫺1. Pro-pranolol was the only API investigated which was found tohave acute toxic effects in the Japanese medaka fish. Theseeffects were observed at a concentration of 0.5 g ml⫺1 (19).
Previous studies have suggested that -blockers do not affect
microbes due to the absence of the API receptors in theseorganisms (10, 23). However, in another study conducted byour group we reported that (⫾)-propranolol significantly re-duced the amount of lipid storage components of the algaMicrasterias hardyi 649/15 and caused a marked reduction inthe cellular protein content (34). In addition, the findings ob-tained by metabolic fingerprinting suggested that the pheno-type was altered during exposure to this API (34). To our
FIG. 3. Partial least-squares regression model for P. putida KT2440
knowledge, no further studies on the metabolic effects of pro-
exposed to various concentrations (0 to 100 g ml⫺1 in steps of 10 gml⫺1) of (⫾)-propranolol. The model was trained with FT-IR spec-
pranolol in aquatic microorganisms have been carried out.
troscopy data using two of the biological replicates and was validated
The effects on the growth dynamics of the bacteria are likely to
using the third biological replicate. The PLS regression model was
reflect changes in the metabolic potential of the cells, and this
built using 10 factors.
WHARFE ET AL.
APPL. ENVIRON. MICROBIOL.
TABLE 3. Quantification of atenolol from HPLC data for bacterial cells exposed to different ratios of (R)-atenolol
to (S)-atenolol at various concentrations
Amt (g ml⫺1)b
Concn of atenolol
(R) enantiomer
(S) enantiomer
(R) enantiomer
(S) enantiomer
P. putida KT2440
P. aeruginosa PA14
a Control experiments (with labeled medium) were performed to determine the experimental effect on the drug concentration (i.e., loss of API during incubation
in growth medium).
b The values are averages for five measurements. The values in parentheses are standard deviations.
growth effect was proportional to the concentration of API
a PC-CVA score plot represents the degree of similarity or
applied (data not shown). Under these conditions it was not
dissimilarity between the samples. A smaller distance indicates
possible to obtain a correlation between the drug concentra-
greater similarity, and a larger distance indicates that there are
tion and the FT-IR spectroscopy data. Therefore, the presence
greater differences between samples. Loading plots provide an
of the drug may have led to more complex biochemical per-
indication of which regions of the spectrum are used to define
turbations in the organisms that we were unable to model using
the patterns of separation, which allows meaningful biochem-
PLS regression.
ical interpretation of the results. FT-IR spectroscopy analysis
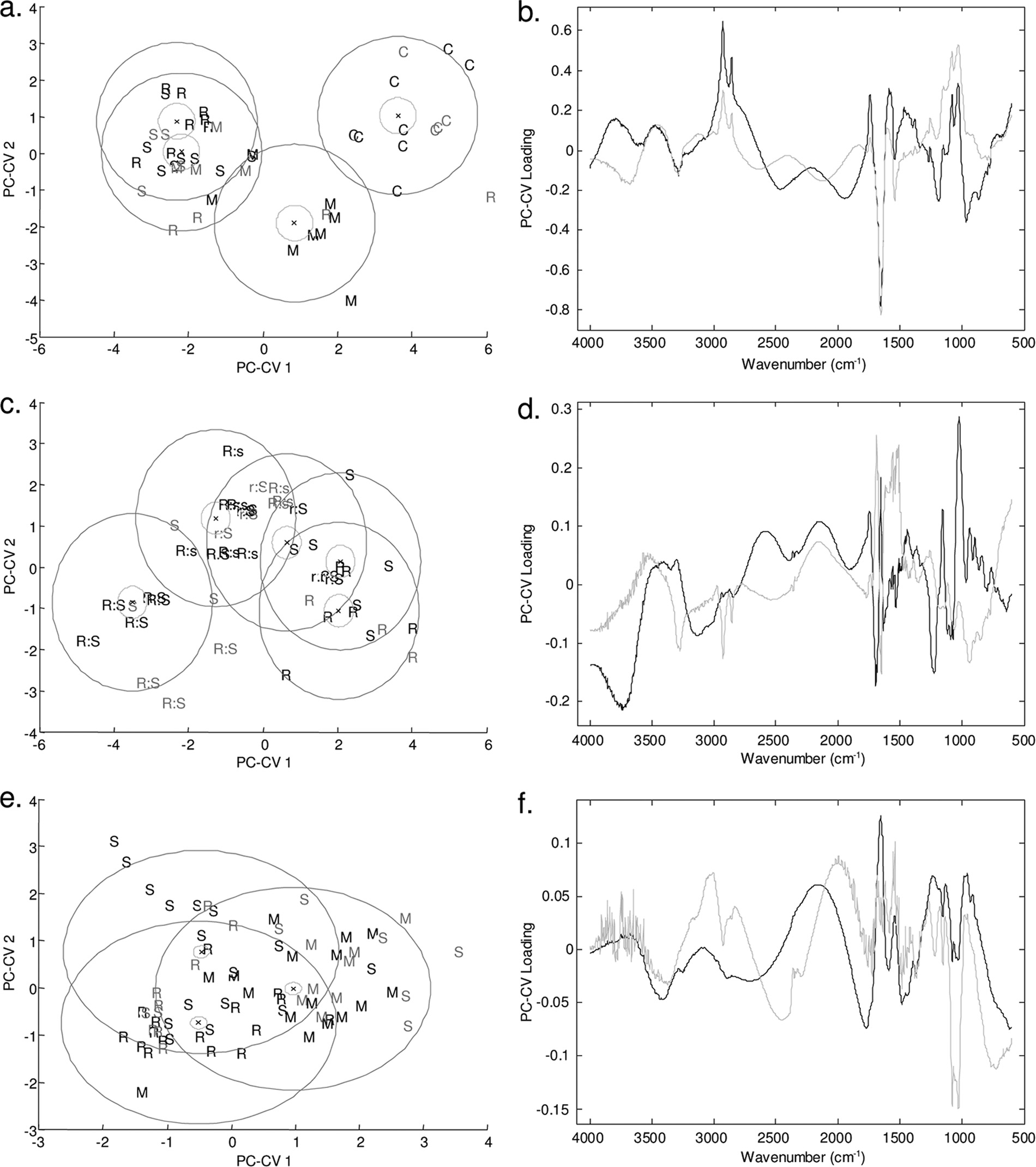
Quantitative analysis of API concentration with HPLC.
demonstrated that each of the propranolol enantiomers had a
Chiral HPLC analysis was performed to quantify the amounts
metabolic effect on the cells of P. aeruginosa PA14 at a con-
of the enantiomers remaining at the end of the growth period.
centration of 80 g ml⫺1 (Fig. 4a) compared to the control.
This analysis was used to explore the effects shown by the
The PC-CVA score plot clearly shows that the control samples
growth rate data. In addition, control experiments were per-
separate across PC-canonical variate 1 (CV1), which accounts
formed to determine the effect of the experiment on the drug
for the greatest variance in the data according to the putative
concentration (i.e., the loss of API during incubation in growth
class assignment; as discussed above, this finding was perhaps
medium). To determine the effects of the enantiomers on the
not surprising given the effect of the APIs on the bacterial
growth of the pseudomonads, a range of ratios of propranolol
growth dynamics (Fig. 2). In addition, the samples exposed to
enantiomers were employed using a concentration of 50 g
(⫾)-propranolol are clearly separate from the samples exposed
ml⫺1. The findings of the HPLC analysis (Table 2) demon-
to each of the enantiomers [(R) and (S)], which in this analysis
strated that neither of the enantiomers was degraded during
showed no separation across the first two PC-CV scores. As
batch growth. In addition, the pseudomonads were exposed to
described above, two of the three biological replicates were
a range of concentrations of the API atenolol (Table 3); the
used for calibration (indicated by black type in Fig. 4), and the
concentrations selected were chosen based on the growth ratedata. There was no notable indication of API degradationduring growth of the bacteria.
TABLE 4. Concentrations of propranolol and atenolol at which a
Effects of chiral APIs on FT-IR spectroscopy metabolic fin-
significant effect on the bacterial phenotype was observed
gerprints. In order to investigate whether there were any
using FT-IR spectroscopy
chirality-specific phenotypic changes in the various aquatic
Concn at which effect was observed
bacteria, a single drug concentration with which there was no
(g ml⫺1)a
observable difference in the growth rate between the enantio-
mers was chosen for investigation.
P. putida KT2440
During the investigation of the chiral API-specific effects on
P. aeruginosa PA14
microorganisms, the four bacteria were exposed to a number of
M. luteus 2.13
drug concentrations (Table 1). A summary of the statistically
B. natatoria 2.1
significant differences in the data for the drug enantiomers,
a Effects were considered significant in PC-CVA plots if confidence regions
enantiomer ratios, and racemate is shown in Table 4.
were statistically different at the 95% 2 limit; that is, below the concentration
PC-CVA was carried out in order to investigate any chiral-
indicated all bacteria had equivalent phenotypes (overlapping clusters) andabove the concentration indicated there was clear differentiation.
ity-specific effects on the microorganisms as determined by
b ND, not determined as propranolol appeared to produce the most notable
FT-IR spectroscopy. The distance between samples plotted on
effects in earlier experiments.
EFFECTS OF PHARMACEUTICALS ON AQUATIC MICROORGANISMS
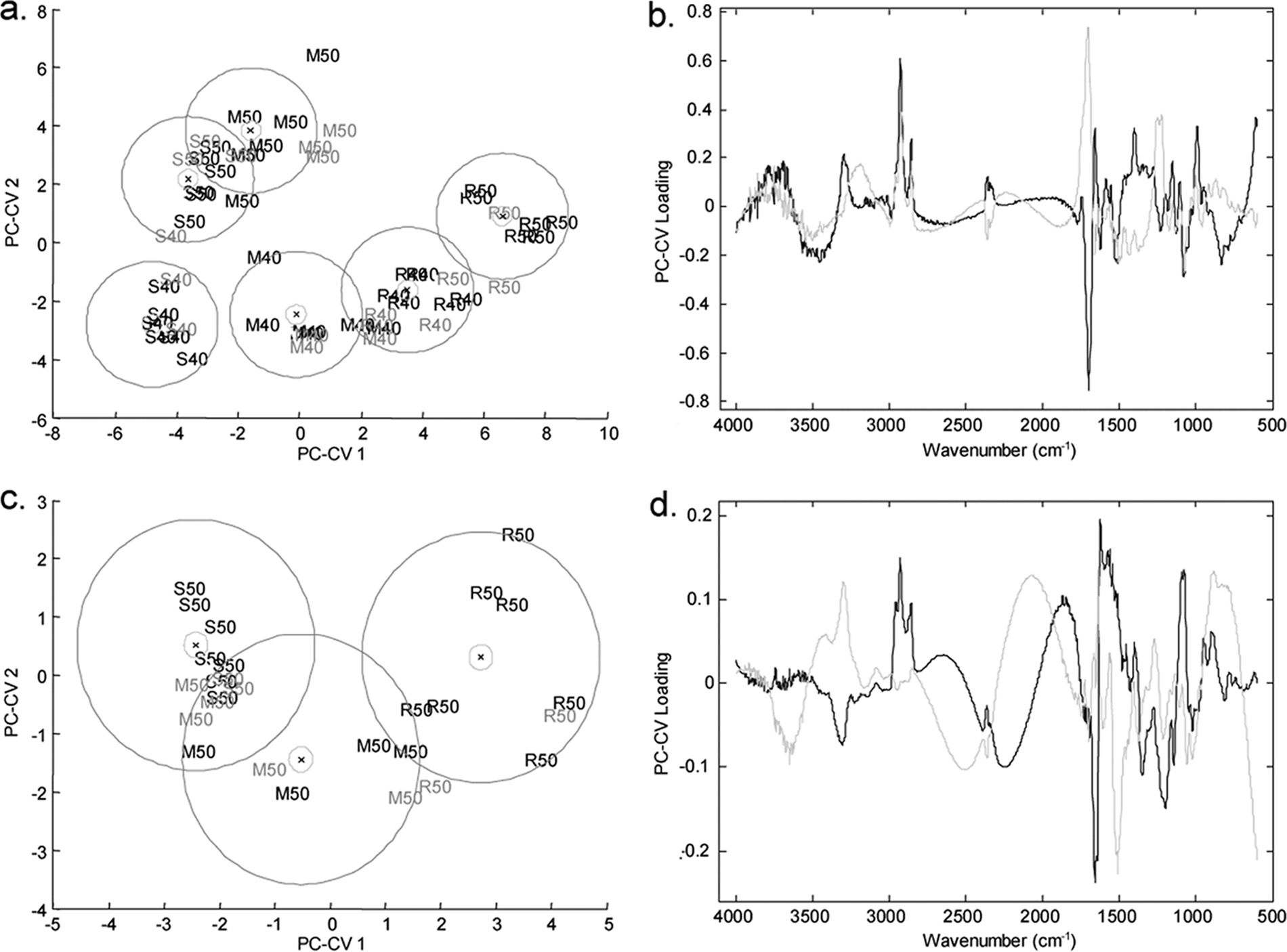
FIG. 4. PC-CVA score (left side) and loading (right side) plots for FT-IR spectroscopy data for P. aeruginosa PA14 exposed to (R)-propranolol,
(S)-propranolol, and (⫾)-propranolol at a concentration of 80 g ml⫺1 (a and b), for P. putida KT2440 exposed to different ratios of (R)-propranolol to(S)-propranolol at a concentration of 50 g ml⫺1 (c and d), and for P. aeruginosa PA14 exposed to (R)-atenolol, (S)-atenolol, and (⫾)-atenolol at aconcentration of 80 g ml⫺1 (e and f). In the score plots black type indicates the two biological replicates used to train the PC-CVA models. Gray typeindicates the third biological replicate, which was used to validate the PC-CVA model. Black circles indicate the 95% confidence interval around thegroup centroid, and gray circles indicate the 95% confidence region around the group sample population. In the loading plots the loading for PC-CV1is indicated by black lines and the loading for PC-CV2 is indicated by gray lines. C, control; M, racemic mixture; R, (R) enantiomer; S, (S) enantiomer;R:s, ratio of (R) enantiomer to (S) enantiomer of 75:25; r:S, ratio of (R) enantiomer to (S) enantiomer of 25:75.
third biological replicate was projected into the model (indi-
the 95% confidence intervals for the groups are also indicated
cated by gray type). The majority of the projected data are
in Fig. 4 and show that for three of the groups there is a distinct
grouped with the appropriate calibration samples, indicating
separation in CVA score space. This analysis demonstrated
that the separation shown in the model was valid. Moreover,
that the microbial cells exposed to (R)- and the microbial cells
WHARFE ET AL.
APPL. ENVIRON. MICROBIOL.
exposed to (S)-propranolol clustered together, indicating that
left side and the results for the 25:75 mixture of (R)-propran-
there were no metabolic differences in the microbial cells ex-
olol and (S)-propranolol (indicated by r:S) and for the pure
posed to the two pure enantiomers. It was very surprising that
enantiomers on the right side. The 75:25 mixture of (R)-pro-
the cultures exposed to the racemate formed a distinct cluster
pranolol and (S)-propranolol (indicated by R:s) falls between
separate from the clusters containing cultures exposed to the
these two groups. The clear separation of P. putida KT2440
pure enantiomers or control samples in the CVA space. The
exposed to the racemate supports the observations on the
loading plot (Fig. 4b) indicates that very specific changes in
effect of propranolol on the metabolic fingerprints of P. aerugi-
the metabolic fingerprints of the microbial cells account for the
nosa PA14 described above.
patterns of separation observed for the control and drug-ex-
In contrast, no phenotypic variation was observed in the
posed samples in the scores plot. The major chemical changes
metabolic fingerprints of the samples exposed to (R)-, (S)-, and
occur in the protein (1,681 to 1,629 cm⫺1) and carbohydrate
(⫾)-atenolol (Fig. 4e and f) as the 95% 2 confidence regions
(1,155 to 999 cm⫺1) regions of the FT-IR spectrum; there was
overlap. This analysis clearly showed that there was no differ-
a less pronounced contribution from lipid species (2,951 to
ence between the three treatments. This result differs from the
2,845 cm⫺1), which changed in the same direction as the car-
results for P. aeruginosa PA14 exposed to propranolol (Fig. 4a)
bohydrates. To investigate the effects of the racemate and
and indicates that atenolol does not have a chirality-specific
enantiomers on the biochemical components of the cells fur-
metabolic effect on P. aeruginosa PA14.
ther, we calculated difference spectra [for example, the meta-
Due to the chirality-specific effects on the pseudomonads
bolic fingerprint of the racemate was subtracted from that of
observed when they were exposed to propranolol, two addi-
the (R) enantiomer] for each combination of interest. The
tional bacteria were used to investigate the effects of propran-
resulting data were then used to determine the relative
olol. Propranolol had a very noticeable metabolic effect on B.
changes in the lipid and amide components of the cells when
natatoria 2.1 at concentrations of 40 and 50 g ml⫺1 (Fig. 5a
they were exposed to drugs. Inspection of the difference spec-
and b) and on M. luteus 2.13 at a concentration of 50 g ml⫺1
tra revealed that the bacterial cells exposed to the racemate
(Fig. 5c and d). The greatest difference observed in these
contained lower levels of amides and higher levels of lipids
analyses was the difference between the control and API-ex-
than the cells exposed to either of the enantiomers. This sug-
posed samples. To investigate the more subtle differences be-
gests that the racemate has less metabolic effect on the bacte-
tween the cultures exposed to the different enantiomers and
rial cells, and this suggestion is supported by the PC-CVA
the racemate, the control samples were removed from the
scores plot, in which the cells exposed to racemate are between
analysis. The B. natatoria 2.1 samples in the PC-CVA score
the control cells and the enantiomer-exposed cells across PC-
plot are separated across the first CV with respect to the
CV1. As discussed above, HPLC analysis suggested that deg-
enantiomers. The (S) and (R) enantiomers are clearly sepa-
radation or significant uptake of the APIs did not occur in the
rated in the CVA space, and the racemate is located between
microbial cells. Therefore, it is unlikely that the increase in the
them. A concentration effect was also observed in the meta-
levels of proteins shown by the FT-IR spectra of propranolol-
bolic fingerprints across CV2. This is in contrast to the chiral-
exposed cells was due to expression of enzymes in order to
ity-specific effects of this API on the two pseudomonads, in
metabolize this API. It is more probable that this effect was
which the greatest variation was found between the cells ex-
due to expression of an efflux system to remove the API from
posed to the racemate and the cells exposed to the enantio-
the bacterial cells. In addition, propranolol is a lipophilic API
mers. The loading data for B. natatoria 2.1 indicate that the
which is known to interact with cell membranes of mammalian
major chemical changes occur in the lipid (2,936 to 2,851
cells, and the observed reduction in the level of lipids in ex-
cm⫺1) region of the FT-IR spectrum and at 1,748 to 1,654
posed cells was likely due to interactions of the API with the
cm⫺1. Vibrations in this region may be attributed to the CAO
bacterial cell wall. Propranolol is routinely administered to
stretching of esters and carboxylic acids; however, this region is
humans as the racemate. The (S) enantiomer accounts for the
dominated by amide I. The FT-IR spectra demonstrate that
majority of the -blocking effect, while the (R) enantiomer has
the cells exposed to (S)-propranolol contained lower levels of
a predominantly membrane-stabilizing effect (3, 17, 36, 51).
lipids but higher levels of amide and carbohydrate than the
We hypothesized that the results for the racemate [(⫾)-pro-
cells exposed to the (R) enantiomer. This suggests that (S)-
pranolol] were different from the results for either of the en-
propranolol has a greater biological effect on the bacterial
antiomers because of the difference in the physical properties
cells. The effect of this API on M. luteus 2.13 and B. natatoria
between the racemate and the enantiomers (8, 42).
2.1 is perhaps more predictable as the different metabolic ef-
To explore the chirality-specific effect observed in the exper-
fects of the enantiomers are linearly additive. The difference
iments described above further, the pseudomonads were ex-
between the phenotypic effects on these organisms following
posed to various ratios of (R)-propranolol to (S)-propranolol
exposure to propranolol and the phenotypic effects on pseudo-
at a concentration of 50 g ml⫺1. The results of the chemo-
monads is probably a consequence of the metabolic differences
metric analysis of the FT-IR spectra also showed that there was
between the bacteria.
a metabolic difference between the microbial cells exposed to
To our knowledge, the -blockers atenolol and propranolol
different ratios of (R)-propranolol to (S)-propranolol and the
have not previously been studied to examine chirality-specific
control cells (data not shown), and the data for the controls
effects in microbial systems. Nevertheless, the effects of APIs in
were removed prior to PC-CVA so that only chirality-specific
these systems are highly relevant, as microorganisms populate
changes were observed. The results of this PC-CVA are shown
the lower trophic levels in food webs. Therefore, differences in
in Fig. 4c, which shows the results for P. putida KT2440 ex-
the population dynamics could represent significant effects
posed to 50 g ml⫺1 (⫾)-propranolol (indicated by R:S) on the
on the whole freshwater community (23).
EFFECTS OF PHARMACEUTICALS ON AQUATIC MICROORGANISMS
FIG. 5. PC-CVA score (left side) and loading (right side) plots for FT-IR spectroscopy data for B. natatoria 2.1 exposed to (R)-propranolol,
(S)-propranolol, and (⫾)-propranolol at concentrations of 40 and 50 g ml⫺1 (a and b) and for M. luteus 2.13 exposed to (R)-propranolol,(S)-propranolol, and (⫾)-propranolol at a concentration of 50 g ml⫺1 (c and d). In the score plots black type indicates the two biological replicatesused to train the PC-CVA models. Gray type indicates the third biological replicate, which was used to validate the PC-CVA model. Black circlesindicate the 95% confidence interval around the group centroid, and gray circles indicate the 95% confidence region around the group samplepopulation. In the loading plots the loading for PC-CV1 is indicated by gray lines and the loading for PC-CV2 is indicated by black lines. M, racemicmixture; R, (R) enantiomer; S, (S) enantiomer.
Conclusion. The growth data clearly showed that propran-
propranolol at concentrations at which no difference in the
olol had a biological effect on all of the microorganisms
growth rates was observed. The FT-IR spectroscopy analysis
studied. At the higher concentrations tested growth was
revealed that propranolol affected both the lipid and protein
retarded, and in most cases the death rate increased; asso-
contents of the bacterial cells. We hypothesize that this was
ciated changes were observed in the metabolic fingerprints.
likely due to the interaction of the APIs with the microbial cell
The loading plots from the PC-CVA of API-exposed and
walls. A more predictable effect on the metabolic fingerprints
unexposed P. aeruginosa PA14 cells (Fig. 4b) indicate that
was noted during the analysis of exposure of B. natatoria 2.1
propranolol has a widespread effect on bacterial cells, and
and M. luteus 2.13 to propranolol, in which the racemate fell
this effect was also observed in the other bacteria studied.
between the (R) and (S) enantiomers in the PC-CVA. Rather
The results of the HPLC analysis showed that this API was
surprisingly, the most significant effect on the two pseudo-
not degraded during the growth period, and this suggests
monads was the effect of the racemate, while the enantiomers
that the observed changes in the multivariate analysis of the
had identical effects on the phenotypes of the cells. It is pos-
metabolic fingerprints were not due to degradation of the
sible that the physical properties of the racemate were signif-
API but more likely were a secondary effect of the drug.
icantly different from those of the (R) and (S) enantiomers and
Despite the genetic similarity of the two pseudomonads
that this was reflected in how the cells responded to exposure
studied, our findings show that propranolol had different
to this API. This possibility will be investigated in the future.
effects in the two species. In contrast, the growth data show
In conclusion, we have shown that chirality-specific effects
that no effects were observed in the atenolol-exposed cul-
do occur in bacteria, which may have implications for environ-
tures, and this finding was reflected in the results of the
mental ecosystems as APIs are regularly found in the aquatic
multivariate analyses of the bacterial fingerprints (Fig. 4e).
environment. We believe that FT-IR spectroscopy with appro-
All four aquatic bacteria were exposed to the enantiomers of
priate chemometrics is a very powerful method for investigat-
WHARFE ET AL.
APPL. ENVIRON. MICROBIOL.
ing the phenotypes and metabolic differences between APIs
23. Jones, O. A. H., N. Voulvoulis, and J. N. Lester. 2002. Aquatic environmental
when they interact with bacterial cells.
assessment of the top 25 English prescription pharmaceuticals. Water Res.
36:5013–5022.
24. Krzanowski, W. J. 1988. Principles of multivariate analysis: a user's perspec-
tive. Oxford University Press, New York, NY.
25. Lees, P., P. M. Taylor, F. M. Landoni, A. K. Arifah, and C. Waters. 2003.
E.S.W. thanks AstraZeneca and UK BBSRC for financial support.
Ketoprofen in the cat: pharmacodynamics and chiral pharmacokinetics. Vet.
C.L.W., R.M.J., and R.G. thank UK BBSRC for funding.
26. Manly, B. F. J. 1994. Multivariate statistical methods: a primer. Chapman
and Hall, London, United Kingdom.
1. Alsberg, B. K., W. G. Wade, and R. Goodacre. 1998. Chemometric analysis
27. Martens, H., and T. Naes. 1989. Multivariate calibration. John Wiley & Sons,
of diffuse reflectance-absorbance Fourier transform infrared spectra using
Chichester, United Kingdom.
rule induction methods: application to the classification of Eubacterium
28. Martens, H., J. P. Nielsen, and S. B. Engelsen. 2003. Light scattering and
species. Appl. Spectrosc. 52:823–832.
light absorbance separated by extended multiplicative signal correction. Ap-
2. Ashton, D., M. Hilton, and K. V. Thomas. 2004. Investigating the environ-
plication to near-infrared transmission analysis of powder mixtures. Anal.
mental transport of human pharmaceuticals to streams in the United King-
dom. Sci. Total Environ. 333:167–184.
29. Martínez-Bueno, M. A., R. Tobes, M. Rey, and J.-L. Ramos. 2002. Detection
3. Barrett, A. M., and V. A. Cullum. 1968. The biological properties of the
of multiple extracytoplasmic function (ECF) sigma factors in the genome of
optical isomers of propranolol and their effects on cardiac arrhythmias. Br. J.
Pseudomonas putida KT2440 and their counterparts in Pseudomonas aerugi-
nosa PA01. Environ. Microbiol. 4:842–855.
4. Bosakova, Z., E. Curinova, and E. Tesarova. 2005. Comparison of vancomy-
30. Mehvar, R., and D. R. Brocks. 2001. Stereospecific pharmacokinetics and
cin-based stationary phases with different chiral selector coverage for enan-
pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm.
tioselective separation of selected drugs in high-performance liquid chroma-
tography. J. Chromatogr. A 1088:94–103.
31. Naumann, D., D. Helm, and H. Labischinski. 1991. Microbiological charac-
5. Buser, H.-R., T. Poiger, and M. D. Muller. 1999. Occurrence and environ-
terizations by FT-IR spectroscopy. Nature 351:81–82.
mental behavior of the chiral pharmaceutical drug Ibuprofen in surface
32. Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. P.
waters and in wastewater. Environ. Sci. Technol. 33:2529–2535.
Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M.
6. Carlsson, C., A.-K. Johansson, G. Alvan, K. Bergman, and T. Kuhler. 2006.
Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O.
Are pharmaceuticals potent environmental pollutants? Part I. Environmen-
White, J. Peterson, H. Khouri, I. Hance, P. C. Lee, E. Holtzapple, D. Scanlan,
tal risk assessments of selected active pharmaceutical ingredients. Sci. Total
K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H.
Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz,
7. Carlsson, C., A.-K. Johansson, G. Alvan, K. Bergman, and T. Ku
J. Eisen, K. N. Timmis, A. Du
¨ft, B. Tu
¨mmler, and C. M. Fraser. 2002.
Are pharmaceuticals potent environmental pollutants? Part II. Environmen-
Complete genome sequence and comparative analysis of the metabolically ver-
tal risk assessments of selected pharmaceutical excipients. Sci. Total Envi-
satile Pseudomonas putida KT2440. Environ. Microbiol. 4:799–808.
33. Nikolai, L. N., E. L. McClure, S. L. MacLeod, and C. S. Wong. 2006.
8. Chickos, J. S., D. L. Garin, M. Hitt, and G. Schilling. 1981. Some solid state
Stereoisomer quantification of the -blocker drugs atenolol, metoprolol, and
properties of enantiomers and their racemates. Tetrahedron 37:2255–2259.
propranolol in wastewaters by chiral high-performance liquid chromatogra-
9. Choi, H.-J., B.-H. Kim, J.-D. Kim, and M.-S. Han. 2005. Streptomyces neya-
phy-tandem mass spectrometry. J. Chromatogr. A 1131:103–109.
gawaensis as a control for the hazardous biomass of Microcystis aeruginosa
34. Patel, S. A., F. Currie, N. Thakker, and R. Goodacre. 2008. Spatial metabolic
(Cyanobacteria) in eutrophic freshwaters. Biol. Control 33:335–343.
fingerprinting using FT-IR spectroscopy: investigating abiotic stresses on
10. CSTEE. 2001. Opinion on draft discussion paper on environmental risk
Micrasterias hardyi. Analyst 133:1707–1713.
assessment of medicinal products for human use. C2/JCD/csteeop/CPMP
35. Pearson, A. A., T. E. Gaffney, T. Walle, and P. J. Privitera. 1989. A stereo-
paperRAssessHumPharm12062001/D(01). European Commission, Brussels,
selective central hypotensive action of atenolol. J. Pharmacol. Exp. Ther.
11. Davies, C. L. 1990. Chromatography of -adrenergic blocking agents.
36. Potter, L. T., and C. B. Sweetland. 1967. Uptake of propranolol by isolated
J. Chromatogr. B Biomed. Sci. Appl. 531:131–180.
guinea-pig atria. J. Pharmacol. Exp. Ther. 155:91–100.
12. Escher, B. I., N. Bramaz, R. I. L. Eggen, and M. Richter. 2005. In vitro
37. Pouliquen, H., H. Le Bris, and L. Pinault. 1992. Experimental study of the
assessment of modes of toxic action of pharmaceuticals in aquatic life.
therapeutic application of oxytetracycline, its attenuation in sediment and
Environ. Sci. Technol. 39:3090–3100.
sea water, and implications for farm culture of benthic organisms. Mar. Ecol.
13. Fent, K., A. A. Weston, and D. Caminada. 2006. Ecotoxicology of human
Prog. Ser. 89:93–98.
pharmaceuticals. Aquat. Toxicol. 76:122–159.
38. Reasoner, D. J., and E. E. Geldreich. 1985. A new medium for the enumer-
14. Fono, L. J., and D. L. Sedlak. 2005. Use of the chiral pharmaceutical pro-
ation and subculture of bacteria from potable water. Appl. Environ. Micro-
pranolol to identify sewage discharges into surface waters. Environ. Sci.
39. Rice, K. C., and K. W. Bayles. 2003. Death's toolbox: examining the molec-
15. Goodacre, R., E. M. Timmins, R. Burton, N. Kaderbhai, A. M. Woodward,
ular components of bacterial programmed cell death. Mol. Microbiol. 50:
D. B. Kell, and P. J. Rooney. 1998. Rapid identification of urinary tract
infection bacteria using hyperspectral whole-organism fingerprinting and
40. Rickard, A. H., S. A. Leach, C. M. Buswell, N. J. High, and P. S. Handley.
artificial neural networks. Microbiology 144:1157–1170.
2000. Coaggregation between aquatic bacteria is mediated by specific-
16. Halling-Sørensen, B., S. Nors Nielsen, P. F. Lanzky, F. Ingerslev, H. C. Holten
growth-phase-dependent lectin-saccharide interactions. Appl. Environ. Mi-
¨tzhøft, and S. E. Jørgensen. 1998. Occurrence, fate and effects of pharma-
ceutical substances in the environment—a review. Chemosphere 36:357–393.
41. Roberts, P. H., and K. V. Thomas. 2006. The occurrence of selected phar-
17. Hanna, G. M., and F. E. Evans. 2000. Optimization of enantiomeric sepa-
maceuticals in wastewater effluent and surface waters of the lower Tyne
ration for quantitative determination of the chiral drug propranolol by 1H-
catchment. Sci. Total Environ. 356:143–153.
NMR spectroscopy utilizing a chiral solvating agent. J. Pharm. Biomed.
42. Secor, R. M. 1963. Resolution of optical isomers by crystallization proce-
dures. Chem. Rev. 63:297–309.
18. Harrigan, G. G., R. H. LaPlante, G. N. Cosma, G. Cockerell, R. Goodacre,
43. Seiler, J. P. 2002. Pharmacodynamic activity of drugs and ecotoxicology—
J. F. Maddox, J. P. Luyendyk, P. E. Ganey, and R. A. Roth. 2004. Application
can the two be connected? Toxicol. Lett. 131:105–115.
of high-throughput Fourier-transform infrared spectroscopy in toxicology
44. Sly, L. I., and M. M. Cahill. 1997. Transfer of Blastobacter natatorius (Sly
studies: contribution to a study on the development of an animal model for
1985) to the genus Blastomonas gen. nov. as Blastomonas natatoria comb.
idiosyncratic toxicity. Toxicol. Lett. 146:197–205.
nov. Int. J. Syst. Bacteriol. 47:566–568.
19. Huggett, D. B., B. W. Brooks, B. Peterson, C. M. Foran, and D. Schlenk. 2002.
45. Stoschitzky, K., G. Egginger, G. Zernig, W. Klein, and W. Lindner. 1993.
Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (B-block-
Stereoselective features of (R)- and (S)-atenolol: clinical pharmacological,
ers) on aquatic organisms. Arch. Environ. Contam. Toxicol. 43:229–235.
pharmacokinetic, and radioligand binding studies. Chirality 5:15–19.
20. Jarvis, R. M., and R. Goodacre. 2004. Discrimination of bacteria using
46. Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J.
surface-enhanced Raman spectroscopy. Anal. Chem. 76:40–47.
Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L.
21. John, D. M., and G. F. White. 1998. Mechanism for biotransformation of
Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L.
nonylphenol polyethoxylates to xenoestrogens in Pseudomonas putida. J.
Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith,
D. Spencer, G. K. S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier,
22. Johnson, H. E., D. Broadhurst, D. B. Kell, M. K. Theodorou, R. J. Merry,
R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome
and G. W. Griffith. 2004. High-throughput metabolic fingerprinting of le-
sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen.
gume silage fermentations via Fourier transform infrared spectroscopy and
chemometrics. Appl. Environ. Microbiol. 70:1583–1592.
47. Tan, M.-W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caeno-
EFFECTS OF PHARMACEUTICALS ON AQUATIC MICROORGANISMS
rhabditis elegans by Pseudomonas aeruginosa used to model mammalian bac-
Stereoselective ring oxidation of propranolol in man. Br. J. Clin. Pharmacol.
terial pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 96:715–720.
48. Ternes, T. A. 1998. Occurrence of drugs in German sewage treatment plants
52. Winder, C. L., E. Carr, R. Goodacre, and R. Seviour. 2004. The rapid
and rivers. Water Res. 32:3245–3260.
identification of Acinetobacter species using Fourier transform infrared spec-
49. Thacker, P. D. 2005. Pharmaceutical data elude researchers. Environ. Sci.
troscopy. J. Appl. Microbiol. 96:328–339.
53. Winder, C. L., S. V. Gordon, J. Dale, R. G. Hewinson, and R. Goodacre. 2006.
50. Timmins, E. M., S. A. Howell, B. K. Alsberg, W. C. Noble, and R. Goodacre.
Metabolic fingerprints of Mycobacterium bovis cluster with molecular type:
1998. Rapid differentiation of closely related Candida species and strains by
implications for genotype-phenotype links. Microbiology 152:2757–2765.
pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy.
54. Yang, Y., B. Su, Q. Yan, and Q. Ren. 2005. Separation of naproxen enantio-
J. Clin. Microbiol. 36:367–374.
mers by supercritical/subcritical fluid chromatography. J. Pharm. Biomed.
51. Walle, T., U. K. Walle, M. J. Wilson, T. C. Fagan, and T. E. Gaffney. 1984.
Source: http://dbkweb.mib.man.ac.uk/pubs/pdfs/Wharfe-AEM2010.pdf
J. Med. Toxicol. (2011) 7:205–212DOI 10.1007/s13181-011-0162-6 2,4-Dinitrophenol (DNP): A Weight Loss Agentwith Significant Acute Toxicity and Risk of Death Johann Grundlingh & Paul I. Dargan &Marwa El-Zanfaly & David M. Wood Published online: 8 July 2011 # American College of Medical Toxicology 2011 Abstract 2,4-Dinitrophenol (DNP) is reported to cause Keywords Dinitrophenol . Weight loss . Toxicity. Fatality
INSTRUCTION MANUAL Super Miniature Variable Power Transmitters With Digital Hybrid Wireless® TechnologyUS Patent 7,225,135 SMQV Dual Battery Model Fill in for your records: Rio Rancho, NM, USA Super-Minature Belt Pack Transmitter General Technical Description The voltage and current requirements of the wide vari- The Digital Hybrid design results in a signal-to-noise ratio








